Targeting SPHK1 in macrophages remodels the tumor microenvironment and enhances anti-PD-1 immunotherapy efficacy in colorectal cancer liver metastasis
Abstract
Background
Colorectal cancer liver metastasis (CRLM) is characterized by an immunosuppressive microenvironment and a blunted response to immunotherapy. Notably, tumor-associated macrophages (TAMs) play a critical role in modulating immune responses and exhibit significant heterogeneity in CRLM. Sphingosine kinase 1 (SPHK1) serves as a pivotal kinase in maintaining the balance between ceramide and sphingosine-1-phosphate (S1P) levels. However, the effects of SPHK1 within TAMs on tumor immune evasion during CRLM remain elusive. This study aimed at investigating the role of TAM-intrinsic SPHK1 in tumor immunosuppressive microenvironment in CRLM.
Methods
SPHK1 expression levels in TAMs were estimated by immunofluorescence and bioinformatics analysis. Several animal models were established to elucidate the role of SPHK1 in tumor immunity reprogramming in vivo. Flow cytometry, cytokine assay, and transwell assay were conducted to investigate the effects of SPHK1 in TAMs in cell-cell communication in vitro. RNA-sequencing, Western blotting, and quantitative real-time polymerase chain reaction were used to explore the molecular mechanism by which SPHK1 activated NLR family pyrin domain containing 3 (NLRP3) inflammasome in TAMs.
Results
We found that SPHK1 was mainly expressed in TAMs and identified SPHK1+ TAMs as associated with CRLM and diminished efficacy of immunotherapy in human patients. These SPHK1+ TAMs exhibited strong immunosuppressive activities by inducing CD8+ T cell exhaustion with high programmed cell death 1 (PD-1) expression via the interaction between TAMs and CRC cells. Mechanistically, SPHK1-produced S1P exerted an autocrine effect to activate NLRP3 inflammasome and interleukin 1 beta (IL-1β) release via nuclear factor-kappa B (NF-κB) and hypoxia inducible factor 1 subunit alpha (HIF-1α) signaling in TAMs. Paracrine IL-1β then upregulated the expression of monocyte chemoattractants and ADAM metallopeptidase domain 17 (ADAM17) sheddase in CRC cells, resulting in TAM infiltration and CD8+ T cell dysfunction in the liver microenvironment. Furthermore, combining SPHK1-targeting treatments with anti-PD-1 therapy or radioimmunotherapy largely stalled liver metastasis and caused a significant extension of lifespan in preclinical mouse models.
Conclusions
Our findings highlighted the role of SPHK1 of TAMs in facilitating CRLM by promoting CD8+ T cell dysfunction and immunosuppressive microenvironment. Combining SPHK1 blockade with anti-PD-1 therapy may be a promising treatment regimen for patients with CRLM.
Abbreviations
-
- AAV
-
- adeno-associated virus
-
- ADAM17
-
- ADAM metallopeptidase domain 17
-
- ALT
-
- alanine transaminase
-
- AST
-
- aspartate transaminase
-
- ATP
-
- adenosine tri-phosphate
-
- BMDMs
-
- bone marrow-derived macrophages
-
- BSA
-
- bovine serum albumin
-
- CCL2
-
- C-C motif chemokine ligand 2
-
- CCL7
-
- C-C motif chemokine ligand 7
-
- CCL8
-
- C-C motif chemokine ligand 8
-
- CD62L
-
- lymphocyte adhesion molecule 62L
-
- CK
-
- creatine kinase
-
- CL
-
- clodronate liposome
-
- CM
-
- conditioned medium
-
- CRC
-
- colorectal cancer
-
- CREA
-
- creatinine
-
- CRLM
-
- colorectal cancer liver metastasis
-
- CSF1R
-
- colony stimulating factor 1 receptor
-
- CTLs
-
- cytotoxic T cells
-
- CXCL12
-
- C-X-C motif chemokine ligand 12
-
- CyTOF
-
- cytometry by time-of-flight
-
- dMMR
-
- mismatch repair-deficient
-
- DAPI
-
- 4',6-diamidino-2-phenylindole
-
- FACS
-
- fluorescence activated cell sorting
-
- GSEA
-
- gene-set enrichment analysis
-
- GZMB
-
- granzyme B
-
- HIF-1α
-
- hypoxia inducible factor 1 subunit alpha
-
- HPF
-
- high-power field
-
- ICIs
-
- immune checkpoint inhibitors
-
- IF
-
- immunofluorescence
-
- IFN-γ
-
- interferon gamma
-
- IHC
-
- immunohistochemistry
-
- IL-10
-
- interleukin 10
-
- IL-1β
-
- interleukin 1 beta
-
- IL-1R
-
- interleukin 1 receptor
-
- IL-2
-
- interleukin 2
-
- IR
-
- irradiation
-
- KEGG
-
- Kyoto encyclopedia of genes and genomes
-
- KIRC
-
- kidney renal clear cell carcinoma
-
- LAG3
-
- lymphocyte activating 3
-
- LPS
-
- lipopolysaccharide
-
- MSI-H
-
- microsatellite instability-high
-
- mUC
-
- metastatic urothelial cancer
-
- NF-κB
-
- nuclear factor-kappa B
-
- NLRP3
-
- NLR family pyrin domain containing 3
-
- P65
-
- RelA, a member of the NF-κB transcription factor family
-
- PBMCs
-
- peripheral blood mononuclear cells
-
- PBS
-
- phosphate buffered saline
-
- PD-1
-
- programmed cell death 1
-
- PD-L1
-
- programmed cell death 1 ligand 1
-
- PMA
-
- phorbol 12-myristate 13-acetate
-
- PMs
-
- peritoneal macrophages
-
- qPCR
-
- quantitative real-time PCR
-
- RNA-seq
-
- RNA sequencing
-
- S1P
-
- sphingosine-1-phosphate
-
- S1PR2
-
- sphingosine-1-phosphate receptor 2
-
- scRNA-seq
-
- single-cell RNA sequencing
-
- SPHK1
-
- sphingosine kinase 1
-
- TAMs
-
- tumor-associated macrophages
-
- TCGA
-
- The Cancer Genome Atlas
-
- TIDE
-
- tumor immune dysfunction and exclusion
-
- TIM3
-
- T-cell immunoglobulin and mucin domain-containing protein 3
-
- TIME
-
- tumor immune microenvironment
-
- TME
-
- tumor microenvironment
-
- UREA
-
- urea nitrogen
-
- WT
-
- wild-type
1 BACKGROUND
The liver is the predominant organ for metastatic spread in colorectal cancer (CRC), with nearly 50% of CRC patients developing colorectal cancer liver metastases (CRLM) throughout the disease process [1]. Most patients with CRLM are not eligible for surgery resection and with a 5-year survival rate as low as 14% [2]. In the past decade, immunotherapy has revolutionized cancer care and has become a promising therapeutic option for cancer patients. Immune checkpoint inhibitors (ICIs) received regulatory approval in 2017 for the treatment of tumors with mismatch repair-deficient (dMMR) or microsatellite instability-high (MSI-H) [3]. However, 15% of CRCs are dMMR or MSI-H, and 4%-5% of advanced CRCs exhibit these features [4]. Moreover, liver metastasis has been currently validated as a negative baseline determinant of ICI efficacy, being associated with an inferior response even in dMMR CRC patients [5], emphasizing the necessity for a more comprehensive understanding of the tumor immune microenvironment (TIME) and its regulators in CRLM.
Across solid tumors, the majority of immune cells in the TIME comprise various immunosuppressive myeloid cells, with tumor-associated macrophage (TAM) being the most abundant [6]. The clinical relevance of TAMs has been investigated in multiple CRC cohorts, indicating TAMs may have context-specific pro-metastatic and anti-metastatic effects. A microarray analysis of 159 CRLM specimens demonstrated that the presence of CD68+ TAMs in CRLM was associated with better prognosis of CRC patients [7]. Conversely, emerging evidence reported the association between TAM density and the presence of CRLM, and identified TAMs as the key determinant of immune tolerance of liver metastasis [8, 9]. Such conflicting results highlight the heterogeneity of TAMs in the TIME of CRLM. Although macrophages are the major population of phagocytes, they fail to successfully destroy tumor cells in the context of cancer. TAMs pave the way to tumor immune evasion through the secretion of immunosuppressive cytokines, like interleukin-10 (IL-10) and transforming growth factor-beta, which facilitate T cell suppression [10]. Cytotoxic T cells (CTLs) are crucial components of adaptive antitumor immunity, and a high CTL infiltration was associated with improved survival in patients after CRLM resection [11]. To promote immune evasion, CTL activity is suppressed through multiple regulators, including TAMs [12]. Increasing evidence suggests that TAM-targeting therapies are of paramount importance to enhance CTL activity and ICI efficacy [13, 14]. For the phenotypic heterogeneity, it is largely unknown how each TAM subpopulation affects adaptive immune response in the context of CRLM. Thus, an in-depth understanding of the roles of TAMs in liver metastasis must be valuable to develop effective immunotherapeutic strategies for CRLM patients.
SPHK1 is a key enzyme that catalyzes the production of sphingosine-1-phosphate (S1P), a bioactive lipid mediator involved in diverse cellular processes, such as inflammation, immune regulation, and tumor progression [15]. Increasing studies have shown that tumor-intrinsic SPHK1 contributes to the development of multiple cancers, including CRC [16, 17]. Recently, preclinical research has reported that tumor-intrinsic SPHK1 promoted tumor growth by suppressing antitumor immunity in immunocompetent melanoma mouse models [18]. However, the specific role of SPHK1 in TAMs during CRLM pathogenesis remains obscure. Here, we evaluated the SPHK1 expression in TAMs in CRLM tissues and the relationship between SPHK1 expression and CRLM patient prognosis. Subsequently, we examined the biological functions of SPHK1 in TAMs through in vitro and in vivo assays. We further investigated the potential mechanism underlying SPHK1-induced CD8+ T cell dysfunction and CRC immune evasion. Finally, we explored the role of combination therapy in sensitizing anti-programmed cell death 1 (PD-1) therapy using a series of animal experiments.
2 MATERIALS AND METHODS
2.1 Cell lines
Mouse colon carcinoma cell lines MC38 and CT26, mouse fibroblast line L929, human colon carcinoma cell line HCT116, and human monocyte macrophage line THP-1 were obtained from the American Type Culture Collection (Manassas, VA, USA). MC38 and L929 were maintained in Dulbecco's modified eagle's medium (DMEM) high glucose (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 U/mL penicillin and 100 µg/mL streptomycin (Gibco). CT26, HCT116 and THP-1 were maintained in Roswell Park Memorial Institute-1640 (RPMI-1640, Gibco) medium supplemented with 10% FBS, 100 U/mL penicillin and 100 µg/mL streptomycin. All cells were cultured at 37°C in a humidified incubator with 5% CO2. To induce differentiation into macrophages, THP-1 cells were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate (PMA, InvivoGen, Toulouse, Occitanie, France) for 48 h. The cell lines used in this study were verified for authenticity through the short tandem repeat genotyping method.
2.2 Mice
Six-week-old wild-type (WT) and SPHK1-knockout (SPHK1−/−) mice on a C57BL/6 background were obtained from Shanghai Model Organisms Center (Shanghai, China). Six- to 9-week-old wild-type C57BL/6 mice and BALB/c mice were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, Guangdong, China). The mice were housed under specific pathogen-free conditions, with room temperature controlled between 22-24°C, a natural light-dark cycle, and free access to food and water. All experimental procedures and protocols were approved by the Animal Care and Use Committee of Southern Medical University (SMUL2022189). Mice were monitored daily for signs of distress, including weight loss, lethargy, or tumor burden. The humane endpoint was defined as a 20% weight loss, or over 1,500 mm3 tumor size, or severe lethargy. Cervical dislocation was used for euthanasia to ensure a quick and painless death.
2.3 Bone marrow-derived macrophage (BMDM) isolation
Six-week-old C57BL/6 mice were euthanized by cervical dislocation, and the legs were dissected. Using aseptic techniques, the tibia and femur bones were separated after the removal of the surrounding muscle. Next, joints of leg bones were cut using a scalpel, and the bone marrow cells were rinsed out with phosphate buffered saline (PBS) at the ends of bones with a 25-gauge needle and a 10 mL syringe. Bone marrow cells were centrifuged at 300 ×g at 4°C for 5 min and resuspended with erythrocyte lysate, followed by adding DMEM medium to terminate the lysis. After a centrifugation at 300 ×g for 10 min at 4°C, the cell pellet was resuspended in DMEM containing 10% FBS and 20% L929 conditioned medium (CM). The medium was changed every 3 days. Generally, BMDMs can be induced to mature after 6-7 days.
2.4 Tumor models
In the orthotopic liver metastasis model, MC38 cells (5 × 105) were injected into the subserous layer of the cecal wall of 6-week-old WT or SPHK1−/− C57BL/6 mice with a 30-gauge needle.
In the spleen-liver metastasis models, the spleen was injected with MC38 cells (5 × 105) and CT26 cells (5 × 105) in 6-week-old C57BL/6 and BALB/c mice, respectively. For endpoint studies, mice were euthanized for assessments of metastatic tumors at 3 to 4 weeks after surgery. For overall survival studies, mice were monitored daily and euthanized for the development of ascites or decreased activity.
In the mouse model with intraportal injection of tumor cells and macrophages, BMDMs were extracted from WT or SPHK1−/− mice before surgery. Subsequently, MC38 cells (3 × 105) and BMDMs (3 × 105) was mixed and injected into the portal vein of wild-type C57BL/6 mice via a 30-gauge needle, as described in previously published protocols [19]. After 3 weeks, mice were euthanized for tumor burden evaluation.
In the mouse model established with subcutaneous and liver tumors simultaneously, MC38 cells (1 × 106) were implanted into the hind flanks of wild-type C57BL/6 mice, and then an intrasplenic injection of MC38 cells (5 × 105) was conducted following an immediate splenectomy. PBS was injected into the spleen in control mice. Mice were assigned to experimental groups when the volume of subcutaneous tumors had grown to 100 mm3. Tumor volume was determined using the formula (length × width × width) / 2.
2.5 Animal treatments
To inhibit SPHK1 activity, C57BL/6 and BALB/c mice with an intrasplenic tumoral injection were given SPHK1 inhibitor PF543 (5 mg/kg; MedChemExpress, Monmouth Junction, NJ, USA) or SKI II (50 mg/kg; MedChemExpress) at day 4 by intraperitoneal injection every three days. Control mice were given dimethyl sulfoxide or β-cyclodextrin, corresponding to PF543 or SKI II treatment, respectively. For in vivo Sphk1 overexpression, adeno-associated virus (AAV) vector expressing mouse Sphk1 (5 × 1010 vg/mL in 2 µL per mouse; Vigene Biosciences, Jinan, Shandong, China) or control vector was injected on day 3 and day 10 via the tail vein after CT26 intrasplenic inoculation.
For in vivo depletion of TAMs, 100 µL clodronate liposomes (FormuMax Scientific, Sunnyvale, CA, USA) on days 6 and 9 and 500 µg anti-colony stimulating factor 1 receptor (CSF1R) antibody clone AFS98 (BioXcell, West Lebanon, NH, USA) on days 5, 8, and 11 were given by intraperitoneal injection. PBS liposomes and isotype controls for anti-CSF1R antibody were administered to control mice. To deplete CD8+ T cells in vivo, C57BL/6 mice were intraperitoneally injected with 200 µg anti-CD8 monoclonal antibody clone YTS 169.4 (BioXcell) on days 6, 9, and 12. Isotype controls were used to control for nonspecific effects.
In the combination treatment with SPHK1 inhibitor and anti-PD-1, C57BL/6 and BALB/c mice with intrasplenic tumoral inoculation were treated with drugs on days 7, 10, 13, and 16. Anti-PD-1 antibody (clone RMP1-14, 200 mg; BioXcell) or isotype IgG (200 mg; BioXcell) combined with or without PF543 (5 mg/kg) or SKI II (50 mg/kg) was injected intraperitoneally into each mouse.
For anti-interleukin 1 receptor (IL-1R) treatment of liver tumors, BALB/c mice with CT26 intrasplenic inoculation were administered 500 µg IL-1R antagonist anakinra (MedChemExpress), by intraperitoneal injection on days 4, 7, 10, 13, and 16.
In the combination treatment with PF543 and ICI and radiotherapy, C57BL/6 mice with simultaneous subcutaneous and liver tumors were divided into the following groups: (1) control treatment group; (2) anti-PD-1 group; (3) anti-PD-1 + irradiation group; (4) anti-PD-1 + irradiation + PF543 group. Mice were treated with 200 µg anti-PD-1 antibody, with or without the addition of 5 mg/kg PF543, on days 7, 10, 13, and 16. Two fractions of 8 Gy of liver-directed irradiation were provided on days 6 and 7 before initiation of drug treatment.
2.6 Human specimens
A tissue microarray containing 155 primary CRC samples from patients who underwent laparoscopic colorectal resection between November 2013 and June 2014 at the Department of General Surgery, Nanfang Hospital, Southern Medical University (Guangzhou, Guangdong, China) was used for immunohistochemistry (IHC). Patients without other concurrent malignant diseases were included and had signed informed consent. The patients were followed till July 2022, with a median follow-up of 96 months (range, 8-103 months). The clinicopathological information, medication details, and follow-up data of these CRC patients are provided in Supplementary Table S1. The criteria for TNM staging were based on the 8th Edition Colorectal Cancer Stage Classification released by the American Joint Committee on Cancer (AJCC). Another tissue microarray with 36 pairs of primary CRC and liver metastasis samples for IHC analysis was obtained from Zhuoli Biotechnology (Shanghai, China), with no information of neoadjuvant and adjuvant therapy (Supplementary Table S2). Additionally, 11 primary CRC and 9 CRLM samples from separate patients for IF staining were obtained from the Department of General Surgery, Nanfang Hospital, Southern Medical University. The specimens were collected after the patients had received chemotherapy and targeted therapy, or had received neither (Supplementary Table S3). Eleven biopsy CRC samples and 1 surgical CRC sample obtained from the Department of Pathology, Nanfang Hospital, were collected for IHC analysis from patients before they received anti-PD-1 therapy, with or without concurrent chemotherapy, targeted therapy, or both (Supplementary Table S4). Human peripheral blood mononuclear cells (PBMCs) and CD8+ T cells were acquired from 6 healthy volunteers.
2.7 Immunofluorescence (IF)
The multi-color IF was performed with a four-color multiple fluorescent immunohistochemical staining kit (Panovue, Beijing, China). In brief, human or mouse tumor tissue sections were subjected to microwave-induced antigen retrieval in Tris-ethylenediaminetetraacetic acid buffer (pH = 9.0) and soaked in 3% hydrogen peroxide for 30 min, followed by incubation with primary antibody for 1 h at room temperature. After washing in PBS 3 times, sections were incubated with horseradish peroxidase-labeled secondary antibody and fluorescent dye diluted by signal amplification reagent for 10 min each. For multiple fluorescence staining, sections were processed, starting from the antigen retrieval step to the incubation with another primary antibody. Counterstaining was performed with 4',6-diamidino-2-phenylindole (DAPI). Images were taken with a Zeiss LSM 880 confocal laser scanning microscope (Zeiss, Oberkochen, Baden-Württemberg, Germany) and analyzed using ZEN microscopy software (Zeiss). The antibodies used for immunofluorescence are listed as follows: SPHK1 (1:400; 10670-1-AP, Proteintech, Rosemont, PA, USA), phospho-SPHK1 (p-SPHK1, Ser225, 1:400; 19561-1-AP, Proteintech), CD68 (1:400; ab213363, Abcam, Cambridge, Cambs, UK), interleukin 1 beta (IL-1β, 1:200; ab9722, Abcam), NLR family pyrin domain containing 3 (NLRP3, 1:200; ab283819, Abcam), F4/80 (1:400; 70076, Cell Signaling Technology, Danvers, MA, USA), CD19 (1:400; 66298-1-lg, Proteintech), CD3 (1:400; 17617-1-AP, Proteintech), CD31 (1:400; 66065-1-lg, Proteintech), alpha smooth muscle actin (αSMA, 1:400; 67735-1-lg, Proteintech), CD11c (1:400; 17342-1-AP, Proteintech), epithelial cell adhesion molecule (EPCAM, 1:400; 21050-1-AP, Proteintech).
2.8 IHC
Paraffin-embedded CRC or CRLM tissue blocks were sectioned to 3 µm thickness and adhered to the glass slices. Sections were deparaffinized with xylene, rehydrated, subjected to heat-mediated antigen retrieval, and blocked with 3% hydrogen peroxide, followed by incubation with primary antibodies overnight at 4°C. Antibodies for IHC used in this study are described as follows: SPHK1 (1:200; PK69865, Abmart, Shanghai, China), CD8 (1:800; ab245118, Abcam), CD8 (1:800; ab209775, Abcam), F4/80 (1:400; 70076, Cell Signaling Technology), C-C motif chemokine ligand 2 (CCL2, 1:400; 26161-1-AP, Proteintech), ADAM metallopeptidase domain 17 (ADAM17, 1:200; ET1703-06, Huabio, Hangzhou, Zhejiang, China). Sections were incubated with secondary antibodies for 1 h at room temperature. The signal was visualized with 3,3’-diamin-obenzidine, and the sections were counterstained with hematoxylin. Images were taken with an OLYMPUS DP22 microscope (Olympus, Hachioji-shi, Tokyo, Japan), and two pathologists assessed the expression of indicated markers. The average number of SPHK1+ cells was calculated in five random fields of view at 400-fold magnification for each CRC sample using ImageJ software (https://imagej.net/ij/). CRC samples were classified into the high group if the number of SPHK1+ cells was greater than the mean value, otherwise, they were classified into the low group.
2.9 Quantitative real-time PCR
Total RNA was extracted from cells with TRIzol reagent (TaKaRa, Otsu, Shiga, Japan) following the manufacturer's instructions and quantified with NanoDrop 8000 (Thermo Fisher Scientific, Waltham, MA, USA). Reverse transcription of RNA into cDNA was performed with PrimeScript RT-PCT Kit (TaKaRa). Real-time PCR was performed under conditions of 95°C for 3 min, and 45 cycles of 95°C for 5 s and 60°C for 30 s, with SYBR Premix Ex Taq (TaKaRa) on a LightCycler 96 Detection System (Roche, Basel, Basel-Stadt, Switzerland). The expression of mRNA was analyzed using β-actin for normalization. The primers used are detailed in Supplementary Table S5.
2.10 Fluorescence activated cell sorting (FACS)
Mouse tumor tissues were cut and digested with 0.5 mg/mL collagenase IV (Sigma-Aldrich, St Louis, MO, USA) and 0.1 mg/mL deoxyribonuclease type I (Sigma-Aldrich) in DMEM medium for 1 h at 37°C in a shaker. After depleting red blood cells by ammonium-chloride-potassium (ACK) buffer (Leagene, Beijing, China), cells were filtered through a 70 µm cell filter to obtain single-cell suspensions. The Fc receptor was blocked by mouse anti-CD16/CD32 antibody or human Fc receptor blocking solution for 15 min. Cells were incubated with fluorophore-conjugated primary antibodies for 30 min at 4°C to stain surface antigens. For intracellular staining, samples were fixed with IC Fixation Buffer (Biolegend, San Diego, CA, USA) for 20 min at room temperature and permeabilized with Intracellular Staining Perm Wash Buffer (Biolegend) according to the manufacturer's instructions. For the detection of intracellular interferon gamma (IFN-γ) and granzyme B (GZMB), cells were stimulated with Cell Activation Cocktail with Brefeldin A (Biolegend) for 4 h at 37°C, and then stained as described above. To assess cell apoptosis, cells harvested from different co-culture plates were washed with cold PBS and stained with annexin V binding buffer (KeyGen BioTech, Nanjing, Jiangsu, China). The data were acquired on BD LSRFortessa X-20 and FACSCanto II using FACSDiva software (version 8.0.2, BD Biosciences, San Jose, CA, USA) and analyzed with FlowJo software (version 10.8.1, BD BioSciences).
2.11 Mass cytometry (CyTOF)
Fresh CRLM tissue samples of mice from WT and SPHK1−/− groups were subjected to CyTOF by PLTTECH (Hangzhou, Zhejiang, China). Cell suspensions were prepared by dissociating tumor tissues with the GentleMACS system (Miltenyi Biotec, Bergisch Gladbach, North Rhine-Westphalia, Germany) according to the manufacturer's instructions. Single-cell suspensions were incubated with Fc receptor blocking reagent for 30 min at 4°C and stained with surface or intracellular antibodies against 41-parameter panels. After washing with flow cytometry buffer 2 times and another quality control step, the final pools were applied to the Helios CyTOF system (Fluidigm, South San Francisco, CA, USA) to detect the metal signals. The data were visualized using the x-shift algorithm and separate t-distributed stochastic neighbor embedding (t-SNE) dimensionality reduction. Antibody clones and suppliers used for this experiment are listed in Supplementary Table S6.
2.12 CD8+ T cell isolation and culture
The mouse spleen was gently ground with the rough end of a syringe plunger and collected in a tube. After a centrifugation at 500 ×g for 10 min at 4°C, the cell pellet was resuspended with ACK buffer to remove red blood cells. The cell suspension was filtered through a 70 µm cell filter, and an appropriate quantity of splenocytes was inoculated with anti-CD8 microbeads (Miltenyi Biotec) for CD8+ T cell isolation. For human CD8+ T cell isolation, 3 mL of peripheral blood from healthy volunteers was diluted 1:1 with RPMI 1640 and transferred to the upper layer of separation solution (Histopaque®-1077, Sigma-Aldrich), which was previously added into the lymphocyte separator SepMate-50 (Stemcell Technologies, Vancouver, BC, Canada). By centrifugation at 800 ×g for 20 min at room temperature, the middle cell layer containing mononuclear cells was collected and washed with PBS. Then, cells were counted by the Countess 3 Automated Cell Counter (Thermo Fisher Scientific) and subjected to CD8+ T-cell sorting using anti-CD8 microbeads. Prior to experiments, the isolated CD8+ T cells were activated with 5 µg/mL anti-CD3 antibody (plate-bound), 2 µg/mL anti-CD28 antibody, and 10 ng/ml IL-2 (Peprotech, Cranbury, NJ, USA) for 3 days.
2.13 Peritoneal macrophage extraction
Six-week-old C57BL/6 mice were euthanized by cervical dislocation. Exposing the intact peritoneal wall with aseptic techniques, 5-10 mL of sterile PBS was injected into the peritoneal cavity. After gently massaging the abdomen for 1 min, we aspirated the peritoneal fluid with a syringe. The peritoneal fluid was centrifuged at 500 × g for 10 min at 4°C, and the supernatant was discarded. The cell pellet was resuspended and incubated at 37°C with 5% CO2 for 1 h. By gently washing with PBS to remove nonadherent cells, the remaining cells were predominantly macrophages.
2.14 Cell treatments
To induce TAM phenotype, BMDMs and PMA-differentiated THP-1 cells were cultured in vitro with MC38-CM and HCT116-CM for 24 h, respectively. For NLRP3 inflammasome activation, BMDM and THP-1-derived TAMs were pretreated with or without 10 µmol/L S1P (Avanti Polar Lipids, Alabaster, AL, USA) for 12 h and then primed with lipopolysaccharide (LPS, 500 ng/mL; Sigma-Aldrich) for 3 h, followed by a stimulation with adenosine tri-phosphate (ATP, 5 mmol/L, 30 min; InvivoGen). For hypoxia inducible factor 1 subunit alpha (HIF-1α) activation by hypoxia treatment, TAMs were cultured in a standard cell culture incubator (20% O₂ and 5% CO₂) or a hypoxia incubator with the anaerobic gas mixture (1% O₂, 5% CO₂, and 94% N₂) for 24 h. To determine which sphingosine-1-phosphate receptors (S1PRs) accounted for S1P-induced NLRP3 expression, TAMs were pretreated with 10 µmol/L S1P for 12 h, followed by treatment with 5 µmol/L inhibitors targeting S1PR1 (W146), S1PR2 (JET013), S1PR3 (TY52156) or S1PR4 (CYM50358) for 24 h. The above-mentioned S1PR inhibitors were purchased from MedChemExpress. For S1PR2 activation, TAMs were treated with 2 or 5 µmol/L S1PR2 agonist CYM5520 (MedChemExpress) for 24 h. For co-culture experiments, 1 × 105 BMDMs or PMA-differentiated THP-1 cells were co-cultured with an equal number of CRC cells and CD8+ T cells for 48 h.
2.15 Cell transfection
A lentiviral vector containing the full-length cDNA sequence of mouse Sphk1 and a lentiviral vector containing small hairpin RNA (shRNA) targeting human SPHK1, mouse Sphk1, or mouse Adam17 were constructed by Vigene Biosciences (Jinan, Shandong, China). A number of 1 × 105 cells was seeded per well in a 6-well plate and incubated with lentivirus at a multiplicity of infection of 10 and a viral titer of 1 × 108 TU/mL. After a 24 h incubation, infected cells were selected by treating the cultures with 2 µg/mL puromycin (Sigma-Aldrich) for 3-5 days. Mouse HIF-1α and P65 siRNAs were produced by RiboBio (Guangzhou, Guangdong, China). Human and mouse S1PR2-overexpressing plasmids and human HIF1A-overexpressing plasmids were synthesized by Vigene Biosciences. The transfection experiments were performed with Lipofectamine 3000 reagents (Invitrogen, Carlsbad, CA, USA). Briefly, 5 µg plasmid or 50 nmol/L siRNA for each well of a 6-well plate was mixed with the diluted Lipofectamine 3000 and incubated at room temperature for 15 min. The mixture was dropped into the cells and replaced with a fresh culture medium after 24 h. After 48 h, the cells were tested for transgene expression or used for subsequent experiments.
2.16 Cytokine assays
THP-1 cells transduced with lentivirus harboring NC vector or shSPHK1 vector were co-cultured with HCT116 and human CD8+ T cells for 48 h. The CM was harvested and centrifuged at 2,000 ×g for 10 min at 4°C to remove unbroken cells and debris. The CM was then utilized to detect the levels of 27 cytokines and chemokines by Bio-Plex Pro Human Cytokine 27-Plex Immunoassay kit (BioRad, Austin, TX, USA). Plates were measured with Bio-Plex Manager version 6.1 (Luminex, Austin, TX, USA) and analyzed by Wayen Biotechnologies, Inc. (Shanghai, China). To measure the cytokine levels in liver tumors with or without PF543 treatment, tumor lysate was incubated with the Mouse Inflammation Antibody Array C1 (Ray Biotech, Norcross, GA, USA). After developing, the images were scanned and quantified using ImageJ software. Intensity was normalized to internal positive controls for comparison.
2.17 Western blotting
Cellular protein was extracted by adding radio immunoprecipitation assay buffer (Amresco, Solon, OH, USA) containing protease and phosphatase inhibitors (Leagene). Protein concentrations were measured with a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Lysates were then separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were immersed in 5% defatted milk for 1 h and incubated with primary antibodies overnight at 4°C. Following incubation with a horseradish peroxidase-labeled secondary antibody for 1 h at 4°C, the protein-antibody complexes were visualized using an enhanced chemiluminescence kit (FDbio-pico ECL, Fude Bio, Hangzhou, Zhejiang, China). All antibodies used for Western blotting are listed as follows: SPHK1 (1:1,000; 10670-1-AP, Proteintech), p-SPHK1 (Ser225, 1:1,000; 19561-1-AP, Proteintech), NLRP3 (1:1,000; 68102-1-lg, Proteintech), IL-1β (1:1,000; 12242, Cell Signaling Technology), P65 (1:1,000; 8242, Cell Signaling Technology), phospho-P65 (p-P65, Ser536, 1:1,000; 3033, Cell Signaling Technology), phospho-inhibitor of nuclear factor kappa B kinase alpha/beta (p-IKKα/β, Ser176/180, 1:1,000; 2697, Cell Signaling Technology), Caspase1 (1:1,000; sc-56036, Santa Cruz, Dallas, TX, USA), HIF-1α (1:1,000; GTX127309, GeneTex, Irvine, CA, USA), extracellular signal-regulated kinase 1/2 (ERK1/2, 1:1,000; ARG20026, Arigobio, Zhubei, Taiwan, China), phospho-ERK1/2 (p-ERK1/2, 1:1,000; ARG20126, Arigobio), ADAM17 (1:1,000; ET1703-06, Huabio), glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:1,000; 60004-1-Ig, Proteintech), and β-actin (1:1,000; 66009-1-Ig, Proteintech).
2.18 Enzyme-linked Immunosorbent Assay (ELISA)
Briefly, the supernatant of macrophages with or without LPS and ATP treatments was collected, followed by removing cell debris through a 0.45 µm filter. The supernatant was subjected to the measurement of IL-1β levels with a human or mouse IL-1β ELISA kit (Dakewe, Shenzhen, Guangdong, China). For the detection of ADAM17 in vitro, mouse CRC cells were stimulated with 50 ng/mL recombinant IL-1β (rIL-1β, SinoBiological, Beijing, China) for 24 h, and then the medium was replaced with fresh medium. After 36 h, the supernatant was collected for detecting ADAM17 levels with a mouse ADAM17 ELISA kit (Cusabio, Wuhan, Hubei, China). To extract tumor interstitial fluid, 0.5-1 g metastatic tissue was rinsed by PBS, wrapped with a nylon mesh of 20 µm, and put into a 1.5 mL centrifuge tube. After centrifuging at 400 ×g for 15 min at 4°C, the tumor interstitial fluid in the bottom of tube was used to detect the levels of IL-1β and ADAM17.
2.19 Proteomics
The proteomics analysis was supported by LC-Bio Technology. Briefly, MC38 cells were treated with 50 ng/mL rIL-1β for 24 h, and then the medium was replaced with serum-free RPMI 1640 medium and cultured for 36 h. We collected supernatant and removed cell debris with a 0.22 µm membrane filter for proteome analysis. A total of 20 µg protein was fractionated on a 12.5% SDS-PAGE gel and visualized with coomassie blue staining. The peptides obtained through protein hydrolysis by trypsin were desalted and concentrated by vacuum centrifugation. The peptides were then subjected to liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. MS raw data were identified and quantified using the MaxQuant software (version 2.1.4.0, https://maxquant.org/). Proteins with |log2 fold change (rIL-1β-treated/blank)| > 1 are considered as differentially expressed.
2.20 Transwell assays
A total of 1 × 105 BMDMs and THP-1 cells were suspended in 200 µL serum-free RPMI 1640 and seeded into 12-µm pore transwell chambers (Corning, Corning, NY, USA). An equal number of MC38 or HCT116 cells was incubated in the lower chambers of 24-well plates supplemented with 10% FBS. After 24 h, the outside surface of upper chambers covered with macrophages was immersed in methanol for fixation and stained with 0.1% crystal violet solution. Images were captured using an OLYMPUS DP22 microscope, and cell counts were determined on five random microscopic fields. For the detection of migration or invasion of CRC cells, 1 × 105 CRC cells was seeded on 8-µm pore transwell chambers pre-coated without or with matrigel (BD Biosciences). Following 24 h (migration assay) or 48 h (invasion assay) of incubation, the upper chambers were collected, stained, and photographed.
2.21 RNA-sequencing (RNA-seq)
Total RNA of macrophages was isolated using TRIzol reagent, and RNA quality was evaluated with Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA). With high-quality RNA, cDNA library was created and the 2 × 150 bp paired-end sequencing (PE150) was conducted on an Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA). In the analysis phase, fastp software (https://github.com/OpenGene/fastp) was applied to remove unnecessary reads and verify sequence quality. HISAT2 software (https://daehwankimlab.github.io/hisat2/) was used in orientation mode to map the clean reads to the reference genome (mus_musculus/Ensembl/v101). StringTie (https://ccb.jhu.edu/software/stringtie/) was employed to assemble and quantify the mapped reads of each sample and estimate the expression levels of all transcripts. The differentially expressed genes (DEGs) were selected with fold change > 1.5 or < 0.66 and P value < 0.05 by R package “edgeR”. The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis and gene-set enrichment analysis (GSEA) were performed with DAVID software (https://david.ncifcrf.gov/) and GSEA4.1.0 software (http://gsea-msigdb.org/gsea/index.jsp/), respectively.
2.22 Bioinformatics analysis
The datasets were retrieved from Gene Expression Omnibus (GEO, https://ncbi.nlm.nih.gov/geo/) with accession codes GSE41568, GSE207194, GSE193594, GSE14095, GSE243245, GSE164522, GSE178318, GSE146771, and GSE17538.
To identify the DEGs that were significantly upregulated in CRLM compared with primary CRC, the bulk RNA-seq data from datasets GSE41568, GSE207194, and GSE193594 were analyzed using the R package “limma” or “edgeR” based on the screening criteria of fold change > 1.5 and P < 0.05. To identify the DEGs preferentially expressed in TAMs from CRLM relative to TAMs from primary CRC, we analyzed single-cell RNA-sequencing (scRNA-seq) data from datasets GSE178318 and GSE164522 using the Scanpy library in Python (version 3.11.5, https://python.org/). The Mann-Whitney U test was applied to select the DEGs with adjusted P < 0.05.
The RNA-seq profiles of GSE41568, GSE14095, and GSE243245 were applied to estimate the tumor immune dysfunction and exclusion (TIDE) value for each sample on the website (http://tide.dfci.harvard.edu/). Based on the default value of 0, all CRLM samples were categorized into TIDE-high group and TIDE-low group. Samples in TIDE-high group were predicted to be non-responders to ICI therapy, whereas those in TIDE-low group were expected to respond favorably. The gene difference analysis was performed to screen the DEGs that were upregulated in TIDE-high CRLM group.
We downloaded the scRNA-seq data of GSE146771 and GSE164522. GSE146771 was utilized for clustering immune cell subsets and visualizing the expression of S1PRs in subcutaneous MC38 tumors with Python. GSE164522 was applied for the comparison of the expression of SPHK1, IL-1B, and NLRP3 among 3 TAM subsets [secreted phosphoprotein 1 (SPP1+) TAMs, complement C1q C chain (C1QC+) TAMs, and NLRP3+ TAMs] in CRC and CRLM tissues. Utilizing the Matplotlib library in Python, the gene expression levels among 3 TAM subsets were plotted. The Kruskal-Wallis test was used to determine significant differences in gene expression between these TAM subsets.
The bulk RNA-seq data and clinical information of GSE17538 (CRC [20]), PMID33020056 (20 types of human solid tumors treated with ICIs [21]), PMID32472114 (kidney renal clear cell carcinoma treated with nivolumab [22]), and IMvigor210 (metastatic urothelial cancer treated with atezolizumab [23]) were utilized to perform survival analysis. Overall and disease-specific survival analyses were performed with data from GSE17538 stratified by the median value of CD68 and SPHK1 expression. We stratified SPHK1 expression levels into high/low groups using an optimal cut-off value determined by the R package “survminer”, and then conducted survival analyses in ICI-treated cohorts via the Kaplan-Meier method and log-rank test.
We loaded the CIBERSORT package and its built-in LM22 file (containing immune cell signature gene profiles) in R software (https://r-project.org/). Through CIBERSORT, we employed the RNA-seq data of CRLM samples in GSE41568 dataset to generate an immune cell infiltration matrix.
The gene expression profiles from The Cancer Genome Atlas-Colon Adenocarcinoma (TCGA-COAD, https://cancer.gov/ccg/research/genome-sequencing/tcga/) were used to perform GSEA stratified by SPHK1 expression. The correlation analysis between the expression of IL1B and ADAM17 was conducted using data from TCGA-COAD and GSE243245. Finally, expression analysis and visualization of SPHK1 in tumor immune cells across different cancer types was conducted using the TISCH2 database (http://tisch.comp-genomics.org/home/) [24].
2.23 Statistical analysis
All data are presented as mean ± standard deviation unless otherwise specified. The analytical processes were completed with GraphPad Prism (version 8.0.1, https://graphpad.com/; Dotmatics, San Diego, CA, USA). Two-tailed unpaired Student's t-test was utilized to assess differences in variables between two groups. One-way analysis of variance was utilized for comparing the means of 3 or more experimental groups. Correlation was determined by calculating the Pearson correlation coefficient. Cox univariate and multivariate analyses were performed using SPSS Statistics (version 27, https://ibmspss.com.cn/; IBM, Armonk, NY, USA) to assess the independent prognostic significance of various factors. A P value lower than 0.05 denoted statistical significance.
3 RESULTS
3.1 SPHK1 was selectively expressed in TAMs from CRLM and predicted poor prognosis in CRC patients
To comprehensively catalog TAMs and their interactions with tumor microenvironment (TME) related to CRLM, the public RNA-seq data of human primary CRC and corresponding CRLM from GSE41568, GSE207194, and GSE193594 were used to identify the genes that were significantly upregulated in CRLM, and 644 DEGs (Set 4) were screened. Next, we utilized TIDE, a tool for predicting patients’ response to ICI therapy based on the tumor TIDE values [25], and identified 397 DEGs (Set 8) with significantly higher expression in TIDE-high CRLM group than in TIDE-low CRLM group, based on datasets GSE41568, GSE14095, and GSE243245. By analyzing the scRNA-seq data from GSE164522 and GSE178318, we identified 147 DEGs (Set 11) that were preferentially expressed in TAMs from CRLM relative to TAMs from primary CRC. Finally, we intersected the above DEGs and identified one gene: SPHK1 (Supplementary Figure S1A), suggesting its potential role as a macrophage marker in tumor immune evasion in the context of CRLM. By clustering immune cells and analyzing SPHK1 expression with scRNA-seq data, we validated that CRLM-derived TAMs displayed higher SPHK1 expression compared to primary CRC-derived TAMs (Figure 1A–C and Supplementary Figure S1B). IF analysis revealed significantly increased infiltration of SPHK1+ TAMs in CRLM tissues compared with normal intestinal mucosa, primary CRC, and normal liver tissues (Figure 1D and Supplementary Figure S1C). In parallel, utilizing the mRNA data from GSE41568 CRLM dataset, tumors with high SPHK1 expression exhibited greater TAM infiltration than tumors with low SPHK1 expression (Supplementary Figure S1D). Moreover, IF staining showed that SPHK1 was predominantly expressed in TAMs relative to other cell types in the CRLM microenvironment (Supplementary Figure S1E).
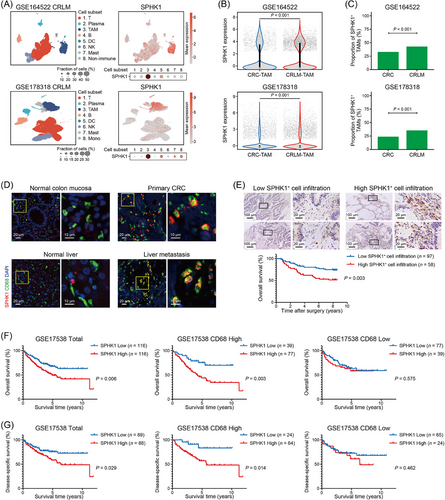
To further explore the clinical relevance of SPHK1 expression in TAMs, we evaluated the number of SPHK1+ cells in the tumor stroma with a human CRC tissue microarray. We found that high infiltration level of intratumor SPHK1+ cells was associated with lymph node metastasis and TNM stage of CRC (Supplementary Table S7). High SPHK1+ cell infiltration was associated with poor overall survival in CRC patients (Figure 1E) and served as an independent risk factor for prognosis (Supplementary Table S8). However, the expression of SPHK1 in tumor cells failed to predict prognosis of CRC patients (Supplementary Figure S1F, G). Furthermore, by analyzing data from the GSE17538 dataset, we revealed associations between high SPHK1 expression in tumor tissues and inferior overall survival and disease-specific survival in CRC patients, particularly in patients with high CD68 expression (Figure 1F, G). Collectively, these data indicated that SPHK1 was highly expressed in TAMs from CRLM and closely related to unfavorable outcomes in CRC patients.
3.2 Pharmacologic and genetic inhibition of SPHK1 in TAMs prevented CRLM
To investigate whether SPHK1 in TAMs plays a role in CRLM progression, we treated spleen-liver metastasis mouse model with intraperitoneal injection of PF543 or SKI II, two SPHK1 inhibitors (Figure 2A). The mice receiving SPHK1 inhibitors showed a reduced number and diameter of liver metastatic tumors (Figure 2B, C and Supplementary Figure S2A, B) and had significantly prolonged survival compared to the control groups (Supplementary Figure S2C, D). IF staining showed that the liver tumors with PF543 or SKI II treatment exhibited a decreased number of phosphorylated SPHK1+ TAMs (activated SPHK1) in the TME (Figure 2D and Supplementary Figure S2E). Given the importance of the SPHK1-S1P axis in a wide range of physiological processes [26], we evaluated the toxicity of PF543 and SKI II on healthy mice and observed no significant adverse effects on mouse weight, liver and renal function, or lymphocyte proportions (Supplementary Figure S3A–D).
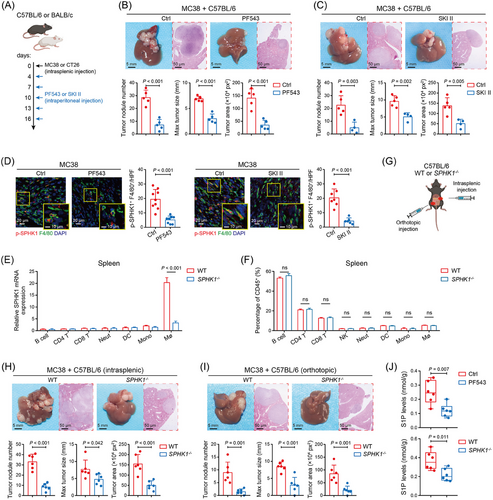
Next, by extracting splenocytes from WT and SPHK1−/− C57BL/6 mice, we validated that SPHK1-knockout caused a significant decline of SPHK1 expression in splenic macrophages and barely affected the baseline proportions of other immune cell types (Figure 2E, F). We then injected orthotopically or intrasplenically with MC38 cells into WT and SPHK1−/- mice (Figure 2G). Our results showed that SPHK1 deficiency significantly decreased the number and diameter of metastatic tumors compared with controls (Figure 2H, I). SPHK1 deficiency largely extended mouse survival time in the spleen-liver metastasis model, but showed no significant difference compared with the controls in the cecum-liver metastasis model (Supplementary Figure S3E). Subsequently, to more concretely validate the role of SPHK1 in TAMs, we extracted BMDMs from WT or SPHK1−/− mice and mixed them with MC38 cells, and then injected them into the portal vein of wild-type C57BL/6 mice. The mice injected with SPHK1−/− BMDMs exhibited fewer metastatic nodules and smaller metastatic tumor diameters than those injected with WT BMDMs (Supplementary Figure S3F). For the basic function of SPHK1 in producing S1P, we detected the concentration of S1P in the liver tumors. We found that the level of S1P was markedly reduced by SPHK1 inhibitor treatments and SPHK1-knockout (Figure 2J and Supplementary Figure S3G). Together, these data indicated that SPHK1 in TAMs promoted the progression of CRLM.
3.3 SPHK1 deficiency in TAMs reversed the immunosuppressive TME of CRLM
To evaluate the impact of SPHK1 on TME, fresh CD45+ immune cells were isolated from the liver metastatic tumors of WT and SPHK1−/− mice, and then subjected to CyTOF analysis. Results showed that the proportions of CD4+ T cells, CD8+ T cells, and conventional dendritic cells were significantly increased, while the proportion of TAMs was significantly decreased in the liver tumor tissues from MC38-injected SPHK1−/− mice compared with controls (Figure 3A–C). Notably, SPHK1 deficiency-induced reduction in TAM frequencies was mainly driven by decreases in the C11, C12, and C13 TAM subpopulations (Supplementary Figure S4A, B). C11 TAMs presented the co-expression of inflammatory (CD11c, CD86) and immune inhibitory markers [programmed cell death 1 ligand 1 (PD-L1)], C12 and C13 TAMs simultaneously displayed high expression of M1 (MHC II) and M2 (CD206) markers, while C13 TAMs specifically presented upregulation of vascular cell adhesion molecule 1 (VCAM1) and C-C motif chemokine receptor 2 (CCR2), signifying enhanced chemotaxis ability (Supplementary Figure S4C–E). In monocytes, SPHK1 loss resulted in a decrease of classical lymphocyte antigen 6 complex, locus C (Ly6C)+CCR2+ monocytes (C09) which were regarded as the precursors of TAMs [27] (Supplementary Figure S4F, G). By FACS analysis, reduced proportions of TAMs and increased proportions of CD8+ T cells were confirmed in SPHK1−/− liver tumors compared with controls (Figure 3D, E). Additionally, we obtained reproducible results through the use of PF543 on mice by FACS and IHC analyses (Supplementary Figure S5A–D). FACS analysis showed that SPHK1 deficiency significantly decreased the expression of exhaustion markers, including PD-1, lymphocyte activating 3 (LAG3), and T-cell immunoglobulin and mucin domain-containing protein 3 (TIM3), and increased the expression of effector molecule IFN-γ in CD8+ T cells (Figure 3F, G and Supplementary Figure S5E, F). CyTOF results also showed a significantly lower expression of PD-1 and higher expression of activation markers [CD44, CD25, and T-box expressed in T cells (T-bet)] in CD8+ T cells from SPHK1−/− liver tumors than those from controls (Figure 3H). These data demonstrated that targeting SPHK1 in TAMs can reverse the immunosuppressive TME of CRLM.
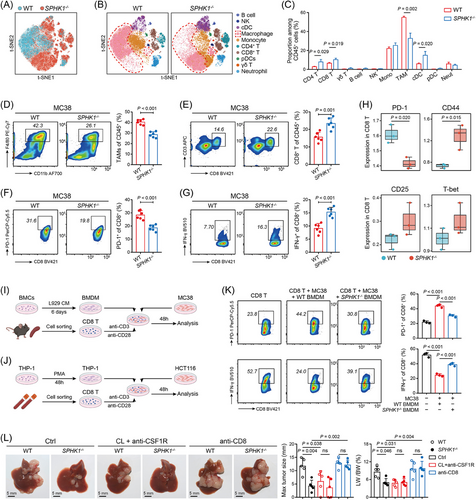
To evaluate whether SPHK1 modulates macrophage survival and M2 polarization, we prepared CRC-derived CM and then cultured macrophages with the CM. We validated that SPHK1 in TAMs had no effect on their apoptosis and M2 polarization (Supplementary Figure S6A, B). The expression of CD206 in TAMs in liver tumors was also not altered by SPHK1-targeting treatment (Supplementary Figure S6C). Subsequently, we stably transduced THP-1 cells with lentivirus to establish a SPHK1-knockdown stable cell line (Supplementary Figure S6D). Via in vitro co-culture experiments, we found that CD8+ T cells co-cultured with SPHK1−/− BMDMs or shSPHK1 THP-1 cells exhibited a less exhausted phenotype with reduced PD-1 expression and an increased expression of IFN-γ and GZMB (Figure 3I–K and Supplementary Figure S7A, B). In parallel, SPHK1 deletion or knockdown in macrophages largely promoted tumor cell apoptosis in the presence of CD8+ T cells (Supplementary Figure S7C), while the CD206 levels in macrophages were not significantly changed (Supplementary Figure S7D). Nevertheless, the phenotype of CD8+ T cells showed no significant changes when co-cultured with TAMs in the absence of CRC cells (Supplementary Figure S7E). Next, to determine whether the inhibitory effect of SPHK1 loss on CRLM progression was dependent on TAMs and CD8+ T cells, we depleted these cells in mice with a spleen-liver metastasis model. The results showed that SPHK1 loss significantly reduced metastatic tumor diameter and the ratio of liver weight to mouse body weight, whereas TAM depletion potently suppressed liver metastasis in WT mice and abolished the differences between WT and SPHK1−/− mice; depletion of CD8+ T cells significantly promoted liver metastasis in SPHK1−/− mice and blocked the inhibitory effect of SPHK1-knockout on liver metastasis (Figure 3L and Supplementary Figure S8A, B). IHC analysis showed that the density of stromal SPHK1+ cells was inversely correlated with that of CD8+ T cells in human CRLM tissues (Supplementary Figure S8C). The above results suggested that SPHK1+ TAMs orchestrated an immunosuppressive effect on CD8+ T cell-mediated antitumor immunity.
3.4 The SPHK1-S1P axis promoted IL-1β secretion through NLRP3 inflammasome in TAMs
To investigate whether the immunosuppressive effect of SPHK1 depends on its metabolite S1P, BMDMs were pretreated with S1P and then co-cultured with MC38 and CD8+ T cells. We found that S1P-pretreated BMDMs potently elicited a reduction in the expression of IFN-γ and GZMB in CD8+ T cells (Supplementary Figure S9A). However, PD-L1 expression, migration, and invasion of CRC cells were unaffected by S1P treatment (Supplementary Figure S9B, C). Liver metastasis in immune-sufficient mice was also unaffected by SPHK1 overexpression or knockdown in CRC cells (Supplementary Figure S9D–F), implying that SPHK1 plays function most likely through S1P acting on TAMs. Next, by performing RNA-seq with TAMs isolated from SPHK1−/− or WT mice and BDMD-derived TAMs with or without S1P treatment in vitro, we found that the SPHK1-S1P axis enhanced the mRNA expression of IL-1β and NLRP3 (Figure 4A and Supplementary Tables S9–S10). Through GSEA, we identified significant downregulations of the NLRP3 inflammasome pathway in SPHK1−/− TAMs (Figure 4B) and SPHK1-low CRCs (Supplementary Figure S9G). By qPCR and inflammatory cytokine array, a significantly lower IL-1β expression was detected in TAMs treated with PF543 compared to control group (Figure 4C, D). IF staining showed that the number of IL-1β+ TAMs was dramatically decreased by SPHK1 inhibitor treatment (Figure 4E and Supplementary Figure S9H). Notably, TAMs isolated from liver tumors exhibited higher IL-1β and NLRP3 expression than BMDMs and peritoneal macrophages (Supplementary Figure S10A). Greater infiltration of TAMs co-expressing SPHK1 and IL-1β was observed in human CRLM specimens than in CRC specimens (Figure 4F).
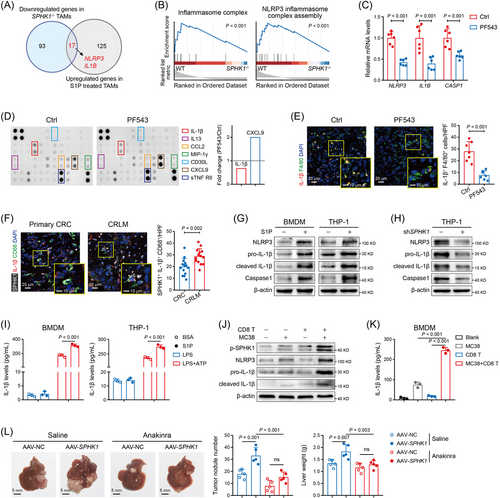
NLRP3 inflammasome activation requires two signals, a priming signal which leads to NF-κB-mediated transcription of inflammasome molecules and pro-IL-1β, and a danger signal inducing inflammasome assembly and Caspase-1 activation [28]. We found that S1P significantly upregulated the mRNA levels (Supplementary Figure S10B) and protein levels (Figure 4G) of NLRP3 inflammasome components in both BMDM and THP-1-derived TAMs. Knockdown of SPHK1 notably decreased the expression of NLRP3 inflammasome molecules (Figure 4H). Moreover, S1P remarkably augmented the mRNA levels of inflammasome molecules in the presence of LPS (Supplementary Figure S10C) and enhanced IL-1β secretion upon LPS and ATP co-stimulation (Figure 4I). Some studies reported that CD8+ T cell-mediated cytotoxicity induced NLRP3 inflammasome activation, thus driving resistance to antitumor immune response [29, 30]. We found that co-incubation of CRC cells with active CD8+ T cells elevated the protein levels of phosphorylated SPHK1, IL-1β, and NLRP3 (Figure 4J) and the secretion levels of IL-1β (Figure 4K and Supplementary Figure S10D). Targeting SPHK1 in macrophages significantly reversed the IL-1β levels in the co-culture supernatant (Supplementary Figure S10E). Next, we confirmed that CRC-derived CM was unable to induce SPHK1 phosphorylation in macrophages (Supplementary Figure S10F), but IFN-γ, mainly released by CD8+ T cells, largely promoted the phosphorylation of SPHK1 and its upstream factor, ERK1/2 [31] (Supplementary Figure S10G). IFN-γ also facilitated the expression of IL-1β and NLRP3 in TAMs, which were reversed when SPHK1 was silenced (Supplementary Figure S10H). These data suggested that CD8+ T cells drove the SPHK1-NLRP3-IL-1β axis in TAMs, and then this process may participate in the adaptive immune evasion of CRLM.
Subsequently, we utilized AAV-SPHK1 to deliver SPHK1 into TAMs. The mice treated with AAV-SPHK1 showed increased metastatic tumor nodules and liver weight, which were significantly rescued by blocking IL-1R with anakinra (Figure 4L). IHC staining showed that anakinra markedly reduced the number of TAMs and increased the number of tumor-infiltrating CD8+ T cells (Supplementary Figure S11A). In in vitro experiments, anakinra largely restored the antitumor activity of CD8+ T cells and repressed surface PD-L1 expression on MC38 cells after co-cultured with SPHK1-overexpressing BMDMs (Supplementary Figure S11B–E). Together, these data indicated that SPHK1 in TAMs promoted liver metastatic progression through IL-1β-mediated immunosuppression.
3.5 The S1P-S1PR2 axis activated NLRP3 inflammasome via NF-κB and HIF-1α signaling
By KEGG enrichment analysis, we found that NF-κB and HIF-1α signaling ranked highly among the significantly enriched pathways in S1P-treated BMDM-derived TAMs (Figure 5A). NF-κB-mediated priming step is indispensable for NLRP3 inflammasome activation, and previous studies fully addressed the effects of S1P on NF-κB activation [32, 33]. In terms of HIF-1α, a study demonstrated that hypoxia was sufficient to prime and activate NLRP3 inflammasome, causing IL-1β release [34], while whether S1P affects HIF-1α expression remains obscure. Therefore, we performed Western blotting and observed that S1P strongly boosted the expression of p-P65, p-IKKα, and HIF-1α in BMDM and THP-1-derived TAMs (Figure 5B). Next, we observed that the expression levels of NLRP3 inflammasome molecules were markedly increased when BMDM- and THP-1-derived TAMs were incubated under hypoxia conditions (Figure 5C, D) or transfected with HIF1A-overexpressing plasmid (Supplementary Figure S12A, B). Furthermore, the effect of S1P on NLRP3 and IL-1β expression was partially rescued by silencing P65 and completely rescued by silencing both P65 and HIF-1α (Figure 5E, F). Currently, NLRP3+ TAMs have been validated as a prominent subpopulation of TAMs that were related to the pro-metastatic functions [35, 36]. Hence, we comprehensively analyzed the expression feature of NLRP3+ TAMs with a human scRNA-seq dataset (GSE164522). We demonstrated that in both CRC and CRLM tissues, the transcription levels of SPHK1, IL-1β, NF-κB1, HIF-1α and HIF-1α-targeted genes were significantly elevated in NLRP3+ TAMs, in contrast to SPP1+ TAMs and C1QC+ TAMs (Figure 5G, H and Supplementary Figure S12C–F). By IF analysis, we confirmed that SPHK1 and NLRP3 were co-expressed in TAMs in human CRLM specimens (Figure 5I). Collectively, these data suggested that SPHK1-S1P axis activated NLRP3 inflammasome via NF-κB and HIF-1α signaling in TAMs.
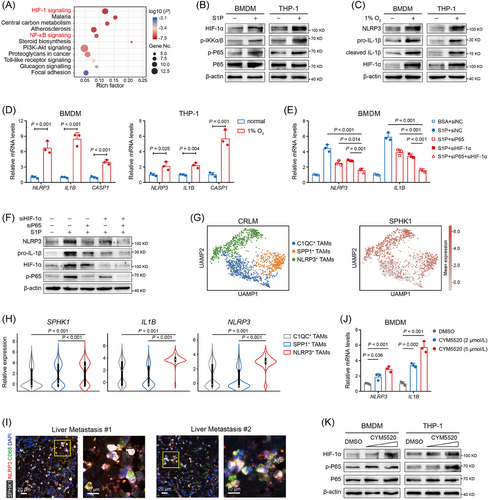
As S1P functions as an essential ligand for activating 5 isoforms of S1P receptors (S1PR1- S1PR5) [37], we continued to define which S1PRs accounted for S1P-induced NLRP3 inflammasome signaling. We treated BMDM-derived TAMs with S1P and S1PR1-S1PR4 inhibitors (S1PR5 is dominantly expressed in natural killer cells [38]), and results showed that targeting S1PR2 largely abrogated the expression of NLRP3 and IL-1β (Supplementary Figure S13A). S1PR2 agonist CYM5520 significantly boosted the expression of IL-1β, NLRP3, P65 and HIF-1α in TAMs (Figure 5J, K). Overexpressing S1PR2 plus S1P treatment resulted in an additive induction of NLRP3 inflammasome members in TAMs (Supplementary Figure S13B). We further found that S1PR2 was highly expressed in myeloid cells compared with other S1PRs by analyzing a public scRNA-seq dataset from subcutaneous MC38 tumors (GSE146771) (Supplementary Figure S13C, D). These results demonstrated that the S1P-S1PR2 signaling facilitated NF-κB and HIF-1α activation in TAMs.
3.6 rIL-1β-treated CRC cells promoted TAM recruitment and CD8+ T cell dysfunction
To further explore how SPHK1+ TAM-derived IL-1β tailors the immunosuppressive TME, we co-cultured shNC or shSPHK1 THP-1 cells with HCT116 and CD8+ T cells. Cytokine array showed that the level of CCL2 in the co-culture supernatant was significantly decreased when SPHK1 was knocked down in macrophages, whereas IL-2, a cytokine known to promote T-cell proliferation [39], showed a notable increase (Figure 6A). We isolated TAMs from co-cultured cells and found that the expression of CCL2 was not altered by either targeting SPHK1 or direct S1P pretreatment (Figure 6B). However, the levels of monocyte chemoattractants in CRC cells, including CCL2, CCL7, CCL8, and C-X-C motif chemokine ligand 12 (CXCL12), were dramatically reduced when co-cultured with SPHK1-knockout or -knockdown macrophages (Figure 6C). Thus, we considered the possibility that IL-1β secreted from SPHK1+ TAMs functions on CRC cells. Indeed, rIL-1β markedly increased the expression of monocyte chemoattractants in CRC cells (Supplementary Figure S14A). Transwell assays showed that SPHK1 deficiency greatly inhibited macrophage migration when co-cultured with CRC cells, but the effect was less pronounced when culturing macrophages alone (Figure 6D). IHC staining showed that CCL2 expressed lower in SPHK1−/− liver tumors than in WT group (Supplementary Figure S14B). These data suggested that SPHK1-expressing TAMs promoted IL-1β secretion and then interacted with CRC cells to recruit more TAMs into the TME.
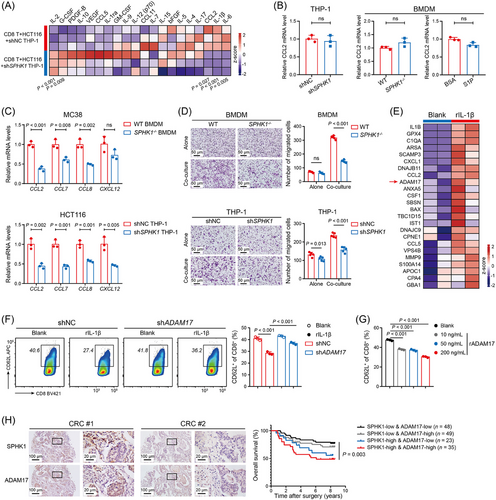
Based on the observation that SPHK1 expression in TAMs had no direct regulation on CD8+ T cell function (Supplementary Figure S7E), we hypothesized SPHK1 may exert its immunosuppressive role indirectly, potentially via rIL-1β-treated CRC cells. By proteomic analysis, we observed that ADAM17 and multiple monocyte chemoattractants (CCL2, CCL5, and CSF1) were enriched in the secretome of rIL-1β-treated MC38-CM (Figure 6E and Supplementary Table S11). The increased secretion of ADAM17 induced by rIL-1β was further confirmed by ELISA (Supplementary Figure S14C). Correlation analysis revealed a positive correlation between IL-1β and ADAM17 expression in CRC datasets TCGA-COAD and GSE243254 (Supplementary Figure S14D). ADAM17 is an important sheddase involved in the cleavage of many transmembrane proteins, such as LAG3, PD-L1, and CD62L in T cells [40]. A prior study demonstrated that myeloid-derived suppressor cells (MDSCs) induced CD62L loss in T cells via ADAM17 [41]. CD62L (L-sellectin) is an adhesion molecule that regulates entry of naive and central memory T cells into lymph nodes and activated CD8+ T cells to tumor sites [42]. Evoked by these, we knocked down ADAM17 expression in MC38 cells (Supplementary Figure S14E, F) and performed co-culture experiments with CD8+ T cells and different MC38 groups. Results showed that rIL-1β-pretreated MC38 cells significantly reduced CD62L expression on the surface of CD8+ T cells, whereas knockdown of ADAM17 in MC38 cells rescued this effect (Figure 6F). Additionally, rIL-1β-pretreated MC38 cells enhanced PD-1 and TIM3 expression in CD8+ T cells, which were reversed when ADAM17 was knocked down in CRC cells (Supplementary Figure S15A). Being stimulated with recombinant ADAM17 (rADAM17), CD8+ T cells exhibited significantly decreased surface expression of CD62L (Figure 6G and Supplementary Figure S15B). in vivo evidence demonstrated that the number of CD62L+CD8+ T cells was higher in SPHK1−/− tumors than in WT tumors by IHC analysis (Supplementary Figure S15C). Furthermore, stimulation of CD8+ T cells with rADAM17 directly induced increased PD-1 and TIM3 expression (Supplementary Figure S15D). The expression of ADAM17 in liver metastatic lesions was markedly reduced in SPHK1−/- mice compared to WT mice (Supplementary Figure S15E). Notably, ADAM17 expression was positively correlated with SPHK1 expression and TIDE score in human CRLM samples (Supplementary Figure S15F). Survival analysis demonstrated that SPHK1-high/ADAM17-high CRC patients exhibited significantly poorer overall survival compared to SPHK1-low/ADAM17-low counterparts (Figure 6H). Collectively, our results suggested that SPHK1+ TAMs induced CRC cells to release ADAM17 via IL-1β, thereby limiting the trafficking and antitumor activity of CD8+ T cells.
3.7 SPHK1-targeting treatment enhanced the efficacy of anti-PD-1 immunotherapy
ICIs are assumed to unleash exhausted CD8+ T cells to drive durable antitumor responses, but the efficacy is marginal in CRLM patients [43]. Notably, the exhausted T-cell populations were heterogeneous, with a hierarchy of differentiation status and distinct responsiveness to ICIs [44]. In the present study, 4 subtypes of tumor-infiltrating CD8+ T cells were distinguished based on PD-1 expression (Figure 7A). Intermediate exhausted CD8+ T cells (C18 and C27) with a moderate PD-1 level and PD-1lowCD8+ T cells (C21) expressing memory T-cell markers (IL-7Ra and Ly6C) were highly increased in SPHK1−/− tumors, while the proportion of PD-1highCD69highCD8+ T cells (C26) was not changed between groups (Figure 7B). Since CD8+ T cells with moderate or low PD-1 expression were reported to be preferentially responsive to ICIs [45], we hypothesized that blocking SPHK1 in TAMs may have an additive antitumor effect with ICIs.
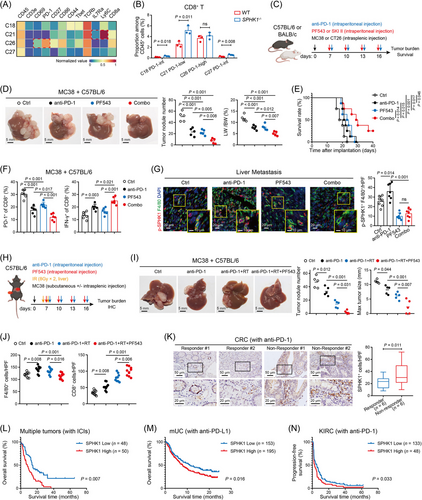
We employed MC38 and CT26 liver metastatic models in immunocompetent mice (Figure 7C), as these models are known to exhibit partial or poor responsiveness to anti-PD-1 therapy [46]. We found that PF543 or anti-PD-1 alone partially repressed CRLM and that the combination treatment resulted in greater inhibition of metastases and prolonged mouse survival (Figure 7D, E). Similar results were observed in the CT26 model receiving SKI II and anti-PD-1 (Supplementary Figure S16A, B). FACS data showed that the combination therapy significantly reduced PD-1 expression and enhanced IFN-γ expression in CD8+ T cells (Figure 7F and Supplementary Figure S16C–E). Furthermore, SPHK1 inhibitors markedly diminished the number of total TAMs (Supplementary Figure S16F) and p-SPHK1+ TAMs (Figure 7G) in the liver TME. The content of IL-1β and ADAM17 in the interstitial fluid of liver tumors was markedly reduced by PF543 treatment (Supplementary Figure S16G). These data indicated that inhibiting SPHK1 can enhance the antitumor responses of PD-1 blockade.
Yu et al. [47] reported that liver metastasis attenuated ICI efficacy systemically by inducing CD8+ T-cell apoptosis via the interaction with liver TAMs. Liver-directed radiotherapy stopped hepatic siphoning of CD8+ T cells by TAMs and restored systemic antitumor effect in models of liver metastasis [47]. Evoked by this, we asked whether SPHK1 inhibitor could provide additional therapeutic benefit if combined with radioimmunotherapy. C57BL/6 mice simultaneously bearing subcutaneous tumors and liver metastases were treated with mono-therapy (anti-PD-1), double-therapy (anti-PD-1 + irradiation), or triplet-therapy (anti-PD-1 + irradiation + PF543) (Figure 7H). We found that liver-directed irradiation largely restored the responsiveness of subcutaneous tumors to anti-PD-1, whereas PF543 did not hinder this effect (Supplementary Figure S17A, B). Importantly, the mice receiving triplet-therapy exhibited the greatest repression of liver metastases and an immunostimulatory liver TME (Figure 7I, J, and Supplementary Figure S17C). Interestingly, irradiation greatly worsened liver damage with anti-PD-1-based treatment, while PF543 ameliorated this worsening with decreased myeloid cell infiltration in the liver (Supplementary Figure S17D) and reduced levels of alanine transaminase (ALT) and aspartate transaminase (AST) in serum (Supplementary Figure S17E).
To further dissect the clinical association between SPHK1 expression and ICI response, we performed IHC analysis and found that SPHK1+ cell infiltration was significantly higher in CRC specimens from anti-PD-1 non-responders than in those from anti-PD-1 responders (Figure 7K). By investigating a public RNA-seq dataset (IMvigor210), we revealed that metastatic urothelial cancer patients with non-response to anti-PD-L1 exhibited significantly higher expression of SPHK1 and NLRP3 compared to those with a favorable response (Supplementary Figure S17F). Furthermore, by analyzing 3 cancer RNA-seq datasets (PMID33020056, IMvigor210, and PMID32472114), patients with high SPHK1 expression had lower overall or progression-free survival rates after ICI therapy compared to patients with low SPHK1 expression (Figure 7L–N). The expression of SPHK1 was positively correlated with the expression of CD68, CD163, and the key molecules in this present study (Supplementary Figure S18A). Utilizing TISCH databases, we observed that SPHK1 was highly expressed in TAMs in many malignant diseases (Supplementary Figure S18B). Taken together, these data demonstrated that SPHK1 in TAMs may be a potential marker for predicting response to ICIs in cancer patients.
4 DISCUSSION
In this study, we found that SPHK1 was prominently expressed in TAMs, and SPHK1+ TAMs were abundantly infiltrated in CRLM lesions. Pharmacologic and genetic inhibition of SPHK1 in TAMs largely impeded CRLM and sensitized the efficacy of anti-PD-1 therapy and radioimmunotherapy. In the liver TME, targeting SPHK1 and SPHK1 inhibitors reduced the number of mixed M1- and M2-like TAMs and increased the number of effector CD8+ T cells. Interestingly, inhibition of SPHK1 effectively mitigated liver damage with decreased levels of ALT, AST, and myeloid cell infiltration when combined with radioimmunotherapy, indicating some unknown mechanisms underlying SPHK1 on these effects. In addition, it is reasonable to assume that SPHK1 in TAMs plays an immunosuppressive function in a broad range of cancer types, as the associations of SPHK1 expression with poor ICI efficacy and high TAM infiltration were also observed in other cancer types.
The metabolite composition of the TME is influenced by the intricate network of metabolic control within and between cells and thus affects both cancer and immune components [48]. S1P has been found to serve as a signaling molecule that mediates the communications between cells in the TME [49]. In this study, we provided in vitro and in vivo evidence that SPHK1-produced S1P exerted an autocrine effect to activate NLRP3 inflammasome and IL-1β release via NF-κB and HIF-1α signaling in TAMs. NLRP3 inflammasome has been a focus of investigation in macrophages and is associated with several inflammatory disorders [50]. Some studies showed that NLRP3 inflammasome signal in tumors responded to CD8+ T cell-mediated immunity, thus driving adaptive resistance to ICIs [29, 30]. Similarly, our study revealed that IFN-γ released from effector CD8+ T cells potentiated NLRP3 inflammasome activation through phosphorylated SPHK1 in TAMs. According to previous studies, S1P has been characterized as an effective chemoattractant for monocytes and macrophages [51]. Our study uncovered the amplifying effect of S1P on TAM recruitment in the context of CRC. We found that IL-1β produced by SPHK1+ TAMs acted on CRC cells to secrete monocyte chemoattractants, such as CCL2, CCL5, and CCL7, thereby recruiting more TAMs to the liver. Although CCL2-CCR2 axis and CSF1-CSF1R axis are implicated in TAM recruitment and targeting these axes augments the antitumor effect of ICIs in preclinical models [52, 53], however, multiple clinical trials have tested CCR2 or CSF1R antagonists combined with ICIs showing without high objective response rate. These outcomes were possibly due to the compensation by MDSCs and a lack of effect on resident TAM populations [54]. Thus, it is still challenging to develop TAM-targeting therapies for effective antitumor response. Our study highlighted a feedforward paracrine circuit of IL-1β/IL-1R/CCL2 between SPHK1+ TAMs and CRC cells that created an interdependence driving highly immunosuppressive microenvironment. Interruption of this circuit by SPHK1-targeting treatment served as an option to remodel the TME and improve the antitumor effectiveness of anti-PD-1 in preclinical models of CRLM. We also noticed that a proportion of MHC II−Ly6C+ monocytes related to monocytic MDSCs was increased in SPHK1−/− tumors, thus the compensation upon eliminating SPHK1+ TAMs also needs to pay attention in future transformative studies.
Note that our study reconfirmed the inessential role of SPHK1 on macrophage M2 polarization. A study on breast cancer found that the knockdown of SPHK2, but not SPHK1, attenuated macrophage M2 polarization after recognition of apoptotic tumor cells [55], which highlighted the limited effect of SPHK1 on M2 polarization. Secondly, we presented evidence that the expression of SPHK1 in tumor cells was lower than that in TAMs, and the SPHK1-S1P axis was not required for CRC invasion and metastasis. Up to now, there is no relevant research supporting tumor-intrinsic SPHK1 could promote liver metastasis of CRC in vivo. Although the roles of tumoral SPHK1 have been studied extensively over time, SPHK1-targeting therapies rarely entered clinical trials for cancer patients. A study once tested new SPHK inhibitors on an array of cancer cells including glioblastoma, melanoma, breast, cervix, and colon cancers, and demonstrated that SPHK activity was negligible for tumor cell viability in vitro and in vivo [56]. In 2011, safingol, a competitive inhibitor but with low selectivity for SPHK1, concluded a phase 1 trial as non-toxic for cancer patients, which included 11 CRC patients [57]. Nevertheless, clinical activity was only seen in 2 patients with adrenal cortical cancer and no further phase 2 trials were reported [57], emphasizing the need for an explicit investigation of how new SPHK1 inhibitors function in CRLM and the predictive markers to identify patients who might benefit most from them.
Furthermore, we found that rIL-1β-treated CRC cells increased ADAM17 secretion, an enzyme that cleaves the ectodomains of transmembrane proteins and leads to release of the soluble ectodomain [40]. Up to now, over 90 ADAM17 substrates have been identified [58]. Given its broad range of substrates, ADAM17 mediates a wide range of immunological functions in multiple immune cell types, specifically in T cells. Upon T-cell receptor stimulation, the enzymatic activity of ADAM17 was upregulated, which involved CD8+ T cell activation and proliferation by cleavage of LAG3 [59], FasL [60], or CD62L [61]. A study demonstrated that targeting ADAM17 enhanced CD8+ T cells effector differentiation and antitumor immunity [62]. Additionally, ADAM17 is involved in the malignant process of many cancer types, including CRC [63]. Li et al. [64] reported that ADAM17 can be loaded in exosomes, thereby promoting pre-metastatic niche formation and distant metastasis of CRC. Our study revealed that rIL-1β-trained CRC represses CD62L expression and activity of CD8+ T cells via ADAM17 secretion. Other studies suggested that CD62L knockout CD8+ T cells were poor at controlling tumor growth and CD62L+ precursors of exhausted T cells as stem-like populations were central to the maintenance of responsiveness to ICIs [65, 66]. In our observations, the proportions of CD62L+ T cells, PD-1int and PD-1low CD8+ T cells were significantly elevated in the liver by targeting SPHK1, which may explain the additive antitumor effect of SPHK1-targeting treatment and anti-PD-1. Human data demonstrated that high expression levels of SPHK1 and ADAM17 were associated with high TIDE values and poor prognosis in CRC patients. Some ADAM17 antibodies exhibited a potent antitumor effect in pancreatic cancer mouse model, and which may provide a strong impetus for clinical applications of ADAM17 targeted therapy.
However, there are several limitations in this study that must be acknowledged. A noticeable limitation is that the SPHK1−/− mice used in this study were whole-body knockout models, which may not precisely represent the role of SPHK1 in TAMs during CRLM. The CRLM mouse models established by tumor cell injection fail to reflect the complexity and multi-phased characteristics of liver metastasis, and this is an issue that scientists around the world are faced with. Although we performed numerous bioinformatics analyses and IF staining with human tumor slices, we had not validated the role of SPHK1 in macrophages in the model of human organoids or humanized animals. Consequently, it remains unclear whether the SPHK1-targeting treatment can reliably reverse the immune suppression of CRLM in human clinical setting. Another limitation of our research is the incomplete exploration of the potential mechanisms of ADAM17-mediated dysfunction of CD8+ T cells. Further investigations on this aspect are necessary to pinpoint additional therapeutic targets for curbing tumor immune evasion. This study also did not delve deeply into the signaling pathways and regulatory mechanisms associated with IL-1β and ADAM17. Elucidating these aspects is pivotal for comprehensively understanding their functions in the progression of CRLM.
5 CONCLUSIONS
Herein, we found that SPHK1-S1P signaling in TAMs promoted the shaping of immunosuppressive TIME with increased TAM infiltration and decreased effector CD8+ T cell infiltration in CRLM. Mechanistically, SPHK1-derived S1P interacted with S1PR2 on TAMs, thus activating NLRP3 inflammasome and IL-1β secretion through NF-κB and HIF-1α signaling. Secreted IL-1β from SPHK1+ TAMs acted on CRC cells to induce TAM recruitment by CCL2 and CD8+ T cell dysfunction by soluble ADAM17. Additionally, high SPHK1 expression in TAMs was associated with inferior immunotherapy efficacy and poor prognosis of CRC patients.
AUTHOR CONTRIBUTIONS
Participated in the conception and design of the study: YF, GXL, DHW, YZZ. Wrote the draft manuscript: YZZ, YF. Performed critical revision of the manuscript for important intellectual content: YZZ, YF. Designed and performed the experiments: YZZ, JSX, ZQZ, YSL. Interpreted data and statistical analysis: YZZ, ZQZ, JYQ. Bred animals and assisted in constructing animal models: JSX, YTH. Collected clinical samples: YTH, YYL, HJD. Obtained funding for the study: YF, ZYS, YZZ. All authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
This work was supported in part by research grants from the National Natural Science Foundation of China (82403009, 82103595); the Guangdong Basic and Applied Basic Research Foundation (2022A1515111142, 2023A1515011789, 2023A1515010980, 2025A1515011911); the Science and Technology Program of Guangzhou (2025A04J4189); Outstanding Youth Cultivation Program of Nanfang Hospital of Southern Medical University (2020J010); the China Postdoctoral Science Foundation (2023M731542).
CONFLICT OF INTERESTS STATEMENT
All authors declare no competing interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Medical Ethics Committee of NanFang Hospital of Southern Medical University (NFEC-2022-430). All participants signed informed consent before participating to this study and the study was performed in accordance with the Declaration of Helsinki. The animal experimental procedures and protocols were approved by the Animal Care and Use Committee of Southern Medical University (SMUL2022189).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors on reasonable request. The sequencing data have been deposited in NCBI under BioProject ID PRJNA1257052.