Targeted intracellular delivery of molecular cargo to hypoxic human breast cancer stem cells
Abbreviations
-
- AnnV
-
- Annexin V
-
- AUC
-
- area under the curve
-
- Bavi
-
- Bavituxumab
-
- BLI
-
- bioluminescent imaging
-
- CSC
-
- cancer stem cell
-
- EGF
-
- epidermal growth factor
-
- FSC
-
- forward scatter
-
- Gla
-
- PS-binding γ-carboxyglutamate domain
-
- GlaS
-
- Gla-binding Protein S (with Gla and epidermal growth factor domains)
-
- IP
-
- intraperitoneal
-
- IV
-
- intravenous
-
- MFI
-
- median fluorescence intensity
-
- PDX
-
- patient derived xenograft
-
- PS
-
- phosphatidylserine
-
- TME
-
- tumor microenvironment
Despite advances in breast cancer therapy, effective targeting of cancer stem cells (CSCs) remains a challenge. CSCs, which have self-renewal, tumorigenic and metastatic properties, are often quiescent and located in hypoxic regions of tumors [1], making them resistant to conventional chemo- and radiotherapies [2]. These characteristics allow CSCs to survive, leading to relapse and metastasis. Studying CSCs under conditions similar to their hypoxic niche is essential for evaluating therapies that target these cells.
We show that CSCs can be targeted via binding to externalized phosphatidylserine (PS). PS, a negatively charged lipid, is typically confined to the inner leaflet of cell membranes [3]. However, its externalization occurs on dying and diseased cells, and as we demonstrated, on stem cells [4]. PS-targeting agents like Annexin V (AnnV) [5] and the monoclonal antibody bavituxumab (Bavi) [6] are under investigation for cancer therapy, but these are limited in their use as they remain surface bound and do not deliver payloads into cells. In contrast, we have found that a truncated protein S comprised of the PS-binding γ-carboxyglutamate (Gla) domain and four epidermal growth factor (EGF) domains (collectively referred to as GlaS), binds PS on the outer leaflet and is internalized after binding [4] enabling delivery of payloads to the cytoplasm of cells with externalized PS, which would include CSC in the hypoxic regions of tumors.
We used patient derived models of human breast cancer which are enriched in CD44+CD24− CSCs (Supplementary Materials and Methods). Following isolation, the CSC were propogated in mice and engineered so they express bioluminescent and fluorescent reporters. Cells collected from the CSC enriched tumors [7] were exposed to an oxygen level in culture that mimics the tumor microenvironment (TME) [8, 9], 4% O2 (hypoxia), and compared to cells exposed to 7% O2 (physoxia) and 21% O2 (hyperoxia, typical cell culture). The percentage of CD44+ cells did not show significant differences at different oxygen levels (F2,36 = 1.32, P = 0.28, Figure 1A-B, Supplemental Figure S1A). However, the percentage of CD44+GlaS+ cells (indicating GlaS binding) varied significantly (F2,36 = 27.69, P < 0.001, Figure 1C-D, Supplemental Figure S1B). Hypoxic conditions showed increased GlaS binding, with 61% of cells being CD44+GlaS+ under hypoxic conditions, compared to 47% in physoxia (P = 0.01) and 28% in hyperoxia (P < 0.001). There were no significant differences in GlaS binding in CD44− cells (P = 0.054), suggesting that oxygen dependent binding is specific to CD44+ cells (Figure 1E-F). These findings were also validated using known PS-binding AnnV, and antibodies, 11.31 and Bavi (Supplemental Figure S2A-F). We confirmed that GlaS binds PS in a similar manner to these proteins, though there were differences in the overlapping populations of cells binding GlaS, AnnV, and PS antibodies (Supplemental Figure S2G-I). However, unlike AnnV and Bavi, GlaS was internalized into the cell, demonstrating its potential for delivering therapeutic payloads to the cytoplasm. This was revealed by confocal microscopy where GlaS can be seen on the cell membrane and in the cytoplasm, in contrast to the membrane-only localization of PS-binding controls (Figure 1G, Supplemental Figure S3); fluorescence localization of GlaS vs AnnV has been quantified previously [4]. This internalization indicated that GlaS can be used to target intracellular pathways, whereas cell surface binding of the other PS binders may be better suited for immune modulation.
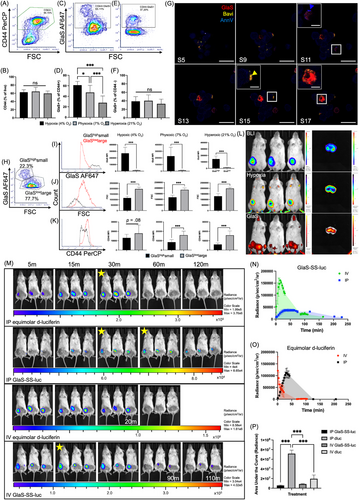
Further analysis revealed two subpopulations within the CD44+ cells based on size, CD44 expression and GlaS staining intensity (Figure 1H-K, Supplemental Figure S4). The smaller cells (low forward-scatter; FSC) with lower CD44 expression, likely representing quiescent CSCs [10], had greater GlaS fluorescence intensity (GlaShighsmall) than the larger cells (GlaSlowlarge). Interestingly, GlaShighsmall cells exhibited significant differences in CD44 median fluorescence intensity (MFI) when exposed to different oxygen conditions (F2,36 = 19.74, P < 0.001), with higher expression under hypoxia versus physoxia and hyperoxia. GlaSlowlarge cells had lower GlaS binding and were 1.5× larger versus the GlaShighsmall cells (P < 0.001 in all oxygen conditions for FSC [t(24) = 13.49; hypoxic, t(24) = 9.58; physoxic and t(16.54) = 9.72; hyperoxic] and GlaS MFI [t(12.05) = 10.45; hypoxic and t(12.11) = 10.47; hyperoxic]. GlaS MFI was 21×, 18×, and 10× higher in the smaller vs larger CD44+ population at hypoxic, physoxic and hyperoxic conditions, respectively. Differences in GlaS MFI relative to oxygen conditions were apparent in the GlaShighsmall population (P < 0.001), but not in the GlaSlowlarge population (F2,36 = 1.63, P = 0.21). These findings underscore the potential of GlaS for selectively targeting the small, tumorigenic CSCs within the hypoxic TME, which are often the most resistant to treatment.
After a systemic (intravenous; IV) administration of GlaS conjugated to a fluorophore (HiLyte750), fluorescent imaging identified accumulation within hypoxic regions of tumors In vivo (Figure 1L). While bioluminescent imaging (BLI) identified viable CSCs throughout the tumor, HypoxySense was used to visualize hypoxic areas, with the tumor cores exhibiting higher levels of hypoxia compared to the surrounding tumor tissue. GlaS accumulated not only within the tumor but also in the gut, knees, ankles, and teeth. In a bisected excised tumor, the localization and spatial resolution of the fluorophores were improved. GlaS localized primarily in the tumor core, where hypoxia was also more pronounced, supporting its potential as a hypoxia-targeted therapeutic agent.
To demonstrate GlaS functional delivery capabilities, we used GlaS conjugated via a disulfide linkage to luciferin (GlaS-SS-luc), with an imaging approach to validate cellular internalization and cargo release (due to intracellular glutathione). Because the CSC in the patient derived xenograft (PDX) models express luciferase, this allowed for real-time tracking of GlaS-mediated delivery using BLI (Figure 1M, Supplemental Figure S5). GlaS-SS-luc showed delayed and sustained bioluminescent signals compared to free D-luciferin (Figure 1N-O), with similar patterns observed after IV and intraperitoneal (IP) administration. After IP administration of D-luciferin, signals peaked at 33 mins, which was similar to the onset of the IP GlaS-SS-luc peak. However, GlaS-SS-luc signals were sustained for 31 mins, while D-luciferin signals declined immediately after peaking despite luciferin concentrations being equimolar. Following IV administration of D-luciferin, the peak signal was detected instantly (< 1 min post-injection), whereas GlaS-SS-luc showed a delayed peak at 15 mins. Overall, peak bioluminescent signals were higher after the administration of equimolar D-luciferin compared to GlaS-SS-luc, with 7.5-fold increase for IV and a 30-fold increase for IP administration. Area under the curve (AUC) was calculated for each treatment (Figure 1P), to quantify luciferin delivered over that time period, with significant differences in AUC between the different materials and injection routes (F3,8 = 99.11, P < 0.001). IV D-luciferin as well as IV and IP GlaS-SS-luc conjugates delivered significantly less luciferin than IP D-luciferin (P < 0.001 for all). There were no differences when GlaS-SS-luc was administered IP or IV (P = 0.908), nor when GlaS-SS-luc or D-luciferin were administered IV (P = 0.133). Together these results indicated that GlaS must first bind to PS, be internalized, and release its cargo inside the cell, compared to free diffusion of D-luciferin. This suggests that GlaS could be used to target and treat CSCs with therapies that affect intracellular signaling pathways, as well as indicating potential for prolonged therapeutic action.
This study highlights GlaS as a promising tool for the targeted delivery of therapies to CSCs in their native, hypoxic niches (further discussed in Supplementary Information). By exploiting PS externalization, particularly in hypoxic CSCs, GlaS could provide a strategy to overcome the challenge of CSC-mediated tumor relapse, metastasis and resistance to conventional treatments. Overall, the study highlights that hypoxia increases GlaS binding to CSCs, particularly on the smaller, more stem-like populations, and GlaS can deliver intracellular therapeutic molecules to these cells effectively both in vitro and In vivo.
AUTHOR CONTRIBUTIONS
Ashley V Makela, Anthony Tundo, Terry Hermiston, and Christopher H Contag conceptualized the study. Ashley V Makela and Anthony Tundo performed experiments, data analysis and statistics. Ashley V Makela, Anthony Tundo, Terry Hermiston, and Christopher H Contg contributed to the analysis and/or interpretation of the data. Doug Schneider and Terry Hermiston helped design the GlaS and GlaS-Fc. Huiping Liu provided the PDX cell lines and technical advice surrounding growth and maintenance. Pavlo Khodakivskyi and Elena Goun developed the GlaS-SS-luc, GlaS-Fc-SS-luc and AnnV-SS-luc materials, and provided guidance on their use. Ashley V Makela drafted the manuscript. All authors revised and edited the manuscript.
ACKNOWLEDGEMENTS
We thank the MSU Flow Cytometry Core for their assistance in experimental planning, acquisition and analysis. We also thank the IQ Microscopy Core for their assistance in confocal microscopy training and help with acquisition and IQ Imaging Core for their support in imaging studies.
CONFLICT OF INTEREST STATEMENT
Huiping Liu is a co-founder of ExoMira Medicine although the current studies are not relevant. Terry Hermiston is founder and CEO of GLAdiator Biosciences. Pavlo Khodakivskyi is affiliated with VitaLume Biotechnologies LLC.
FUNDING INFORMATION
This work was supported in part by GLAdiator Biosciences and The James and Kathleen Cornelius Endowment (to Christopher Contag). This work is partially supported by NIH/NCI R01CA245699 and American Cancer Society ACS0137006 (to Huiping Liu).
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This research was conducted in accordance with the protocols approved by MSU IACUC (PROTO202400041). The patient derived xenograft models were established previously at Stanford University and Northwestern University, with informed consent obtained from the patients.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting this study are available upon reasonable request from the corresponding author.