BRAF mutation is not predictive of long-term outcome in papillary thyroid carcinoma
Abstract
The BRAF mutation occurs commonly in papillary thyroid carcinoma (PTC). Previous investigations of its utility to predict recurrence-free survival (RFS) and disease-specific survival (DSS) have reported conflicting results and its role remains unclear. The purpose of this retrospective study was to determine the incidence of the BRAF mutation and analyze its relationship to clinicopathologic risk factors and long-term outcomes in the largest, single-institution American cohort to date. BRAF mutational status was determined in 508 PTC patients using RFLP analysis. The relationships between BRAF mutation status, patient and tumor characteristics, RFS, and DSS were analyzed. The BRAF mutation was present in 67% of patients. On multivariate analysis, presence of the mutation predicted only for capsular invasion (HR, 1.7; 95% CI, 1.1–2.6), cervical lymph node involvement (HR, 1.7; 95% CI, 1.1–2.7), and classic papillary histology (HR, 1.8; 95% CI 1.1–2.9). There was no significant relationship between the BRAF mutation and RFS or DSS, an observation that was consistent across univariate, multivariate, and Kaplan–Meier analyses. This is the most extensive study to date in the United States to demonstrate that BRAF mutation is of no predictive value for recurrence or survival in PTC. We found correlations of BRAF status and several clinicopathologic characteristics of high-risk disease, but limited evidence that the mutation correlates with more extensive or aggressive disease. This analysis suggests that BRAF is minimally prognostic in PTC. However, prevalence of the BRAF mutation is 70% in the general population, providing the opportunity for targeted therapy.
Introduction
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy 1. The incidence of PTC has increased over the past several decades and now comprises 88% of all thyroid carcinomas 2-5. The course is fairly indolent and treatment is curative, typically involving surgery followed by radioactive Iodine131 (RAI) administration 2, 6. However, a select patient population displays a more aggressive phenotype marked by disease recurrence or death due to thyroid cancer 2. Efforts have been made to identify patients at risk and to develop methods to distinguish markers, such as somatic mutations, that might predict for good versus poor prognosis.
One such mutation that has been extensively studied is the BRAF gene mutation. The majority of these mutations involve a transversion from T to A at nucleotide 1799, leading to a valine to glutamic acid change in codon 600. This results in the BRAFV600E mutation which is the most common mutation in PTC. It occurs in about 70% of patients 7-9. Additional activating mutations in V600, such as V600K and V600D are well described but account for only a minority of cases 10-12. Their exact incidence in a thyroid cancer-specific setting is not established. Multiple publications have reported associations between BRAF V600 mutations (hereafter referred to collectively as BRAF) and poor prognosis 13-16. It has been reported that the BRAF mutation correlates with increased disease recurrence and disease-specific mortality 9, 17-19. However, several recent studies have failed to corroborate these findings, leaving the overall significance of the BRAF mutation unclear 20-24.
Much of the literature investigating the role of the BRAF mutation in outcomes of PTC has been limited by the shortcomings of meta-analysis or has been otherwise limited by low patient numbers or short follow-up. In a disease such as PTC, where negative events such as recurrence and disease-related mortality are rare and often occur late, a large patient cohort and extended follow-up time are critical to the strength of analysis. As such, while some groups have suggested that PTC patients should be routinely tested for the BRAF mutation in order to guide therapy, the prognostic utility of the BRAF mutation as a predictor of recurrence or mortality has not been strongly established 25, 26.
This retrospective study, with the largest cohort of patients and longest follow-up time of any single institution in the United States to date, analyzed the relationship between the BRAF mutation and PTC. Clinical and pathological outcomes, including disease recurrence and disease-specific mortality, and BRAF mutational status were analyzed for possible correlations.
Materials and Methods
Patient identification and clinicopathologic data collection
This retrospective study was approved by the Human Research Protection Office at our institution, including retrospective chart review (protocol number 201010705) with waiver of consent. Records of 1712 patients with thyroid cancer who were referred to the department at our facility from 1974 to 2009 were queried. The data set was interrogated for patients with thyroid carcinoma of follicular cell origin who met the following criteria: underwent either partial or total thyroidectomy, received follow-up care, had classic papillary or follicular variant histologic subtypes of PTC, and had available tumor specimens. A total of 508 patients met criteria. Thyroid tumor specimens were obtained from an archived bank of formalin-fixed, paraffin-embedded (FFPE) thyroid tissue. Anaplastic and undifferentiated thyroid carcinomas were excluded from the study. Data abstracted from patient records included histological subtype, treatment records, and clinicopathologic outcomes. Review of records indicated that none of these individuals had any history of therapeutic radiation exposure. BRAF mutational status was determined after surgical and medical treatments of all patients were concluded and did not affect treatment decisions.
PTC histological classification
Hematoxylin and eosin stained slides were examined by study pathologists to identify areas with classic characteristics of PTC, including papillary architecture, typical PTC nuclei (enlarged, overlapping, irregular, ground-glass empty nuclei with nuclear grooves), psammoma bodies, and stromal reaction 27. Histologically, the 508 cases were comprised of classical (n = 383) and follicular variant (n = 131) subtypes of PTC, classified using standard criteria 27-29. Areas of carcinoma were marked on the glass slides to guide collection of tissue cores from the corresponding FFPE tumor blocks of the case.
DNA extraction and BRAF mutation analysis
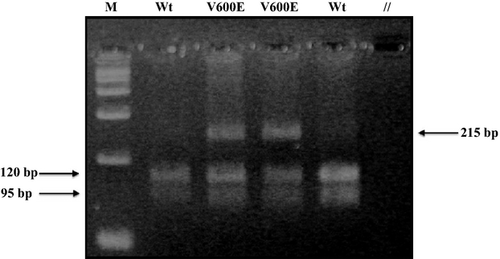
Two tissue cores of 1 mm diameter were extracted from the areas of tumor by means of disposable biopsy punches with plungers (Miltex®, York, PA). Samples were incubated in xylene for three minutes at 50°C; xylene aspiration was followed by two washes with 100% ethanol. Subsequently, samples were incubated for 48 h with 0.5 mg/mL proteinase K (Quiagen®, Germantown, MD), with a mid-interval addition of 0.5 mg/mL proteinase K. DNA was extracted from each sample via a commercial kit (Puregene®, Minneapolis, MN) according to the manufacturer's instructions. Following extraction, DNA was stored at 4°C. Polymerase chain reaction (PCR) was utilized to amplify the 215 base pair (bp) BRAF exon 15, using previously published primers and Platinum Taq DNA Polymerase (Invitrogen®, Carlsbad, CA), according to the manufacturer's instructions. Primers (Invitrogen®) were: BRAF exon 15F (forward): 5′-TCATAATGCTTGCTCTGATAGGA-3′, BRAF exon 15R (reverse): 5′-GGCCAAAAATTTAATCAGTGGA-3′. PCR conditions consisted of 35 cycles with 1 min of denaturation at 94°C, 1 min of annealing at 51°C, and 1 min of extension at 72°C 30. Samples were then subjected to restriction fragment length polymorphism (RFLP) analysis by the enzyme TspRI (Invitrogen®), using the buffer conditions recommended by the manufacturer. TspRI cuts the wild-type 215 bp amplification product into two fragments of 120 and 95 bp. Oncogenic BRAF V600 point mutations disrupt the restriction site, thereby blocking the cleavage of the amplification product. After the restriction digest, the DNA bands were resolved by agarose gel electrophoresis and visualized by ethidium bromide staining.
Assay validation
Restriction fragment length polymorphism (RFLP) analysis has been used by numerous other laboratories to evaluate BRAF exon 15 30-33. We performed several steps to validate the assay for use in our own laboratory. To establish test accuracy, we compared our results with those of direct DNA sequencing by a commercial clinical reference laboratory (GenPath, Elmwood Park, NJ). For 40 cases of PTC diagnosed between 2009 and 2011 (20 cases with and 20 cases without a BRAF V600 mutation) there was 100% concordance between our test and the outside laboratory. These 40 test cases did not overlap with the 508 cases presented here, due to the relative paucity of follow-up data for such recent cases. To establish the reproducibility of the assay, all 40 cases in the validation set were tested three times (in a blinded fashion); there was 100% concordance between test runs. To establish the sensitivity of the test, PCR-amplified DNA from known BRAF mutation samples (at 100 nmol/ml) was diluted into BRAF-negative DNA (also at 100 nmol/mL); dilution ratios were 1:0, 1:1, 1:2, 1:3, 1:5, 1:10, and 1:20 of BRAF mutation positive to negative. These dilutions were then subjected to restriction enzyme analysis and gel electrophoresis, as previously described. The mutant allele could be reproducibly identified when present at an allele frequency of at least 10% in the sample.
Treatment and follow-up
Most patients underwent total thyroidectomy (97%), 3% had a lobectomy or partial resection, and 0.6% of patients had a thyroid biopsy, only. Surgery for metastatic lymph nodes was performed in 57% of patients, consisting of selective nodal dissection in 46% and modified radical neck dissection in 11%. The remaining 43% had no surgical removal of neck lymph nodes. Postoperative 131I was administered to 94% of patients. Nine additional patients were treated with 131I after recurrence. The administered activity of 131I for treatment was based on standard guidelines for adult patients 34, 35. Surveillance consisted of physical examination and laboratory studies including thyroid stimulating hormone (TSH), triiodothyronine, and free thyroxine with the addition of thyroglobulin levels in the latter years of the study.
Statistical analysis
Clinical and pathological outcomes and BRAF mutational status were analyzed for significant associations. P < 0.05 was considered statistically significant and all P values were two-tailed. T tests were used for comparison of data with continuous variables, while Chi-squared tests were used for dichotomous data. All variables associated with the BRAF mutation, disease recurrence, or disease-specific survival (DSS) at the P < 0.05 level were entered into multivariate logistic regression models for BRAF positivity. To remove redundancy and improve the predictive value of the multivariate analysis, complex variables such as AJCC Stage were reduced to the variables they comprise, such as histologic tumor size, extrathyroidal extension, and location of disease at diagnosis. Kaplan–Meier analysis was also performed to estimate the recurrence-free survival and DSS probabilities for BRAF mutation positive versus negative patient groups. Statistical analyses were performed using SAS, Version 9.2 (SAS Institute, Inc., Cary, NC).
Results
Patient characteristics
A total of 508 patients meeting eligibility criteria with evaluable tissue underwent BRAF gene analysis. Patient and tumor characteristics are illustrated in Table 1. Average age at diagnosis was 45.5 ± 15.1 years (range 5.8–84.6 years, median 45.1 years) and mean follow-up time was 9.8 years with a median follow-up time of 8.0 years (range of 0.1–40.1 years). Pathological findings included thyroid capsular invasion in 47%, vascular invasion in 15%, microscopic positive margins in 28%, soft tissue invasion in 30%, and bilateral thyroid involvement in 36%. The average histologic tumor size was 2.0 ± 1.7 cm at the largest dimension. The majority of patients had disease limited to the thyroid only (54.8%, 278/507), 42.2% had disease in the neck nodes and thyroid, 2.8% had distant metastases to the lung, and 0.2% had disease in the thyroid and bone metastases.
| Characteristic | Number (%) or median (range) | BRAFV600 positive (%) | BRAFV600 negative (%) | P value |
|---|---|---|---|---|
| All patients | 508 (100) | 340 (66.9) | 168 (33.1) | NA |
| Gender | ||||
| Male | 125 (24.6) | 88 (70.4) | 37 (29.6) | 0.342 |
| Female | 383 (75.4) | 252 (65.7) | 131 (34.2) | |
| Age at diagnosis (years) | 45.1 (5.8–84.6) | 45.5 (5.8–81.6) | 45.1 (13.9–84.6) | 0.679 |
| Race | ||||
| White | 423 (83.3) | 285 (67.4) | 138 (32.6) | 0.714 |
| Black | 50 (9.8) | 33 (66.0) | 17 (34.0) | |
| Asian | 25 (4.9) | 17 (68.0) | 8 (32.0) | |
| Hispanic | 10 (2.0) | 5 (50.0) | 5 (50.0) | |
| Histology | ||||
| Classic papillary | 377 (74.2) | 266 (70.6) | 111 (29.4) | 0.003 |
| Follicular variant | 131 (25.8) | 74 (56.5) | 57 (43.5) | |
| Pathological features | ||||
| Thyroid capsule invasion | 240 (47.3) | 181 (59.2) | 59 (30.5) | 0.001 |
| Soft tissue invasion | 151 (29.8) | 118 (78.2) | 33 (21.9) | <0.001 |
| Vascular invasion | 74 (14.6) | 54 (73.0) | 20 (27.0) | 0.223 |
| Positive margins | 144 (28.4) | 110 (76.4) | 34 (23.6) | 0.004 |
| Tumor size (cm) | 1.5 (0.1–13.0) | 1.5 (0.1–9.2) | 1.5 (0.2–13.0) | 0.600 |
| Multifocal | 233 (46.0) | 153 (65.7) | 80 (34.3) | 0.597 |
| Cervical LN involvement | 228 (45.0) | 169 (74.1) | 59 (25.9) | 0.002 |
| Extent of disease | 0.004 | |||
| Thyroid only | 278 (54.8) | 170 (61.2) | 108 (38.9) | 0.002 |
| Thyroid and cervical LN | 214 (42.2) | 161 (75.2) | 53 (24.8) | <0.001 |
| Lung metastases | 14 (2.8) | 7 (50.0) | 7 (50.0) | 0.172 |
| Bone metastases | 1 (0.2) | 1 (100) | 0 (0) | 0.482 |
| AJCC tumor stage | ||||
| T1 | 217 (39.8) | 139 (63.4) | 78 (36.6) | 0.037 |
| T2 | 126 (26.3) | 77 (58.0) | 49 (42.0) | |
| T3 | 55 (12.1) | 42 (69.6) | 13 (30.4) | |
| T4 | 106 (21.9) | 80 (71.2) | 26 (28.8) | |
| AJCC nodal stage | ||||
| N0 | 279 (57.5) | 172 (58.1) | 107 (41.9) | 0.015 |
| N1a | 164 (30.8) | 119 (72.2) | 45 (27.8) | |
| N1b | 62 (11.7) | 48 (76.1) | 14 (23.9) | |
| Initial I-131 dose (mCi) | 150 (0–980) | 150 (0–980) | 150 (0–950) | 0.498 |
| Total I-131 dose (mCi) | 150 (0–980) | 150 (0–980) | 150 (0–950) | 0.681 |
| Follow-up time (years) | 8.0 (0.1–40.1) | 8.0 (0.1–30.2) | 7.8 (1.1–40.1) | 0.306 |
- LN, lymph node; AJCC, American Joint Committee on Cancer.
BRAF analysis
The BRAF mutation was present in 66.9% of patients (340/508). Mutation status was identified by RFLP, which displayed two, wild-type DNA bands (120 and 95 bp) digested by the TspRI restriction enzyme when the BRAF V600 mutation was absent, and three DNA bands (215, 120, and 95 bp) when the BRAF V600 mutation was present (heterozygote). Representative cases are illustrated in Figure 1. Follow-up time did not differ between BRAF-positive and BRAF-negative patients (P = 0.507). Initial RAI dose also did not differ between BRAF-positive and -negative patients (P = 0.824). The presence of the BRAF mutation was associated with classic papillary histology (P = 0.003), capsular invasion (P = 0.001), soft tissue invasion (P < 0.001), positive margins after surgery (P = 0.004), cervical lymph node involvement (P = 0.002), and tumor location at diagnosis (P = 0.004) (Table 1). On multivariate analysis, only capsular invasion (HR, 1.7; 95% CI, 1.1–2.6), classic papillary histology (HR, 1.8; 95% CI 1.1–2.9), and cervical lymph node involvement (HR, 1.7; 95% CI, 1.1–2.7) at the time of diagnosis were independently predictive of the BRAF mutation.
Patient outcome
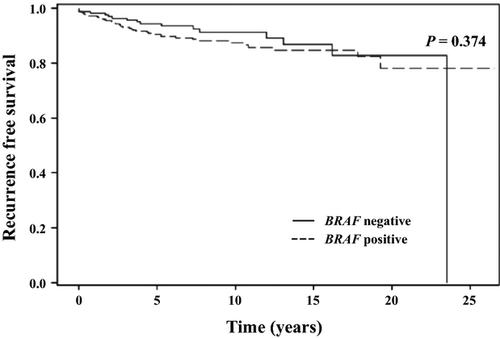
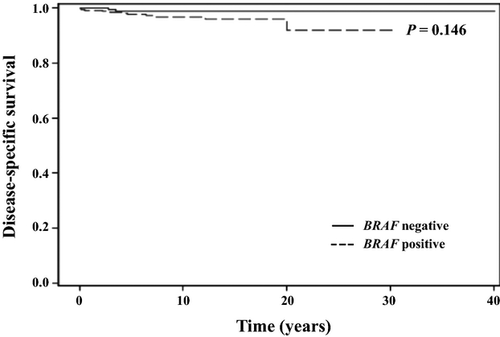
Overall survival for the entire cohort at 10 and 20 years was 95.4% and 84.5%, respectively. A total of 49 patients died during the study period and 13 of these deaths (27%) were due to thyroid cancer. Disease-specific survival at 10 and 20 years was 97.4% and 96.8%, respectively. Recurrence-free survival (RFS) at 10 and 20 years was 88.8% and 80.3%, respectively. There was no difference in the probability of RFS in patients with the BRAF mutation than in patients without the mutation (Fig. 2). Similarly, the probability of DSS was not different in patients with or without the BRAF mutation (Fig. 3). These findings were consistent with the lack of association observed between the BRAF mutation and increased risk of recurrence or cancer-related mortality on univariate and multivariate analyses.
Numerous known correlates of high-risk disease were associated with increased risk for recurrence and disease-specific mortality (Table 2). For recurrence and cancer-specific death, significantly associated pathological features included capsular invasion, vascular invasion, soft tissue invasion, positive surgical margins, and histologic tumor size (Table 2). Tumor involvement of the cervical lymph nodes or lung at diagnosis was also associated with increased recurrence and mortality, whereas tumor confined to the thyroid was negatively correlated with both outcomes (Table 2). Male gender was associated with disease recurrence (P = 0.009) only, while increasing age was associated with increased risk of cancer-specific death (P < 0.001).
| Risk of recurrence | Risk of death | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Characteristic | Hazard ratio for recurrence (95% CI) | P value | Hazard ratio for recurrence (95% CI) | P value | Hazard ratio for disease-specific mortality (95% CI) | P value | Hazard ratio for disease-specific mortality (95% CI) | P value |
| BRAF mutation | 1.3 (0.7–2.3) | 0.376 | Eliminated | NS | 2.9 (0.6–13.1) | 0.165 | Eliminated | NS |
| Age at diagnosis | 1.0 (0.98–1.01) | 0.653 | Eliminated | NS | 1.1 (1.0–1.1) | <0.001 | 8.2 (1.8–37.3) | <0.001 |
| Multifocal disease | 1.5 (0.9–2.6) | 0.112 | Eliminated | NS | 3.4 (0.9–12.6) | 0.066 | Eliminated | NS |
| Classic papillary histology | 2.7 (1.1–6.2) | 0.024 | 2.6 (1.1–6.4) | 0.035 | 3.5 (0.5–27.0) | 0.230 | Eliminated | NS |
| Male gender | 2.0 (1.2–3.5) | 0.009 | Eliminated | NS | 2.6 (0.9–7.7) | 0.088 | Eliminated | NS |
| Disease location | ||||||||
| Thyroid only | 0.2 (0.1–0.4) | <0.001 | 0.1 (0.01–0.6) | 0.013 | ||||
| Nodal involvement | 2.8 (1.6–5.0) | <0.001 | Eliminated | NS | 2.0 (0.6–6.0) | 0.235 | Eliminated | NS |
| Lungs | 7.8 (3.3–18.3) | <0.001 | 22.5 (6.7–75.1) | <0.001 | ||||
| Bone | 0 (0) | 0.986 | 0 (0) | 0.993 | ||||
| Soft tissue invasion | 3.5 (2.1–6.0) | <0.001 | Eliminated | NS | 5.0 (1.5–16.6) | 0.009 | Eliminated | NS |
| Positive margins | 2.9 (1.8–4.6) | <0.001 | Eliminated | NS | 7.5 (2.0–27.8) | 0.002 | 8.2 (1.8–37.3) | 0.006 |
| Thyroid capsule invasion | 4.6 (2.4–8.6) | <0.001 | 2.2 (1.1–4.4) | 0.028 | 13.0 (1.7–100.7) | 0.014 | Eliminated | NS |
| Vascular invasion | 3.6 (2.0–6.2) | <0.001 | 2.1 (1.2–3.8) | 0.01 | 3.8 (1.2–12.0) | 0.023 | 3.7 (1.05–13.6) | 0.042 |
| Cervical LN involvement | 4.6 (2.5–8.8) | <0.001 | 2.8 (1.4–5.4) | 0.004 | 13.7 (1.8–105.6) | 0.012 | Eliminated | NS |
| Histologic tumor size | 1.3 (1.2–1.4) | <0.001 | 1.2 (1.2–1.5) | <0.001 | 1.4 (1.2–1.6) | <0.001 | Eliminated | NS |
- LN, lymph node.
On multivariate analysis, only histologic tumor size (HR, 1.3; 95% CI 1.2–1.5), capsular invasion (HR, 2.2; 95% CI 1.1–4.4), vascular invasion (HR, 2.1; 95% CI 1.2–3.8), classic papillary histology (HR, 2.6; 95% CI 1.1–6.4), and cervical lymph node involvement (HR, 2.8; 95% CI 1.4–5.4) remained independent risk factors for recurrence. Independent predictors of disease-specific mortality included vascular invasion (HR, 3.8; 95% CI 1.05–13.55), positive surgical margins (HR, 8.2; 95% CI 1.8–37.3), and increased age at diagnosis (HR, 1.1; 95% CI 1.1–1.2).
Discussion
This study sought to clarify the relationship between the BRAF mutation and long-term outcome in PTC. Previously, the BRAF mutation has been reported to indicate a poorer prognosis in PTC although there have been significant inconsistencies between various studies 17-24. In our analysis, we found a consistent lack of relationship between the BRAF mutation and either disease recurrence or disease-specific mortality. This was uniformly observed across univariate, multivariate, and Kaplan–Meier analyses.
The method we used to test for the V600E mutation was restriction fragment polymorphism analysis (RFLP). It was the method used by some large reference labs until early 2009 and thus has demonstrated clinical accuracy. It is noteworthy that during most of the time frame covered by the studies in our paper discussing correlations in outcome with BRAF mutations had a physician outside of an academic medical center ordered mutational analysis, it would likely have been done by RFLP analysis. Testing has shifted from RFLP to direct DNA sequence analysis in large reference laboratories because a test kit that has FDA approval has become available, not because of accuracy concerns with RFLP.
A majority of the patients in our cohort (67%) had the BRAF mutation, which is consistent with populations represented in the literature 7-9. It has been suggested that this high prevalence of the BRAF mutation in PTC suggests an important role for the mutation in PTC-related morbidity and mortality 18. However, we propose the opposite. Given the rarity of recurrence and disease-specific mortality in PTC (10% and 5% of patients, respectively) and the contrastingly high prevalence of the mutation (70%, on average, in the literature), an absence of a link between the BRAF mutation and these negative events seems logical 2, 7-9. Importantly, the lack of prognostic value of the BRAF mutation observed in this study is not attributable to difference in follow-up time or treatment between BRAF-positive and -negative patient groups (Table 1).
We also evaluated the association between known clinicopathologic factors of poor prognosis and the BRAF mutation. While multiple poor prognostic factors correlated with BRAF mutations on univariate analysis (Table 1), only capsular invasion, cervical lymph node involvement, and classic papillary histology remained independently predictive of the BRAF mutation on multivariate analysis 13-16. All three have previously been described to predict for BRAF mutations 13-16, 19. The significant association between the BRAF mutation and the classic papillary histologic sub-type of PTC (the most common sub-type) in this analysis, in comparison to the follicular variant sub-type, is a well-documented relationship 13, 20, 21, 24, 26, 36.
Compared to studies that used univariate analysis, our multivariate analysis revealed few risk factors that correlated with the BRAF mutation. A recent publication detailing the University of California, San Francisco experience has called into question the relationship between the BRAF mutation and poor clinicopathologic factors. In the analysis of a cohort of 429 patients, Gouveia, et al. found a minimal association between the mutation and negative prognostic indicators on multivariate analysis 24, 37.
In addition, we analyzed the associations between common clinicopathologic features, RFS, and DSS. The strongest predictor of increased risk on multivariate analysis for recurrence was cervical lymph node involvement, followed by classic papillary histology, capsular invasion, vascular invasion, and then histologic tumor size. These are well-documented risk factors and are components of the current AJCC staging system 9, 13, 19, 24, 34, 35. Our results suggest that the current means of initial prognostication for PTC already accounts for the greatest established predictors of recurrence. For cancer-specific death, the strongest predictors were older age at diagnosis, positive surgical margins, and vascular invasion 9, 17-19, 22. Advanced age and positive margins were associated with an eightfold increase of disease-specific mortality.
The greatest strengths of this analysis are its extensive follow-up time (mean 9.8 years, median 8.0 years, range 40.1 years) and large cohort. The bulk of recurrences and disease-specific deaths in our cohort occurred at least 2.5 years (and up to 23.5 years) after diagnosis. These events were also rare, occurring in only 11.0% and 2.6% of patients, respectively. If the range of follow-up time of our study had been limited to 67 months, the follow-up range in one of the most-cited meta-analyses, we would have failed to capture 25% of disease recurrences and 54% of the cancer-specific deaths in our cohort 19. Thus, the importance of having a large cohort and extensive follow-up data in this study cannot be emphasized strongly enough. One weakness of this analysis is its retrospective nature. However, all patients were treated homogeneously, following guidelines at a single, academic institution.
Our data confirm the high incidence of the BRAF mutation in our population. Approximately 70% of patients will have the mutation. Although the presence of the mutation is not independently predictive of poor outcome, a significant percent of BRAF-positive patients will develop recurrence and metastatic disease. Therefore, treatments guided by presence of the mutation are warranted. Such therapies might include increased cervical nodal dissection and use of BRAF inhibitors. Both in this patient cohort and in previous reports, preoperative BRAF mutation status in cytology specimens has correlated with lymph node status, and some suggest it could guide the extent of node removal during surgery 38. We propose that the clear role for BRAF testing lies in patients who suffer multiple recurrences or develop radioactive iodine (RAI) resistance. Approximately two-thirds of those patients (those with the BRAF mutation) might benefit from targeted therapy with BRAF inhibitors, which have been developed for treatment of metastatic melanoma. BRAF inhibitors have shown promising results against thyroid cancer cells with BRAF mutations in vivo 39. Additionally, preliminary results from the phase III DECISION trial demonstrate that Sorafenib, a non-specific active BRAF inhibitor, significantly improves PFS compared to placebo in patients with progressive RAI-refractory differentiated thyroid carcinoma 40. However, the DECISION trial included both BRAF-positive and BRAF-negative patients, a factor that is likely to significantly reduce the observed efficacy of BRAF inhibitors in this population. A randomized trial to evaluate the safety and efficacy of BRAF inhibitors to improve progression-free survival in progressive RAI-refractory PTC patients, with stratification of patients into sub-groups on the basis of BRAF mutational status is warranted.
Acknowledgments
No NIH grant funding was used in this project.
Conflict of Interest
None declared.




