Synthesis and Biological Evaluation of Matijin-Su Derivatives as Potential Antihepatitis B Virus and Anticancer Agents
Abstract
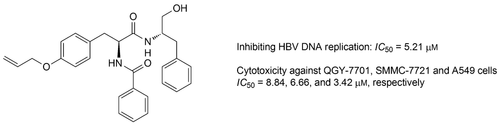
A series of Matijin-Su (MTS, (2S)-2-{[(2S)-2-benzamido-3-phenylpropanoyl]amino}-3-phenylpropyl acetate) derivatives were synthesized and evaluated for their anti-HBV and cytotoxic activities in vitro. Six compounds (4g, 4j, 5c, 5g, 5h and 5i) showed significant inhibition against HBV DNA replication with the IC50 values in range of 2.18 – 8.55 μm, which were much lower than that of positive control lamivudine (IC50 82.42 μm). In particular, compounds 5h (IC50 2.18 μm; SI 151.59) and 5j (IC50 5.65 μm; SI 51.16) displayed relatively low cytotoxicities, resulting in high SI values. Notably, besides the anti-HBV DNA replication activity, compound 4j also exhibited more potent in vitro cytotoxic activity than 5-fluorouracil in two hepatocellular carcinoma cell (HCC) lines (QGY-7701 and SMMC-7721), indicating that 4j may be a promising lead for the exploration of drugs with dual therapeutic effects on HBV infection and HBV-induced HCC.