Reaction Product Variability and Biological Activity of the Lactoperoxidase System Depending on Medium Ionic Strength and pH, and on Substrate Relative Concentration
Françoise Bafort
Integrated and Urban Plant Pathology Laboratory, Gembloux Agro-Bio Tech, Liège University, Passage des Déportés 2, 5030 Gembloux, Belgium
Search for more papers by this authorChristian Damblon
Structural Biological Chemistry Laboratory (SBCL), Liège University, 4000 Liège, Belgium
Search for more papers by this authorNicolas Smargiasso
Molecular Systems Research Unit, Mass Spectrometry Laboratory, Liège University, 4000 Liège, Belgium
Search for more papers by this authorEdwin De Pauw
Molecular Systems Research Unit, Mass Spectrometry Laboratory, Liège University, 4000 Liège, Belgium
Search for more papers by this authorMohamed Haïssam Jijakli
Integrated and Urban Plant Pathology Laboratory, Gembloux Agro-Bio Tech, Liège University, Passage des Déportés 2, 5030 Gembloux, Belgium
Search for more papers by this authorFrançoise Bafort
Integrated and Urban Plant Pathology Laboratory, Gembloux Agro-Bio Tech, Liège University, Passage des Déportés 2, 5030 Gembloux, Belgium
Search for more papers by this authorChristian Damblon
Structural Biological Chemistry Laboratory (SBCL), Liège University, 4000 Liège, Belgium
Search for more papers by this authorNicolas Smargiasso
Molecular Systems Research Unit, Mass Spectrometry Laboratory, Liège University, 4000 Liège, Belgium
Search for more papers by this authorEdwin De Pauw
Molecular Systems Research Unit, Mass Spectrometry Laboratory, Liège University, 4000 Liège, Belgium
Search for more papers by this authorMohamed Haïssam Jijakli
Integrated and Urban Plant Pathology Laboratory, Gembloux Agro-Bio Tech, Liège University, Passage des Déportés 2, 5030 Gembloux, Belgium
Search for more papers by this authorAbstract
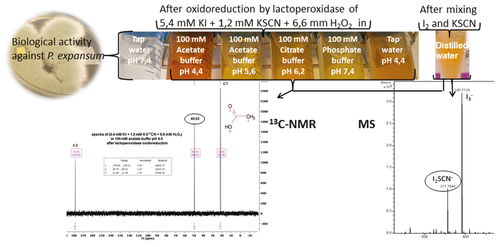
The potential of ions produced in water by the lactoperoxidase system against plant pests has shown promising results. We tested the bioactivity of ions produced by the lactoperoxidase oxidation of I− and SCN− in several buffers or in tap water and characterized the ions produced. In vitro biological activity was tested against Penicillium expansum, the causal agent of mold in fruits, and the major cause of patulin contamination of fruit juices and compotes. In buffers, the ionic concentration was increased 3-fold, and pathogen inhibition was obtained down to the 1:15 dilution. In tap water, the ionic concentration was weaker, and pathogen inhibition was obtained only down to the 1:3 dilution. Acidic buffer increased ion concentrations as compared to less acidic (pH 5.6 or 6.2) or neutral buffers, as do increased ionic strength. 13C-labelled SCN− and MS showed that different ions were produced in water and in buffers. In specific conditions the ion solution turned yellow and a product was formed, probably diiodothiocyanate (I2SCN−), giving an intense signal at 49.7 ppm in 13C-NMR. The formation of the signal was unambiguously favored in acidic media and disadvantaged or inhibited in neutral or basic conditions. It was enhanced at a specific SCN−: I− ratio of 1:4.5, but decreased when the ratio was 1:2, and was inhibited at ratio SCN−>I−. We demonstrated that the formation of the signal required the interaction between I2 and SCN−, and MS showed the presence of I2SCN−.
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under https://doi.org/10.1002/cbdv.201700497.
| Filename | Description |
|---|---|
| cbdv201700497-sup-0001-supinfo.pdfPDF document, 2.6 MB |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. M. Sanzani, M. Reverberi, M. Punelli, A. Ippolito, C. Fanelli, ‘Study on the Role of Patulin on Pathogenicity and Virulence of Penicillium expansum’, Int. J. Food Microbiol. 2012, 153, 323 – 331.
- 2S. P. Snini, J. Tannous, P. Heuillard, S. Bailly, Y. Lippi, E. Zehraoui, C. Barreau, I. P. Oswald, O. Puel, ‘Patulin is a Cultivar-Dependent Aggressiveness Factor Favouring the Colonization of Apples by Penicillium expansum’, Mol. Plant Pathol. 2016, 17, 920 – 930.
- 3A. Welk, C. Meller, R. Schubert, C. Schwahn, A. Kramer, H. Below, ‘Effect of Lactoperoxidase on the Antimicrobial Effectiveness of the Thiocyanate Hydrogen Peroxide Combination in a Quantitative Suspension Test’, BMC Microbiol. 2009, 9, 134.
- 4P. Moskwa, D. Lorentzen, K. J. D. A. Excoffon, J. Zabner, P. B. McCray Jr, W. M. Nauseef, C. Dupuy, B. Bánfi, ‘A Novel Host Defense System of Airways is Defective in Cystic Fibrosis’, Am. J. Respir. Crit. Care Med. 2007, 175, 174 – 183.
- 5H. Välimaa, M. Waris, V. Hukkanen, M. F. J. Blankenvoorde, A. V. Nieuw Amerongen, J. Tenovuo, ‘Salivary Defense Factors in Herpes Simplex Virus Infection’, J. Dent. Res. 2002, 81, 416 – 421.
- 6K. D. Kussendrager, A. C. M. van Hooijdonk, ‘Lactoperoxidase: Physico-Chemical Properties, Occurrence, Mechanism of Action and Applications’, Br. J. Nutr. 2000, 84, Suppl 1, S19 – S25.
- 7K. M. Pruitt, J. O. Tenovuo, ‘ The Lactoperoxidase System: Chemistry and Biological Significance’, Immunol. Ser. 27, Dekker, New York and CRC Press, 1985.
- 8T. M. Aune, E. L. Thomas, ‘Accumulation of Hypothiocyanite Ion During Peroxidase-Catalyzed Oxidation of Thiocyanate Ion’, Eur. J. Biochem. 1977, 80, 209 – 214.
- 9T. M. Aune, E. L. Thomas, ‘Oxidation of Protein Sulfhydryls by Products of Peroxidase-Catalyzed Oxidation of Thiocyanate Ion’, Biochemistry 1978, 17, 1005 – 1010.
- 10P. Nagy, G. N. L. Jameson, C. C. Winterbourn, ‘Kinetics and Mechanisms of the Reaction of Hypothiocyanous Acid with 5-Thio-2-nitrobenzoic Acid and Reduced Glutathione’, Chem. Res. Toxicol. 2009, 22, 1833 – 1840.
- 11J. Kalmár, K. L. Woldegiorgis, B. Biri, M. T. Ashby, ‘Mechanism of Decomposition of the Human Defense Factor Hypothiocyanite Near Physiological pH’, J. Am. Chem. Soc. 2011, 133, 19911 – 19921.
- 12L. M. Wolfson, S. S. Sumner, ‘Antibacterial Activity of the Lactoperoxidase System: A Review’, J. Food Prot. 1993, 56, 887 – 892.
- 13M. Huwiler, H. Kohler, ‘Pseudo-Catalytic Degradation of Hydrogen Peroxide in the Lactoperoxidase/H2O2/Iodide System’, Eur. J. Biochem. 1984, 141, 69 – 74.
- 14F. Bafort, O. Parisi, J.-P. Perraudin, M. H. Jijakli, ‘Mode of Action of Lactoperoxidase as Related to Its Antimicrobial Activity: A Review’, Enzyme Res. 2014, Article ID 517164.
- 15E. L. Thomas, T. M. Aune, ‘Oxidation of Escherichia coli Sulfhydryl Components by the Peroxidase-Hydrogen Peroxide-Iodide Antimicrobial System’, Antimicrob. Agents Chemother. 1978, 13, 1006 – 1010.
- 16A. Virion, J.-L. Michot, D. Deme, J. Pommier, ‘NADPH Oxidation Catalyzed by the Peroxidase/H2O2 System. Iodide-Mediated Oxidation of NADPH to Iodinated NADP’, Eur. J. Biochem. 1985, 148, 239 – 243.
- 17W. A. Prütz, R. Kissner, T. Nauser, W. H. Koppenol, ‘On the Oxidation of Cytochrome c by Hypohalous Acids’, Arch. Biochem. Biophys. 2001, 389, 110 – 122.
- 18W. A. Prütz, R. Kissner, W. H. Koppenol, H. Rüegger, ‘On the Irreversible Destruction of Reduced Nicotinamide Nucleotides by Hypohalous Acids’, Arch. Biochem. Biophys. 2000, 380, 181 – 191.
- 19S. L. Taylor, L. R. Fina, J. L. Lambert, ‘New Water Disinfectant: An Insoluble Quaternary Ammonium Resin-Triiodide Combination That Releases Bactericide on Demand’, Appl. Microbiol. 1970, 20, 720 – 722.
- 20O. Wyss, F. B. Strandskov, ‘The Germicidal Action of Iodine’, Arch. Biochem. 1945, 6, 261 – 268.
- 21Y.-C. Hsu, S. Nomura, C. W. Krusé, ‘Some Bactericidal and Virucidal Properties of Iodine not Affecting Infectious RNA and DNA’, Am. J. Epidemiol. 1965, 82, 317 – 328.
- 22B. Carroll, ‘The Relative Germicidal Activity of Triiodide and Diatomic Iodine’, J. Bacteriol. 1955, 69, 413 – 417.
- 23M. L. Anson, W. M. Stanley, ‘Some Effects of Iodine and Other Reagents on the Structure and Activity of Tobacco Mosaic Virus’, J. Gen. Physiol. 1941, 24, 679 – 690.
- 24L. R. Fina, N. Hassouna, G. L. Horacek, J. P. Lambert, J. L. Lambert, ‘Viricidal Capability of Resin-Triiodide Demand-Type Disinfectant’, Appl. Environ. Microbiol. 1982, 44, 1370 – 1373.
- 25V. Sambhy, B. R. Peterson, A. Sen, ‘Multifunctional Silane Polymers for Persistent Surface Derivatization and Their Antimicrobial Properties’, Langmuir 2008, 24, 7549 – 7558.
- 26M. Ahariz, P. Courtois, ‘Candida albicans Susceptibility to Lactoperoxidase-Generated Hypoiodite’, Clin. Cosmet. Investig. Dent. 2010, 2, 69 – 78.
- 27A. Slungaard, J. R. Mahoney Jr., ‘Thiocyanate Is the Major Substrate for Eosinophil Peroxidase in Physiologic Fluids: Implications for Cytotoxicity’, J. Biol. Chem. 1991, 266, 4903 – 4910.
- 28R. Ihalin, V. Loimaranta, M. Lenander-Lumikari, J. Tenovuo, ‘The Effects of Different (Pseudo)Halide Substrates on Peroxidase-Mediated Killing of Actinobacillus Actinomycetemcomitans’, J. Periodontal Res. 1998, 33, 421 – 427.
- 29J. N. de Wit, A. C. M. van Hooydonk, ‘Structure, Functions and Applications of Lactoperoxidase in Natural Antimicrobial Systems’, Ned. Melk Zuiveltijdschr. 1996, 50, 227 – 244.
- 30E. H. Bosch, H. Van Doorne, S. De Vries, ‘The Lactoperoxidase System: The Influence of Iodide and the Chemical and Antimicrobial Stability Over the Period of About 18 Months’, J. Appl. Microbiol. 2000, 89, 215 – 224.
- 31F. Bafort, O. Parisi, J.-P. Perraudin, M. H. Jijakli, ‘The Lactoperoxidase System: A Natural Biochemical Biocontrol Agent for Pre- and Postharvest Applications’, J. Phytopathol. 2017, 165, 22 – 34.
- 32D. Schlorke, J. Atosuo, J. Flemmig, E.-M. Lilius, J. Arnhold, ‘Impact of Cyanogen Iodide in Killing of Escherichia coli by the Lactoperoxidase-Hydrogen Peroxide-(Pseudo)Halide System’, Free Radic. Res. 2016, 50, 1287 – 1295.
- 33D. Schlorke, J. Flemmig, C. Birkemeyer, J. Arnhold, ‘Formation of Cyanogen Iodide by Lactoperoxidase’, J. Inorg. Biochem. 2016, 154, 35 – 41.
- 34C. L. Schmidt, D. Fischer, H.-J. Himmel, R. Köppe, H. Schnöckel, M. Jansen, ‘To the Knowledge of Interpseudohalogens: A Contribution to Cyanogen Isocyanate (NC–NCO)’, Z. Anorg. Allg. Chem. 2009, 635, 1172 – 1178.
- 35N. N. Greenwood, A. Earnshaw, ‘ Chemistry of the Elements’, ( 2nd ed.), Butterworth-Heinemann, Oxford, 1997.
- 36F. Bernheim, ‘Effect of Cell Size on the Reaction of Uranium Acetate, Mercuric Chloride, and Cyanogen Iodide with a Strain of Pseudomonas aeruginosa’, Microbios 1971, 4, 87 – 92.
- 37F. Bernheim, ‘The Effect of Cyanogen Iodide and Mercuric Chloride on the Permeability of Cells of Pseudomonas aeruginosa and the Antagonistic Action of Sulfhydryl Compounds’, Proc. Soc. Exp. Biol. Med. 1971, 138, 444 – 447.
- 38B. Vanelslander, C. Paul, J. Grueneberg, E. K. Prince, J. Gillard, K. Sabbe, G. Pohnert, W. Vyverman, ‘Daily Bursts of Biogenic Cyanogen Bromide (BrCN) Control Biofilm Formation Around a Marine Benthic Diatom’, Proc. Natl Acad. Sci. USA 2012, 109, 2412 – 2417.
- 39C. Lewis, D. A. Skoog, ‘Spectrophotometric Study of a Thiocyanate Complex of Iodine’, J. Am. Chem. Soc. 1962, 84, 1101 – 1106.
- 40C. Long, D. A. Skoog, ‘A Thiocyanate Complex of Iodine(I)’, Inorg. Chem. 1966, 5, 206 – 210.
- 41I. Országh, G. Bazsa, M. T. Beck, ‘Spectrophotometric Study of the Reversible Iodine-Thiocyanate Interaction’, Inorg. Chim. Acta 1972, 6, 271 – 274.
- 42J. S. McIndoe, D. G. Tuck, ‘Studies of Polyhalide Ions in Aqueous and Non-Aqueous Solution by Electrospray Mass Spectrometry’, Dalton Trans. 2003, 244 – 248.
- 43R. P. Magnusson, A. Taurog, M. L. Dorris, ‘Mechanisms of Thyroid Peroxidase- and Lactoperoxidase-Catalyzed Reactions Involving Iodide’, J. Biol. Chem. 1984, 259, 13783 – 13790.
- 44M. Huwiler, U. Bürgi, H. Kohler, ‘Mechanism of Enzymatic and Non-Enzymatic Tyrosine Iodination. Inhibition by Excess Hydrogen Peroxide and/or Iodide’, Eur. J. Biochem. 1985, 147, 469 – 476.
- 45M. T. Ashby, ‘Inorganic Chemistry of Defensive Peroxidases in the Human Oral Cavity’, J. Dent. Res. 2008, 87, 900 – 914.
- 46M. T. Ashby, ‘Hypothiocyanite’, Adv. Inorg. Chem. 2012, 64, 263 – 303.
- 47E. L. Thomas, T. M. Aune, ‘Lactoperoxidase, Peroxide, Thiocyanate Antimicrobial System: Correlation of Sulfhydryl Oxidation with Antimicrobial Action’, Infect. Immun. 1978, 20, 456 – 463.
- 48G. D. Vogels, L. Uffink, C. van der Drift, ‘Cyanate Decomposition Catalyzed by Certain Bivalent Anions’, Recl. Trav. Chim. Pays-Bas 1970, 89, 500 – 508.
- 49I. A. Kemp, G. Kohnstam, ‘The Decomposition of Inorganic Cyanates in Water’, J. Chem. Soc. 1956, 893 – 895.
- 50T. I. Crowell, M. G. Hankins, ‘The Hydrolysis of Thiocyanic Acid. I. Dependence of Rate on Acidity Function’, J. Phys. Chem. 1969, 73, 1380 – 1383.
- 51O. Barbosa-Filho, A. J. Monhemius, ‘Leaching of Gold in Thiocyanate Solutions – Part 1: Chemistry and Thermodynamics’, Trans. Inst. Min. Metallurg. C 1994, 103, C105 – C110.
- 52A. R. Amell, ‘Kinetics of the Hydrolysis of Cyanic Acid’, J. Am. Chem. Soc. 1956, 78, 6234 – 6238.
- 53M. B. Jensen, ‘On the Kinetics of the Decomposition of Cyanic Acid’, Acta Chem. Scand. 1959, 12, 1657 – 1670.
10.3891/acta.chem.scand.12-1657 Google Scholar
- 54P. G. Furtmüller, W. Jantschko, G. Regelsberger, C. Jakopitsch, J. Arnhold, C. Obinger, ‘Reaction of Lactoperoxidase Compound I with Halides and Thiocyanate’, Biochemistry 2002, 41, 11895 – 11900.
- 55C. J. van Dalen, M. W. Whitehouse, C. C. Winterbourn, A. J. Kettle, ‘Thiocyanate and Chloride as Competing Substrates for Myeloperoxidase’, Biochem. J. 1997, 327, 487 – 492.
- 56E. L. Thomas, T. M. Aune, ‘Peroxidase-Catalyzed Oxidation of Protein Sulfhydryls Mediated by Iodine’, Biochemistry 1977, 16, 3581 – 3586.
- 57W. C. Bray, H. A. Liebhafsky, ‘Reactions Involving Hydrogen Peroxide, Iodine and Iodate Ion. I. Introduction’, J. Am. Chem. Soc. 1931, 53, 38 – 44.
- 58R. O. Griffith, A. McKeown, ‘Kinetics of the Reaction Between Potassium Thiocyanate and Iodine in Aqueous Solution’, Trans. Faraday Soc. 1935, 31, 868 – 875.
- 59G. A. Bowmaker, D. A. Rogers, ‘Synthesis and Vibrational Spectroscopic Study of Compounds Containing the I(SCN)2–, I2(SCN)–, and I(SeCN)2– Ions’, J. Chem. Soc. Dalton Trans. 1981, 1146 – 1151.
10.1039/DT9810001146 Google Scholar
- 60C. M. Gerritsen, M. Gazda, D. W. Margerum, ‘Non-Metal Redox Kinetics: Hypobromite and Hypoiodite Reactions with Cyanide and the Hydrolysis of Cyanogen Halides’, Inorg. Chem. 1993, 32, 5739 – 5748.
- 61J. R. Pollock, H. M. Goff, ‘Lactoperoxidase-Catalyzed Oxidation of Thiocyanate Ion: A Carbon-13 Nuclear Magnetic Resonance Study of the Oxidation Products’, Biochim. Biophys. Acta 1992, 1159, 279 – 285.
- 62M. Huwiler, H. Jenzer, H. Kohler, ‘The Role of Compound III in Reversible and Irreversible Inactivation of Lactoperoxidase’, Eur. J. Biochem. 1986, 158, 609 – 614.
- 63P. G. Furtmüller, J. Arnhold, W. Jantschko, M. Zederbauer, C. Jakopitsch, C. Obinger, ‘Standard Reduction Potentials of All Couples of the Peroxidase Cycle of Lactoperoxidase’, J. Inorg. Biochem. 2005, 99, 1220 – 1229.
- 64F. A. Fonteh, A. S. Grandison, M. J. Lewis, ‘Factors Affecting Lactoperoxidase Activity’, Int. J. Dairy Technol. 2005, 58, 233 – 236.
- 65S. Okazaki, Y. Uchimura, M. Goto, S. Furusaki, ‘Surfactant–Lactoperoxidase Complex Catalytically Active in Organic Media’, Biochem. Eng. J. 2000, 6, 103 – 107.
- 66Y. L. Khmelnitsky, S. H. Welch, D. S. Clark, J. S. Dordick, ‘Salts Dramatically Enhance Activity of Enzymes Suspended in Organic Solvents’, J. Am. Chem. Soc. 1994, 116, 2647 – 2648.
- 67F. Bafort, J.-P. Barthelemy, O. Parisi, J.-P. Perraudin, H. Jijakli, ‘Development of a Colorimetric Method for the Dosage of OI− Anions and I2 in Aqueous Media’, Commun. Agric. Appl. Biol. Sci. 2014, 79, 155 – 160.
- 68H. S. Kouassi, M. Bajji, Y. Brostaux, A. Zhiri, A. Samb, P. Lepoivre, M. H. Jijakli, ‘Development and Application of a Microplate Method to Evaluate the Efficacy of Essential Oils Against Penicillium italicum Wehmer, Penicillium digitatum Sacc. and Colletotrichum musea (Berk. & M.A. Curtis) Arx, Three Postharvest Fungal Pathogens of Fruits’, Biotechnol. Agron. Soc. Environ. 2012, 16, 325 – 336.