A prospective, phase II, neoadjuvant study based on chemotherapy sensitivity in HR+/HER2- breast cancer-FINEST study
Li Chen and Wen-Ya Wu contributed equally to this work.
Abstract
Background
Hormone receptor-positive (HR+)/humaal growth factor receptor 2-negative (HER2-) breast cancer, the most common breast cancer type, has variable prognosis and high recurrence risk. Neoadjuvant therapy is recommended for median-high risk HR+/HER2- patients. This phase II, single-arm, prospective study aimed to explore appropriate neoadjuvant treatment strategies for HR+/HER2- breast cancer patients.
Methods
Eligible female patients with newly diagnosed, untreated HR+/HER2- breast cancer received 2 cycles of nab-paclitaxel and carboplatin (nabPCb). Magnetic resonance imaging (MRI) was performed to assess tumor responses, and 40% regression of the maximal tumor diameter was deemed chemo-sensitive. Chemo-sensitive patients continued nabPCb for 4 more cycles (group A). Chemo-insensitive patients were randomized to groups B, C, and D at a ratio of 1:3:1 to receive a new chemotherapy for 4 cycles or endocrine-immune-based therapy (dalpiciclib, letrozole and adebrelimab, with goserelin if patients were premenopausal) for 4 cycles or to undergo surgery. Peripheral blood and core-needle biopsy (CNB) samples were collected before treatment, followed by a next-generation sequencing (NGS) panel detection and similarity network fusion (SNF) typing through digital pathology data. The primary endpoint was the pathological complete response (pCR) rate, and the secondary endpoint was the clinical objective response rate (ORR).
Results
A total of 121 patients were enrolled (67.8% with stage III disease), with 76, 9, 27, and 9 patients in groups A, B, C and D, respectively. The total pCR rate was 4.1%, and all patients who received pCR were in group A. Group C had a better ORR than Group B (81.5% vs. 66.7%). Exploratory analysis revealed that patients with the SNF4 subtype were the most sensitive to nabPCb (pCR rate of 21.1% vs. 1.8% in group A), whereas patients in group C with the SNF2 subtype were more sensitive to endocrine-immune-based therapy (Miller-Payne grade 4-5, 45.5% vs. 6.3%).
Conclusions
Converting to endocrine-immune-based therapy improved the ORR, but not the pCR rate in chemo-insensitive patients. Neoadjuvant chemotherapy and endocrine therapy are not mutually exclusive. The SNF4 subtype of HR+/HER2- breast cancer was more chemo-sensitive, whereas the SNF2 subtype might be more sensitive to immunotherapy.
List of abbreviations
-
- HR
-
- hormone receptor
-
- HER2
-
- human epidermal growth factor receptor 2
-
- nabPCb
-
- nab-paclitaxel and carboplatin
-
- CNB
-
- core-needle biopsy
-
- NGS
-
- next-generation sequencing
-
- SNF
-
- similarity network fusion
-
- pCR
-
- pathological complete response
-
- ORR
-
- objective response rate
-
- ET
-
- endocrine therapy
-
- NAC
-
- neoadjuvant chemotherapy
-
- NET
-
- neoadjuvant endocrine treatment
-
- CDK4/6
-
- cyclin-dependent kinase 4 and 6
-
- AI
-
- aromatase inhibitor
-
- OFS
-
- ovarian function suppression
-
- RCB
-
- residual cancer burden
-
- MP
-
- Miller&Payne system
-
- AUC
-
- area under the curve
-
- TRAE
-
- treatment-related adverse event
-
- RFS
-
- recurrence-free survival
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- FN
-
- febrile neutropenia
-
- ACTH
-
- adrenocorticotropic hormone
-
- irAE
-
- immune-related adverse event.
1 BACKGROUND
Hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-) breast cancer accounts for 70% of all invasive breast cancer cases [1]. Neoadjuvant therapy is a well-established therapeutic option for patients with locally advanced disease, as this therapy often allows downstaging, facilitates breast-conserving therapy, and enables the assessment of in vivo biomarkers to identify proof-of-principle activity or test new treatment strategies [2, 3]. Achieving a pathological complete response (pCR) to chemotherapy is less common in HR+/HER2- breast cancer patients than in patients with other subtypes of breast cancer [4, 5]. The major challenge in the neoadjuvant treatment of HR+/HER2- breast cancer is identifying the appropriate initial strategy for chemotherapy or endocrine therapy (ET) and further defining intertumoral heterogeneity to guide treatment strategies.
Neoadjuvant chemotherapy (NAC) is recommended for median-high-risk HR+/HER2- patients, and pCR rates after NAC are in the range of 7%-16% [6]. Recent research has focused mainly on further increasing the pCR rate in HR+ patients. One method is to combine targeted therapy with cytotoxic drugs. Immune checkpoint inhibitors (ICIs) are attracting extensive attention for the treatment of breast cancer [7, 8]. Two large phase III clinical trials, CheckMate 7FL and KEYNOTE-756, have evaluated the efficacy of adding an ICI to NAC in grade 3 stage II-III HR+/HER2- breast cancer and have shown that the addition of immunotherapy greatly increased pCR rates [9, 10]. Another approach is to adjust cytotoxic drugs according to patients’ chemo-sensitivity. However, GeparTrio demonstrated that extending cycles in chemo-sensitive patients or converting to different chemotherapy regimens in chemo-insensitive patients did not increase the pCR rate in HR+ patients [11].
A recent meta-analysis reported similar response rates in HR+/HER2- BC patients treated with neoadjuvant endocrine treatment (NET) or NAC [12]. Additionally, NET yielded lower toxicity, suggesting that NET should be further considered as an option in the appropriate setting. Given the antiproliferative effects of cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors, neoadjuvant studies on combined therapy with CDK4/6 inhibitors have been performed, and the results showed that the addition of a CDK4/6 inhibitor to an aromatase inhibitor (AI) in the neoadjuvant setting significantly decreased Ki-67 levels [13, 14].
The optimal strategy for NET in HR+ patients is still unknown. One research direction is the initial use of neoadjuvant ET in HR+ early BC patients and the evaluation of endocrine sensitivity. For example, in the ADAPT studies, preoperative ET was performed for 2 to 4 weeks, and patients with Ki-67 10% and a recurrence score 25 were exempted from chemotherapy [15]. However, in general, this strategy is suitable only for breast cancer patients with low tumor burdens (T1-2N0-1). The ALTERNATE trial tested a different treatment strategy: first, NET was used; then, in patients who were less sensitive to NET (Ki-67 was greater than 10% at week 4 or week 12), conversion to chemotherapy was performed but resulted in a poor pathological response, with a pCR rate of 4.8% [16]. Similar results were reported in the Z1031B trial, which demonstrated the relative ineffectiveness of chemotherapy for ET-resistant tumors [17].
Hence, it is essential to explore the intrinsic characteristics of HR+ tumors to guide treatment strategies. The FINEST trial aimed to explore whether switching to ET combined with immunotherapy and CDK4/6 inhibitors could improve the response rate of chemo-insensitive patients with median-high risk HR+/HER2- breast cancer. To better explore the sensitivities and identify suitable target populations for different treatments, we collected additional samples and conducted multi-omics analysis.
2 MATERIALS AND METHODS
2.1 Study design and participants
The FINEST trial was an investigator-driven, phase II, single-arm trial conducted at the Fudan University Shanghai Cancer Center (FUSCC) (NCT04215003). Eligible patients were females aged 18 years with newly diagnosed, previously untreated, histologically confirmed HR+/HER2- invasive breast cancer. The patients had stage II to III breast cancer and were considered candidates for NAC based on local multidisciplinary evaluation. Other key inclusion criteria were Eastern Cooperative Oncology Group performance status of 0-1 and normal bone marrow, liver, and renal function. The key exclusion criteria included stage IV breast cancer, contraindications for chemotherapy, and other common exclusion criteria in immunotherapy trials. This study was conducted in accordance with the Good Clinical Practice Guidelines and the World Medical Association Declaration of Helsinki. Written informed consent was obtained from all patients. The trial protocol and amendments were approved by a competent ethics committee.
2.2 Procedures
Patients were administered intravenous nab-paclitaxel 100 mg/m2 and carboplatin area under the curve (AUC) 2 mg/mL/min on day 1, day 8, and day 15 every 4 weeks for two cycles. Magnetic resonance imaging (MRI) was performed to assess tumor responses. Patients with 40% MRI regression of the maximal tumor diameter after two cycles of nab-paclitaxel and carboplatin (nabPCb) were considered as chemo-sensitive, and were assigned to group A. These patients completed four subsequent cycles of nabPCb. Chemo-insensitive patients (< 40% MRI regression of the maximal tumor diameter after two cycles of nabPCb) were randomly assigned (1:3:1) to groups B, C, and D. Group B patients were switched to different chemotherapies, including EC (intravenous epirubicin 100 mg/m2 and cyclophosphamide 600 mg/m2 on day 1 every 3 weeks) and NX (intravenous vinorelbine 25 mg/m2 on day 1 and day 8 every 3 weeks, and per oral administration of capecitabine 1,000 mg/m2 twice a day for 14 days every 3 weeks). Group C received 125 mg of oral dalpiciclib daily on a 21 days-on, 7 days-off schedule, 2.5 mg of oral letrozole daily and eight 14-day cycles of 600 mg of intravenous adebrelimab, with the addition of 3.75 mg of intramuscular goserelin every 28 days for premenopausal patients. Letrozole could be used until surgery, at the discretion of the investigators. Reasons to discontinue treatments included unacceptable toxicity, loss to follow-up, noncompliance, physician decision, progressive disease, protocol deviation, and death. The patients in group D underwent direct surgery. Adjuvant therapy was administered at the discretion of the physician.
At baseline, patients underwent radiological tumor assessment via breast MRI, ultrasound, mammography, physical examination, routine work-up staging, assessment of vital signs, laboratory tests, and 12-lead electrocardiography. Laboratory tests, vital signs assessment, and physical examinations were performed before and after every cycle or before surgery. At weeks 1, 9, 17, and 25, the primary breast and axillary lymph nodes were assessed via clinical breast examination, breast MRI, and ultrasound. The objective response for all assessment modalities was defined by modified Response Evaluation Criteria in Solid Tumors (version 1.1) (RECIST 1.1), in which malignant lymph nodes were assessed as non-target lesions, because size alone might not adequately characterize the disease status. The type of surgery indicated in the absence of neoadjuvant treatment was recorded at baseline.
2.3 Outcomes
According to the Bayesian approach, the primary endpoint was to observe whether the pCR rate in group C (switching to ET plus immunotherapy and CDK4/6 inhibitors) was superior to that in group B (switching to different chemotherapies). pCR was determined by local pathological evaluation and was defined as the absence of invasive cancer cells in the breast and axilla (ypT0/is, ypN0). We evaluated the pCR rate after every 10 patients in group C received combined therapy. The key secondary endpoint reported here was the clinical objective response rate (ORR). The ORR was defined as a partial or complete response according to RECIST 1.1 based on breast MRI data obtained at baseline and before surgery.
For the safety evaluation, adverse events were graded according to National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI CTCAE; version 5.0). Immune-related adverse events (irAEs) were defined as adverse events caused by ICIs [18, 19].
2.4 Next-generation sequencing (NGS) analysis
FUSCC has developed a clinical NGS panel for the detection of somatic and germline mutations in 513 breast cancer-specific genes in clinical settings. Breast cancer patients with FUSCC who agreed to the detection of multiple genes were referred to the Precision Cancer Medicine Center, and tumor tissue and peripheral blood samples were collected from these patients. DNA sequencing and data analysis were subsequently performed. Using NGS data, we analyzed and identified the genomic landscape, mutation characteristics, and potential correlations with clinical data efficacy.
2.5 Digital pathology data collection and preprocessing for similarity network fusion (SNF)-subtyping
Based on a multi-omics dataset, our center successfully divided all HR+/HER2- populations into four clusters, namely, the SNF subtypes [20]. Since the clinical implementation of multi-omics profiling is difficult because of its high cost, long turnaround time, and complicated technological processes, inexpensive, fast, and convenient approaches are needed to extrapolate our SNF-subtyping system. In this study, we employed classifiers based on digital pathology data.
2.6 Statistical analysis
The associations between molecular subtypes and clinical efficacy were studied via univariate logistic regression or the χ2 test. The Mann-Whitney U test was used to compare biomarkers between the pCR and non-pCR groups. Statistical significance was set at P < 0.05. Data were analyzed via IBM SPSS Statistics (version 27) and R software (version 4.0.3).
3 RESULTS
3.1 Patient baseline characteristics
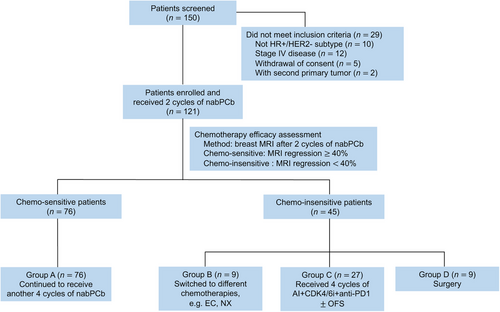
From November 16, 2020, to June 10, 2022, 150 patients were assessed for eligibility, and 121 patients were ultimately enrolled (Figure 1). Seventy-six patients with better efficacy (≥40% MRI regression after 2 cycles of nabPCb) completed the subsequent 4 cycles of nabPCb (group A). Another 45 chemo-insensitive patients (< 40% MRI regression after 2 cycles of nabPCb) were randomly assigned to groups B (n = 9), C (n = 27), and D (n = 9).
The patient characteristics are reported in Table 1. The median patient age was 48 years. The majority (67.8%) of patients presented with clinical stage III breast cancer, 96.7% had lymph node involvement, and most had a high Ki-67 index (Ki-67 index ≥20%; 79.6%). Moreover, 60.3% of patients had a histologic grade of 3.
| Characteristic |
Group A (n = 76) Chemo-sensitive |
Group B (n = 9) Chemo-switched |
Group C (n = 27) AI+CDK4/6i+anti PD-1 OFS |
Group D (n = 9) Surgery |
|---|---|---|---|---|
| Age, years, mean (range) | 46 (31-64) | 54 (32-63) | 62 (31-70) | 50 (36-58) |
| Tumor size, n % | ||||
| T1 | 3 (3.9) | 0 (0) | 0 (0) | 0 (0) |
| T2 | 36 (47.4) | 3 (33.3) | 9 (33.3) | 7 (77.7) |
| T3 | 31 (40.8) | 4 (44.4) | 13 (48.1) | 2 (22.3) |
| T4 | 6 (7.9) | 2 (22.3) | 5 (18.5) | 0 (0) |
| Lymph node status, n % | ||||
| N0 | 3 (3.9) | 2 (22.3) | 0 (0) | 0 (0) |
| N1 | 42 (55.3) | 4 (44.4) | 13 (48.1) | 4 (44.4) |
| N2 | 24 (31.6) | 2 (22.3) | 9 (33.3) | 5 (55.6) |
| N3 | 7 (9.2) | 1 (11.0) | 5 (18.5) | 0 (0) |
| Baseline tumor stage, n % | ||||
| IIA | 5 (6.6) | 0 (0) | 0 (0) | 1 (11.2) |
| IIB | 23 (30.3) | 2 (22.3) | 4 (14.9) | 4 (44.4) |
| IIIA | 38 (50.0) | 4 (44.4) | 16 (59.3) | 4 (44.4) |
| IIIB | 3 (3.9) | 2 (22.3) | 2 (7.4) | 0 (0) |
| IIIC | 7 (9.2) | 1 (11.0) | 5 (18.5) | 0 (0) |
| SNF subtype, n % | ||||
| 1 | 23 (30.3) | 2 (22.3) | 6 (22.2) | 4 (44.4) |
| 2 | 15 (19.7) | 2 (22.3) | 11 (40.8) | 2 (22.3) |
| 3 | 17 (22.4) | 3 (33.3) | 5 (18.5) | 2 (22.3) |
| 4 | 21 (27.6) | 2 (22.3) | 5 (18.5) | 1 (11.2) |
| Histological grade, n % | ||||
| 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 2 | 30 (39.5) | 3 (33.3) | 12 (44.4) | 3 (33.3) |
| 3 | 46 (60.5) | 6 (66.7) | 15 (55.6) | 6 (66.7) |
| Ki-67 expression, n % | ||||
| < 14% | 14 (18.4) | 1 (11.1) | 4 (14.8) | 2 (23.3) |
| ≥14% | 62 (81.6) | 8 (88.9) | 23 (85.2) | 7 (77.7) |
| Luminal subtype, n % | ||||
| Luminal A | 25 (32.9) | 4 (44.4) | 9 (33.3) | 2 (22.3) |
| Luminal B | 51 (67.1) | 5 (55.6) | 18 (66.7) | 7 (77.7) |
| Miller&Payne, n % | ||||
| 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 2 | 12 (15.8) | 4 (44.4) | 9 (33.3) | 3 (33.3) |
| 3 | 40 (52.6) | 5 (55.6) | 12 (44.5) | 3 (33.3) |
| 4 | 15 (19.7) | 0 (0) | 5 (18.5) | 2 (22.3) |
| 5 | 9 (11.9) | 0 (0) | 1 (3.7) | 1 (11.2) |
| RCB categories, n % | ||||
| 0 | 5 (6.6) | 0 (0) | 0 (0) | 0 (0) |
| 1 | 18 (23.7) | 0 (0) | 3 (11.1) | 0 (0) |
| 2 | 31 (40.8) | 4 (44.4) | 9 (33.3) | 2 (22.3) |
| 3 | 22 (28.9) | 5 (55.6) | 15 (55.6) | 7 (77.7) |
| Breast conserving surgery, n % | 15 (19.7) | 2 (22.3) | 4 (14.8) | 0 (0) |
- Abbreviations: CDK4/6i, cyclin-dependent kinase 4 and 6 inhibitors; AI, aromatase inhibitor; OFS, ovarian function suppression; SNF, similarity network fusion; RCB, residual cancer burden.
According to the Bayesian approach, the primary endpoint was to evaluate whether the pCR rate of group C was superior to that of group B. Since that the first 20 patients in group C failed to achieve pCR, we speculated that the FINEST study did not meet the primary endpoint; thus, we suspended the enrollment. Ultimately, 27 patients were included in group C, and the total number of patients included in our study was 121.
3.2 Efficacy
Five out of 121 patients achieved pCR (4.1%), and all five were in group A (5/76, 6.7%). The ORR was 81.8% in the total cohort, with rates of 100%, 66.7%, 81.5%, and 0% in groups A, B, C, and D, respectively (Figure 2A). The Ki-67 index is an important indicator of neoadjuvant therapy sensitivity in patients with HR+/HER2- breast cancer. The Ki-67 index decreased significantly between the baseline and postoperative tumor samples in groups A, B, and C (P < 0.001, Figure 2B). The decrease in the Ki-67 index in groups B and C was statistically significant (P < 0.001, Figure 2C).
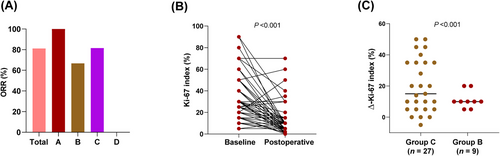
The ORR and alterations of the Ki-67 index in every group. (A) The ORR in each group. (B) The Ki-67 index between baseline and postoperative tumor specimens in in group A, B and C. (C) Decrease of the Ki-67 index between group B and C. The data was compared via paired t-tests. Abbreviations: ORR, objective response rate.
3.3 Safety
Overall, 94% of the patients received at least 4 courses of adebrelimab. Three patients permanently discontinued adebrelimab for safety reasons (including grade 3 hyperglycemia [n = 1], grade 3 liver toxicity [n = 1], and grade 2 liver toxicity [n = 1]). Three patients discontinued dalpiciclib after three courses owing to grade 3 febrile neutropenia (FN). The most common grade 3 treatment-related adverse events (TRAEs) during chemotherapy were neutropenia (33.1%), fatigue (32.2%), and nausea (27.3%) (Table 2). Increases in neutropenia (59.3%), alanine aminotransferase (ALT, 22.2%), aspartate aminotransferase (AST, 22.2%), and hypothyroidism (14.8%) were the most common TRAEs of any grade. The most frequent irAEs during adebrelimab treatment were endocrinopathies, including hypothyroidism (14.8%), hyperthyroidism (7.4%), decreased adrenocorticotropic hormone (ACTH, 3.7%), and hyperglycemia (3.7%, grade 3) (Table 3). Serious adverse events during chemotherapy included FN (n = 2).
| TRAEs occurring in > 5% of patients (any grade) | All grade, n (%) | G1-2, n (%) | G3, n (%) | G4, n (%) |
|---|---|---|---|---|
| Chemotherapy, n = 121 | ||||
| Neutropenia | 40 (33.1) | 15 (12.4) | 15 (12.4) | 10 (8.3) |
| Fatigue | 39 (32.2) | 39 (32.2) | 0 (0) | 0 (0) |
| Nausea | 33 (27.3) | 33 (27.3) | 0 (0) | 0 (0) |
| AST increased | 31 (25.6) | 22 (18.2) | 9 (7.4) | 0 (0) |
| ALT increased | 30 (24.8) | 22 (18.2) | 8 (6.6) | 0 (0) |
| Anemia | 18 (14.9) | 17 (14.0) | 1 (0.9) | 0 (0) |
| Vomiting | 12 (9.9) | 12 (9.9) | 0 (0) | 0 (0) |
| Adebrelimab phase, n = 27 | ||||
| Neutropenia | 16 (59.3) | 6 (22.2) | 6 (22.2) | 4 (14.8) |
| ALT increased | 6 (22.2) | 4 (14.8) | 2 (7.4) | 0 (0) |
| AST increased | 6 (22.2) | 4 (14.8) | 2 (7.4) | 0 (0) |
| Nausea | 4 (14.8) | 4 (14.8) | 0 (0) | 0 (0) |
| Fatigue | 3 (11.1) | 3 (11.1) | 0 (0) | 0 (0) |
| Anemia | 3 (11.1) | 3 (11.1) | 0 (0) | 0 (0) |
- Abbreviations: TRAEs, treatment-related adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
| Potentially irAEs (any incidence, any grade) | All grade, n (%) | G1-2, n (%) | G3, n (%) | G4, n (%) |
|---|---|---|---|---|
| Adebrelimab phase, n = 27 | ||||
| Hypothyroidism | 4 (14.8) | 4 (14.8) | 0 (0) | 0 (0) |
| Hyperthyroidism | 2 (7.4) | 2 (7.4) | 0 (0) | 0 (0) |
| Infusion-related reaction | 2 (7.4) | 2 (7.4) | 0 (0) | 0 (0) |
| ACTH decreased | 1 (3.7) | 1 (3.7) | 0 (0) | 0 (0) |
| Hyperglycemia | 1 (3.7) | 0 (0) | 1 (3.7) | 0 (0) |
- Abbreviations: irAE, immune-related adverse event; ACTH, adrenocorticotropic hormone.
3.4 Exploratory analysis
We established an NGS cohort comprising 98 patients (n = 54 for group A; n = 9 for group B; n = 26 for group C; n = 9 for group D). Our results revealed that the most prevalent breast cancer-related variation observed in our cohort was PIK3CA (41%), followed by mutations in TP53 (28%) and GATA3 (17%). Other top-ranking mutated genes are shown in Supplementary Figure S1. This finding was consistent with the genetic mutation profile of previously reported HR+/HER2- breast cancers at our center [21]. Our cohort had a higher BRCA2 mutation rate (8%). Through carcinogenic pathway analysis, we detected greater mutation rates in RTK-RAS, NOTCH, and PI3K-AKT pathways (Supplementary Figure S2). We also explored the correlation between the key clinicopathological characteristics and genetic profiles in each treatment group. No specific gene mutations were found to influence the efficacy of chemotherapy. The small sample sizes of groups B and D might limit the identification of specific driving genetic events.
Furthermore, we analyzed the internal SNF subtype of each patient to determine whether the SNF subtype was correlated with treatment efficacy. In group A (i.e., the chemo-sensitive group), the overall effective rate was the highest, and patients with the SNF4 subtype had the highest rate of pCR (21.1% [4/19] vs. 1.8% [1/57]) (Figure 3A). In group C, although no pCR was achieved, there were still more patients with tumor remission than in group B. The high proportion of Miller-Payne (MP) grade 4-5 or residual cancer burden (RCB) categories 0-1 in the SNF2 subtype suggests that the SNF2 subtype is intrinsically sensitive to immunotherapy (MP grade 4-5, 45.5% [5/11] vs. 6.3% [1/16]; RCB categories 0-1, 18.2% [2/11] vs. 6.3% [1/16]) (Figure 3B-C).
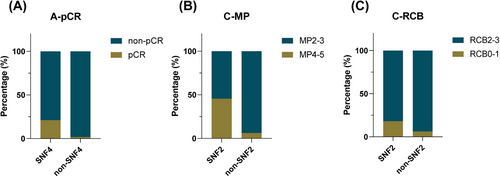
The correlation between the SNF subtype and clinical efficacy in groups. (A) The correlation between the SNF4 and non-SNF4 subtype and clinical efficacy in group A. (B, C) The correlation between the SNF2 and non-SNF2 subtype and clinical efficacy in group C. Abbreviations: pCR, pathological complete response; SNF, similarity network fusion; MP, Miller&Payne system; RCB, residual cancer burden.
4 DISCUSSION
We report the results of a clinical trial that adopted an early prediction of outcome based on therapy response and adjusted treatment strategy in patients with early HR+/HER2- breast cancer. In chemo-sensitive patients, an ongoing full course of NAC resulted in the highest pCR rate and ORR. In chemo-insensitive patients, changing cytotoxic regimen or ET did not increase pCR rates, whereas converting to endocrine-immune-based therapy resulted in a higher ORR and decreased Ki-67 levels. Patients with the SNF4 subtype of HR+/HER2- breast cancer were more chemo-sensitive, whereas patients with the SNF2 subtype might be more sensitive to immunotherapy.
The ADAPT trial suggested neoadjuvant ET or exemption from chemotherapy for HR+/HER2- patients with a low tumor burden [15]. NAC is recommended for median-high risk HR+/HER2- patients, and the KEYNOTE-756 trial noted that combining pembrolizumab with NAC could significantly increase the pCR rate of early-stage high-risk ER+/HER2- breast cancer [10]. However, there is an unmet clinical need to determine effective treatment options for patients who fail to benefit from NAC. With the emergence of CDK4/6 inhibitors and immunotherapy, our study aimed to explore whether switching to ET plus immunotherapy and CDK4/6 inhibitors could increase the response rate in chemo-insensitive patients.
Neoadjuvant therapy provides early information on the response to treatment [22], and this response can serve as a prognostic factor for long-term outcomes and guide subsequent therapy [23]. The FINEST trial was a response-guided neoadjuvant therapy exploration for median-high risk patients with stage II-III breast cancer. All patients received 2 cycles of nabPCb. Predictive methods and cutoff values for evaluating treatment sensitivity may help us further explore therapy-sensitive patients. There is currently no consensus on the criteria for discriminating responders after chemotherapy by imaging. The PHERGain study employed a 40% reduction in SUVmax in 18F-fluorodeoxyglucose (18F-FDG)-PET scans after two cycles of chemotherapy as the threshold to define the responders [24]. The GeparTrio trial instead adopted ultrasonic reduction ≥50% of the primary breast tumor as the effective threshold to identify responders [11]. Additionally, the ACRIN 6657 trial have demonstrated that MRI is the most accurate imaging method for assessing tumor response to neoadjuvant therapy, and outperforms mammography, ultrasound, and clinical examination [25]. According to RESIST 1.1, partial response (PR) requires at least a 30% decrease in the diameter of the tumor lesion [26]. Therefore, in our study, we set a stricter standard in which ≥40% regression of the maximal tumor diameter assessed by MRI after two cycles of nabPCb was considered to indicate chemo-sensitivity.
Treatment strategies can be modified to improve the overall prognosis depending on the patient's response to initial therapy. There are two ways to improve the survival outcomes in chemo-insensitive patients with HR+/HER2- breast cancer. One approach is to convert to another regimen of chemotherapy, and the other is to combine ET with other therapies, such as CDK4/6 inhibitors and immunotherapy [9, 10, 12-14]. Neoadjuvant ET combined with CDK4/6 inhibitors has synergistic effects. For example, the neoMONARCH study demonstrated that the combination of nastrozole and abemaciclib induced cell cycle arrest (defined as Ki-67 < 2.7% or natural logarithm < 1) more potently than did anastrozole alone (66% vs. 15%) [27]. Additionally, the KEYNOTE-756 and CheckMate 7FL trials revealed that the addition of immunotherapy to NAC greatly increased the pCR rate [9, 10]. Some studies have indicated that CDK4/6 inhibitors exert long-term anti-T-cell immunity and increase immunogenicity, which may provide a rationale for new combination strategies combining CDK4/6 inhibitors and immunotherapies for cancer treatment [28]. A decrease in Ki-67 from baseline in response to ET has been validated as a marker of treatment efficacy, and measurement of Ki-67 after two weeks of ET has been shown to improve the prediction of recurrence-free survival (RFS) [17, 29]. Therefore, we used AI plus a CDK4/6 inhibitor and a PD-1 inhibitor in chemo-insensitive patients in group C. We found that for endocrine-immune-based therapy, the ORR rate was 81.5%; furthermore, the Ki-67 index decreased in 96.3% of patients.
Generally, the pCR rate after NAC is 7%-16% and is related to the tumor burden of enrolled patients [6]. We previously reported that weekly paclitaxel plus carboplatin was an effective NCT for ER+/HER2–breast cancer, yielding a pCR rate of 10% [30]. There was no clear association between the pCR rate and improved outcomes in patients with HR+/HER2- breast cancer [31]. However, pCR is still recommended as the primary endpoint for neoadjuvant clinical trials by NeoSTEEP [32] and the US Food and Drug Administration (FDA) [33]. As a result, we adopted pCR as the primary endpoint for the FINEST trial. The pCR rate in our study was relatively low since it was difficult for us to predict the efficacy of the endocrine-immune-based therapy before the prospective enrollment. Additionally, the FINEST trial included more patients with stage III HR+/HER2- breast cancer (67.8%) than other neoadjuvant clinical trials, including ALTERNATE (27.3% patients with T3-4c tumors) [16] and KEYNOTE-756 (36.2% patients with T3/T4 tumors) [10].
According to the results of our trial, the ALTERNATE trial and the Z1031B trial in HR+ breast cancer, chemo-insensitive patients are not sensitive to endocrine-based therapy, and vice versa [16, 17]. Hence, the intrinsic characteristics of HR+ tumors should be further investigated to guide treatment strategies. We found that mutations in ASXL1, INSR and TBX3 may be associated with limited efficacy of chemotherapy, but due to the limited sample size, the P value was not statistically significant. We also examined the crosstalk between the androgen receptor (AR) and estrogen receptor (ER), which may lead to resistance to ET [34], but we failed to find an association. Our center divided all HR+/HER2- breast cancer patients into 4 SNF subtypes, derived from multi-omics clustering [18]. Furthermore, we found that the SNF subtype identified by digital pathology was correlated with the efficacy index. The overall effective rate was the highest in group A, and most of the patients who achieved pCR were SNF4 subtype. In group C, a higher ORR and lower Ki-67 index were associated with the SNF2 subtype, an intrinsic subtype that is sensitive to immunotherapy. Ongoing trials (NCT05582499 and NCT06561022) have focused on HR+/HER2- breast cancer patients at higher risk of relapse based on intrinsic SNF subtypes rather than on treatment sensitivity.
This study has several limitations. This was a nonrandomized phase II trial with a limited sample size, and tissue samples from each time point were not available for all included patients. MRI regression with a cutoff of 40% is worth refining. The greatest limitation is that the FINEST trial was a single-institution study. The strengths of this study include the study design, the implementation of a pilot study to test immunotherapy as part of the neoadjuvant treatment of HR+/HER2- breast cancer after two cycles of NAC, and the evaluation of strategies for early assessment and prediction.
5 CONCLUSION
In chemo-sensitive patients, a full course of NAC resulted in the highest pCR rates and ORR. In chemo-insensitive patients, a cytotoxic regimen or ET did not increase the pCR rate; however, conversion to endocrine-immune-based therapy resulted in a higher ORR and lower Ki-67 levels. The SNF subtype system has potential clinical value for guiding treatment strategies in HR+ patients, and clinical trials are needed for validation.
AUTHOR CONTRIBUTIONS
Jun-Jie Li and Zhi-Ming Shao designed and supervised the FINEST trial. Guang-Yu Liu, Ke-Da Yu, Jiong Wu, Gen-Hong Di, Jun-Jie Li, and Zhi-Ming Shao collected patient samples. Lei Fan and Zhong-Hua Wang conducted therapeutic efficacy evaluation. Li Chen and Wen-Ya Wu collected raw data, performed statistical analysis, and wrote the manuscript. Fei Liang was responsible for statistical review. All authors contributed to administrative support, provision of study materials or patients, collection and interpretation of data. All authors participated in the revision and final approval of the manuscript. The corresponding authors take full responsibility for the credibility of the descriptions and data presented in this work.
ACKNOWLEDGEMENTS
The authors are grateful to the staff of Precision Cancer Medicine Center for assisting with performing NGS panel sequencing, and the staff of the Department of Breast Surgery for their careful patient care. Authors also thank all the patients and their family members who participated in this study. This work was sponsored by grants from Jiangsu Hengrui Pharmaceuticals Co. Ltd. and the National Natural Science Foundation of China (grant number: 82172576).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was performed in accordance with the Declaration of Helsinki and was approved by the institutional independent ethics committee, namely the Approval Committees of Fudan University Shanghai Cancer Center (1908205-21). Trial registration: ClinicalTrials.gov (NCT04215003). Each participant signed an informed consent before participating to this study.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




