Current status and perspectives of esophageal cancer: a comprehensive review
Abstract
Esophageal cancer (EC) continues to be a significant global health concern, with two main subtypes: esophageal squamous cell carcinoma and esophageal adenocarcinoma. Prevention and changes in etiology, improvements in early detection, and refinements in the treatment have led to remarkable progress in the outcomes of EC patients in the past two decades. This seminar provides an in-depth analysis of advances in the epidemiology, disease biology, screening, diagnosis, and treatment landscape of esophageal cancer, focusing on the ongoing debate surrounding multimodality therapy. Despite significant advancements, EC remains a deadly disease, underscoring the need for continued research into early detection methods, understanding the molecular mechanisms, and developing effective treatments.
List of abbreviations
-
- 5-FU
-
- 5-fluorouracil
-
- AAPC
-
- Average Annual Percent Change
-
- ACG
-
- American College of Gastroenterology
-
- ADC
-
- Antibody-drug conjugates
-
- AFI
-
- Autofluorescence imaging
-
- AKT
-
- Serine/threonine-protein kinases
-
- ASCO
-
- American Society of Clinical Oncology
-
- ASDR
-
- Age-standardized death rate
-
- ASIR
-
- Age-standardized incidence rate
-
- ATO
-
- Arsenic trioxide
-
- BE
-
- Barrett's esophagus
-
- BLI
-
- Blue light imaging
-
- BQ-T
-
- Betel quid without tobacco
-
- c-KIT
-
- Cellular-mesenchymal epithelial transition factor
-
- CCD
-
- Charge-coupled devices
-
- CCND1
-
- Cell cycle mediator cytosolic cyclin D1
-
- cCR
-
- Clinical complete response
-
- cCRT
-
- Concurrent chemoradiotherapy
-
- CDKN2A
-
- Cyclin-dependent kinase inhibitor 2A
-
- cfDNA
-
- Cell-free DNA
-
- CI
-
- Confidence interval
-
- CIN
-
- Chromosomally unstable
-
- CLE
-
- Confocal laser microendoscope
-
- CNA
-
- Copy number alterations
-
- COX2
-
- Cyclooxygenase 2
-
- CPS
-
- Combined positive score
-
- CRT
-
- Chemoradiotherapy
-
- CTLA-4
-
- Cytotoxic T-lymphocyte-associated protein 4
-
- CUL3
-
- Cullin 3
-
- DALY
-
- Disability-adjusted life year
-
- DCF
-
- Docetaxel + cisplatin + fluorouracil
-
- dCRT
-
- Definitive CRT
-
- DFS
-
- Disease-free survival
-
- dMMR
-
- Mismatch repair protein deficiency
-
- EAC
-
- Esophageal adenocarcinoma
-
- EC
-
- Esophageal cancer
-
- ECF/ECX
-
- Epirubicin, cisplatin plus either fluorouracil or capecitabine
-
- EFS
-
- Event-free survival
-
- EGFR
-
- Epidermal growth factor receptor
-
- EGJ
-
- Esophagogastric junction
-
- EMR
-
- Endoscopic mucosal resection
-
- EP
-
- Epithelium
-
- ER
-
- Endoscopic resection
-
- ERBB2
-
- Erb-B2 Receptor Tyrosine Kinase 2
-
- ESCC
-
- Esophageal squamous cell carcinoma
-
- ESD
-
- Endoscopic submucosal dissection
-
- ESGE
-
- European Society of Gastrointestinal Endoscopy
-
- EUS
-
- Endoscopic ultrasound
-
- EVs
-
- Extracellular vehicles
-
- FGFR
-
- Fibroblast growth factor receptor
-
- FISH
-
- Fluorescence in situ hybridization
-
- FLOT
-
- Fluorouracil, leucovorin, oxaliplatin, and docetaxel
-
- G/GEJ
-
- Gastric or gastro-esophageal junction
-
- GAC
-
- Gastric adenocarcinoma
-
- GBD
-
- Global Burden of Disease
-
- GEJ
-
- Esophageal or gastroesophageal junction
-
- GERD
-
- Gastroesophageal reflux disease
-
- GOF
-
- Gain-of-function
-
- GWAS
-
- Genome-Wide Association Studies
-
- HER2
-
- Human epidermal growth factor receptor 2
-
- HGD
-
- High-grade dysplasia
-
- HGIN
-
- High-grade intraepithelial neoplasia
-
- HPV
-
- Human papillomavirus
-
- HRE
-
- High-resolution endoscopes
-
- HSP90
-
- Heat shock protein 90
-
- ICIs
-
- Immune checkpoint inhibitors
-
- ICT/RT
-
- Immunotherapy combined with chemo/radiotherapy
-
- IPCL
-
- Intraepithelial papillary endocapillary loops
-
- JES
-
- Japanese Esophageal Society
-
- KDR
-
- Kinase insert domain receptor
-
- KEAP1
-
- Kelch-like epichlorohydrin (ECH)-associated protein 1
-
- KMT2D
-
- Histone-lysine N-methyltransferase 2D
-
- KRAS
-
- Kristen rat sarcoma viral oncogene homolog
-
- LAG 3
-
- Lymphocyte activation gene 3
-
- LCE
-
- Lugol chromoendoscopy
-
- LGD
-
- Low-grade dysplasia
-
- LGIN
-
- Low-grade intraepithelial neoplasia
-
- LN
-
- Lymph node
-
- LNM
-
- Lymph node metastasis
-
- LPM
-
- Lamina propria mucosa
-
- LVI
-
- Lymphovascular invasion
-
- M2
-
- Alternatively activated macrophages
-
- MAPK
-
- Mitogen-activated protein kinase
-
- MEK
-
- Mitogen-activated extracellular signal-regulated kinase
-
- MIE
-
- Minimally invasive esophagectomy
-
- miRNA
-
- Micro RNA
-
- MM
-
- Mucosal muscle
-
- mMDSCs
-
- Monocytic myeloid-derived suppressor cells
-
- mOS
-
- Median OS
-
- MSI-H
-
- High microsatellite instability
-
- mTOR
-
- Mammalian target of rapamycin
-
- NBI
-
- Narrow-band imaging
-
- NCCN
-
- National Comprehensive Cancer Network
-
- nCRT
-
- Neoadjuvant chemoradiotherapy
-
- nCT
-
- Neoadjuvant chemotherapy
-
- NF1
-
- Neurofibromin 1
-
- NFE2L2
-
- Nuclear factor erythroid 2-related factor 2
-
- nICRT
-
- Neoadjuvant immunotherapy combined with chemoradiotherapy
-
- nICT
-
- Neoadjuvant immunotherapy combined with chemotherapy
-
- NOTCH1
-
- Neurogenic locus notch homolog protein 1
-
- NPV
-
- Negative predictive value
-
- NSAIDs
-
- Nonsteroidal anti-inflammatory drugs
-
- OCT
-
- Optical coherence tomography
-
- OE
-
- Optical enhancement
-
- ORR
-
- Objective response rate
-
- OS
-
- Overall survival
-
- pCR
-
- Pathological complete response
-
- PD-L1
-
- Programmed cell death ligand 1
-
- PEITC
-
- Phenethyl isothiocyanate
-
- PET
-
- Positron emission computed tomography
-
- PF/CF
-
- Fluorouracil + cisplatin
-
- PFS
-
- Progression-free survival
-
- PI3K
-
- Phosphatidylinositol-3-kinase
-
- PIK3CA
-
- Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha
-
- PPI
-
- Proton pump inhibitor
-
- PTEN
-
- Phosphatase And Tensin Homolog
-
- RAMIE
-
- Robotic-assisted minimally invasive esophagectomy
-
- RFA
-
- Radiofrequency ablation
-
- RFS
-
- Recurrence-free survival
-
- RTKs
-
- Receptor tyrosine kinases
-
- SBRT
-
- Stereotactic body radiotherapy
-
- scRNA-seq
-
- Single-cell RNA sequencing
-
- SFI
-
- Spectral fusion imaging
-
- SM
-
- Submucosa
-
- SOX-2
-
- Sex determining region Y-box 2
-
- SPRY1
-
- Sprouty RTK signaling antagonist 1
-
- STK11
-
- Serine/threonine kinase 11
-
- tDCs
-
- Tolerogenic dendritic cells
-
- TFF3
-
- Trilobal factor family protein 3
-
- THE
-
- Transhiatal esophagectomy
-
- TIGIT
-
- T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domain
-
- TIME
-
- Tumor immune microenvironment
-
- TKI
-
- Tyrosine kinase inhibitor
-
- TP53
-
- Tumor protein p53
-
- TP63
-
- Tumor protein p63
-
- TPS
-
- Tumor proportion score
-
- TR
-
- Time ratio
-
- Tregs
-
- Regulatory T cells
-
- TRG
-
- Tumor regression grade
-
- TROP2
-
- Trophoblastic cell-surface antigen 2
-
- TTP
-
- Time to progress
-
- VEGFA
-
- Vascular endothelial growth factor A
-
- VEGFR
-
- Vascular endothelial growth factor receptor
-
- VLE
-
- Volumetric laser endomicroscopy
-
- WHO
-
- World Health Organization
-
- WLE
-
- White light endoscopy
1 BACKGROUND
Esophageal cancer (EC) is the eleventh most common cancer and the seventh leading cause of cancer-related deaths worldwide, according to updated statistics from GLOBOCAN 2022, which accounts for 2.6% of all new cancer cases and 4.6% of cancer deaths [1, 2]. This highlights the aggressive nature of the disease and the challenges associated with its management.
The two main histological subtypes are esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), with ESCC making up 90% of all cases [3]. However, in certain regions, particularly in Western countries, the incidence of EAC has been rising rapidly, partly due to lifestyle factors such as obesity, gastroesophageal reflux disease (GERD), and Barrett's esophagus [4]. This shift in epidemiology underscores the importance of understanding the distinct etiological factors driving these subtypes, as they have significant implications for prevention, diagnosis, and treatment strategies.
Over the past two decades, there have been substantial advancements in the diagnosis and treatment of EC, mainly due to the increasing adoption of multimodal therapy. These therapies, which combine surgery, chemotherapy, radiotherapy, and, more recently, immunotherapy, have led to improvements in survival rates for patients with both ESCC and EAC. However, the overall prognosis remains poor, with a 5-year survival rate of approximately 20% across all stages [5]. This low survival rate is primarily because many patients are diagnosed at an advanced stage when curative treatment options are limited.
One of the most promising developments in the treatment of EC has been the introduction of immunotherapy. This approach has revolutionized the therapeutic landscape for many types of cancer, including EC. Immunotherapy offers hope for improved outcomes, particularly for patients with advanced disease, and is an area of active research and clinical trials.
Continued advances in understanding the biological and genetic characteristics of EC are crucial for developing novel therapeutic strategies. Research into the molecular mechanisms underlying EC has revealed significant differences between ESCC and EAC, not only in terms of their etiology but also in their response to treatment. These insights are guiding the development of more targeted and personalized treatment approaches, which are expected to improve outcomes for patients in the future.
This review summarized the current trends in epidemiology and advances in the prevention and treatment of EC. We explored the differences between ESCC and EAC, particularly in their etiology, prognosis, and treatment strategies. By providing a detailed overview of the current state of EC research and clinical practice, we aim to contribute to the ongoing efforts to improve outcomes for patients with this challenging disease.
2 PATHOLOGY
2.1 Histopathological subtypes
Histopathological subtypes of esophageal and esophagogastric junction tumors encompass a range of categories, including epithelial, neuroendocrine, and non-epithelial tumors, as classified by the World Health Organization (WHO) [6]. The State Key Laboratory of Esophageal Cancer Prevention & Treatment in China identified 32 primary histopathological types of esophageal malignant tumors with the database established from September 1973 to December 2020. ESCC predominated, accounting for 97.1% of cases, while EAC accounted for 2.3%. The remaining 0.6% of cases included rare types such as small cell esophageal cancer, malignant melanoma, neuroendocrine cancer, and undifferentiated cancer [7].
2.2 Molecular pathological characteristics in carcinogenesis
EC progression is a multistep process, from hyperplasia to invasive carcinoma [8, 9]. However, the distinct histopathological subtypes exhibit notable pathological differences (Figure 1). ESCC emanates from epithelial dysplasia, characterized by shared clones with high cloning frequency and key mutations [10]. Barrett's esophagus (BE), a metaplasia of the esophageal epithelium, is a precancerous condition for EAC but shares less than 20% of EAC-specific mutations [11]. The evolution of precancerous lesions depends on their inherent mutations and the mutational landscape of adjacent normal tissue. When the fitness of distinct clones is comparable, and they collide, the mutant cell rate returns to normal tissue homeostasis, suggesting a novel approach to cancer prevention [12]. Genomic structural changes could identify patients at risk for progression from precursor lesions characterized by substantial mutations and stable clonal diversity, enabling early screening to shift from cytology to the genetic level [13-15]. Molecular characteristics with malignant potential help stratify the risk of dysplasia and provide valuable insights for early intervention.
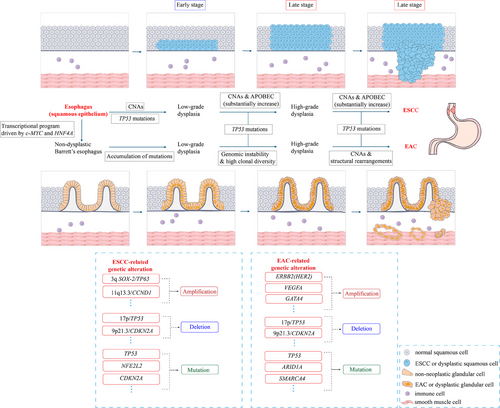
Histopathological progression of esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). ESCC and EAC possess distinct carcinogenic and pathologic characteristics. From the early stages of dysplasia, both have exhibited a relatively stable state with high-frequency clones and mutations. The differences lie in: during the progression of ESCC, CNAs and TP53 mutations occur in the early precancerous stage, while CNAs and APOBEC mutagenesis substantially increase in the late stage.
EAC evolution is characterized by accumulating mutations (such as ARID1A and SMARCA4) in the early stage; TP53 mutations, genomic instability, high clonal diversity, frequent CNAs, and complex large-scale structural rearrangements dominate the late stage. The genetic alterations associated with ESCC and EAC are described in the box at the bottom center of the figure. The histology of cells represented by various colours and shapes is shown in the box at the bottom right. Abbreviations: APOBEC, Apolipoprotein B mRNA editing enzyme catalytic polypeptide-like; ARID1A, AT rich interaction domain 1A; CCND1, Cyclin D1; CDKN2A, Cyclin-dependent kinase inhibitor 2A; c-MYC, Cancer-myelocytomatosis viral oncogene homolog; CNAs, Copy number alterations; ERBB2 (HER2), human epidermal growth factor receptor 2; GATA4, GATA binding protein 4; HNF4A, Hepatocyte nuclear factor 4, Alpha; NFE2L2, Nuclear factor erythroid 2-related factor 2; SMARCA4, SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily A, member 4; SOX-2, SRY-box 2; TP53, Tumor protein 53; TP63, Tumor protein 63; VEGFA, vascular endothelial growth factor A.
Parallels exist in the general progression patterns of ESCC and EAC. Both frequently involve tumor protein 53 (TP53) inactivation, a crucial event that drives cancer progression by undermining genomic stability and cell cycle control [16, 17]. However, their genomic landscapes and evolutionary trajectories differ. Copy number alterations (CNAs) are markedly present in the precursor lesions and persist throughout the progression of ESCC, distinguishing it from EAC, where CNA emerges later [11, 18]. Most characterized in ESCC was the amplification of the squamous cell lineage-associated transcription factors sex determining region Y-box 2 (SOX2) (at 3q26) and tumor protein p63 (TP63) (at 3q28), as well as the genes encoding the cell cycle mediator cytosolic cyclin D1 (CCND1) and multiple fibroblast growth factors (FGF) receptor ligands (FGF19, FGF4, and FGF3) on chromosome 11q13 [10, 16, 19]. CNAs in cyclin-dependent kinase inhibitor 2A (CDKN2A), cyclin-dependent kinase inhibitor 2B (CDKN2B), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), and epidermal growth factor receptor (EGFR), along with mutations in nuclear factor erythroid 2-related factor 2 (NFE2L2), histone-lysine N-methyltransferase 2D (KMT2D), neurogenic locus notch homolog protein 1 (NOTCH1), and PIK3CA, are more prevalent in ESCC as well [19]. Whereas EAC features chromosomal instability (CIN), with large-scale genomic rearrangements and gains or losses of genomic regions [20, 21]. It shows higher mutation rates in CDKN2A and AT-rich interaction domain 1A (ARID1A) and more frequent amplifications of human epidermal growth factor receptor 2 (HER2), vascular endothelial growth factor A (VEGFA), and kristen rat sarcoma viral oncogene homolog (KRAS) [19]. Genomic profiling revealed that ESCC resembles head and neck squamous cell carcinoma, while EAC shows a greater molecular resemblance to gastric adenocarcinoma, especially the CIN subtype [16].
TP53 biallelic loss is the prerequisite for malignant initiation, subsequently leading to mutations and frequent amplifications of key oncogenic driver genes, which in turn drive cascade malignant progression [22, 23]. Although p53 has been traditionally considered ‘undruggable’, new therapeutic strategies have emerged targeting mutant p53. These strategies include restoring wild-type activity [e.g., APR-246 (Eprenetapopt), phenethyl isothiocyanate (PEITC), arsenic trioxide (ATO)], promoting selective degradation [e.g., heat shock protein 90 (HSP90) inhibitors, statins, ATO/triciribine, vorinostat], and inhibiting gain-of-function (GOF) interactions (e.g., efavirenz, statins/zoledronic acid) [24]. Ongoing pan-tumor clinical trials are exploring therapies for patients with mutant p53, though none have yet received regulatory approval [24, 25]. Deep sequencing identified approximately 40% of ESCC patients have druggable genetic alterations, like the 11q13 amplicon, present in 17.8% of patients, which is potentially targetable by FGFR or CDK4/6 inhibitors; NFE2L2/kelch-like ECH-associated protein 1 (KEAP1)/cullin 3 (CUL3) pathway mutations, detected in 17% of patients, which are suitable for glutaminase inhibitor; PIK3CA mutations, observed in 11.9% of patients, which can be targeted by alpelisib; and mutations in neurofibromin 1 (NF1), serine/threonine kinase 11(STK11), and phosphatase and tensin homolog (PTEN), which is potentially responsive to mitogen-activated extracellular signal-regulated kinase (MEK) or mammalian target of rapamycin (mTOR) inhibitors [26]. EGFR is the most investigated target in ESCC, yet phase III studies (NCT00686114, NCT01627379) for locally advanced and advanced stages reported conflicting efficacy outcomes [27, 28]. In EAC, the ToGA study (NCT01041404) demonstrated the therapeutic benefit of the Erb-B2 receptor tyrosine kinase 2 (ERBB2) monoclonal antibody trastuzumab in the strongly HER2-amplified gastric or gastro-esophageal junction (G/GEJ) cancer [29]. However, most subsequent prospective studies failed to achieve similar survival benefits [30, 31]. Resistance to EGFR- and HER2-targeted therapy is caused by tumor heterogeneity, with the existence of low expressing tumor clones, alternative receptors upregulation, co-amplifications of tyrosine kinase receptors, (re)activation of downstream signaling pathways like phosphatidylinositol-3-kinase (PI3K) / serine/threonine-protein kinases (AKT) pathway and mitogen-activated protein kinase (MAPK) pathway, and alterations in cell cycle-related genes [32-35]. Frequent genetic alterations in receptor tyrosine kinases (RTKs) and their targets in EC suggest a need for combination kinase inhibitors for effective cell suppression [20, 35].
2.3 Tumor environment and immunobiology
Immunotherapy has emerged as a pivotal component in treating EC and in reshaping cancer management approaches. Its clinical success hinges on a deep understanding of the tumor immune microenvironment (TIME) and immune landscape [36].
Historically, tumor immunology focused on genomic and transcriptomic profiling at the cellular level or on the identification of specific molecular markers. However, the heterogeneity and complexity of the tumor immune environment have made these methods insufficient, prompting a shift toward more comprehensive analyses [36].
The TIMEs of ESCC and EAC exhibit marked heterogeneity in immune infiltration and suppression mechanisms. In ESCC, the TIME is characterized by both inflammation and immune suppression. Li et al. utilized consensus clustering to delineate immune gene expression profiles, and identified six immune subtypes, ranging from immune-cold to immune-hot phenotypes, and seven gene modules, each with distinct gene expression patterns and clinical outcomes [37]. This work offers a conceptual framework that could inform the development of novel immunotherapy strategies and combination treatments. High-dimensional single-cell RNA sequencing (scRNA-seq) has revealed a TIME dominated by exhausted T cells, NK cells, regulatory T cells (Tregs), alternatively activated macrophages (M2), and tolerogenic dendritic cells (tDCs) in ESCC. These immune-suppressive cells create a hostile environment for effective immune responses, contributing to immune surveillance failure, and targeting these pathways could help reactivate antitumor immunity [38]. For instance, a subset of exhausted CD8+ T cells expressing sprouty RTK signaling antagonist 1 (SPRY1) has been identified as a predictor of response to immune checkpoint inhibitors and serves as a potential biomarker for therapeutic selection [39].
On the other hand, EAC is characterized by an even more profoundly immunosuppressive TIME compared to ESCC. EAC is characterized by higher levels of suppressive cells, such as monocytic myeloid-derived suppressor cells (mMDSCs), and reduced infiltration of active immune cells like proliferating CD8+ T cells [40]. This immune suppression contributes to the reduced effectiveness of immunotherapies like checkpoint inhibitors in EAC. Studies have shown that complete responders to neoadjuvant chemotherapy (nCT) exhibit higher levels of immune-stimulating cells and lower levels of suppressive factors such as programmed cell death ligand 1 (PD-L1)-expressing mMDSCs [41, 42]. Additionally, scRNA-seq analyses of EAC have revealed the complexity of its immune microenvironment, highlighting exhausted T cells and fibroblasts as key factors influencing immune evasion [41].
These findings underscore the distinct immune landscapes of EC and the theoretical basis for immunotherapy strategies. With the continuous deepening of research, we believe that combination immunotherapies based on TIME regulation mechanisms may play an increasingly important role in cancer treatment.
3 EPIDEMIOLOGY
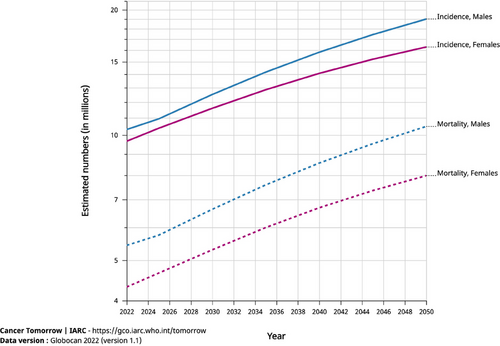
According to the latest GLOBOCAN 2022 data, EC ranks 11th worldwide in incidence and 7th in mortality, a decrease in both morbidity and mortality from previous rankings [1]. EC exhibits diverse global epidemiological trends. According to the 2019 Global Burden of Disease (GBD) study, the age-standardized incidence (ASIRs) and death (ASDRs) rates have declined from 1990 to 2019. However, overall global incidence, mortality, and disability-adjusted life years (DALYs) have increased by 1.4 to 1.7 times [43]. What is concerning is that, according to current trends, the GLOBOCAN 2022 database predicts that the global incidence and mortality rates of EC will continue to rise through 2050 (Figure 2).
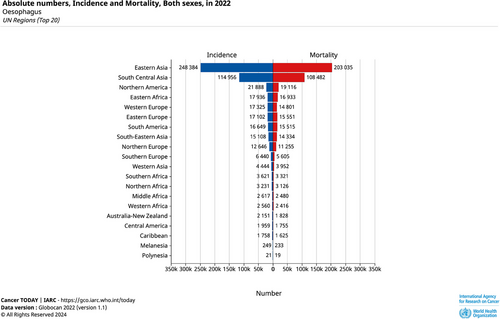
Geographically, Asia shoulders a significant portion of the global EC burden, accounting for 75.0% of cases and 74.1% of deaths worldwide. By 2040, the incidence and mortality rates in the region are projected to increase by 63% and 72%, respectively [44]. In contrast, Oceania and Central America are less affected, as illustrated in Figure 3 from GLOBOCAN 2022. The Caribbean, North Africa, Middle East, Western Sub-Saharan Africa, and North Europe have defied the global decelerating trend, persisting with an upward trajectory from 2010 to 2019 [45]. The Netherlands and Japan also show a continued upward trend in ASIRs, as illustrated in Figure 4. Notably, 47.4% of EC cases occurred in East Asia, with China alone accounting for 43.8% of the global caseload and 42.1% of EC-related deaths, declining compared to data from GLOBOCAN 2020 [45]. In addition to the considerable disease burden attributed to high-risk factors, distinctive screening programs implemented in the region help discern individuals afflicted by EC. However, known environmental risk factors cannot fully explain geographical variations in EC. Mutational signatures interaction with an exogenous exposure still needs to be further explored [46].
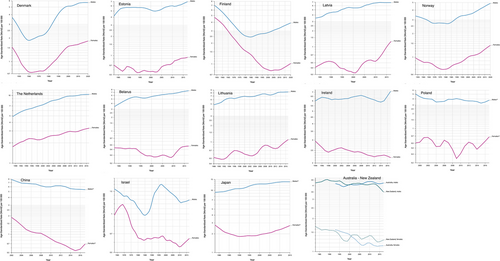
Subtype-wise, geographical disparities are also evident. Northern Europe and North America report the highest incidence rates of EAC, while East Asia, Central and South Asia, and South Africa dominate ESCC statistics [45]. Notably, a shift in epidemiological distribution is occurring. In Western countries, EAC incidence has sharply increased, including cases at the esophagogastric junction (EGJ), likely driven by the rising prevalence of risk factors such as GERD, BE, abdominal obesity, and high-fat diets [4]. Conversely, ESCC incidence is declining in these areas, attributable to reductions in smoking and alcohol consumption. For example, smoking rates in the United States dropped from 20.9% in 2005 to 12.5% in 2020 [47]. Similarly, in Asia, where ESCC accounts for over 90%, ASRs are decreasing more rapidly than in other regions [48]. In China, the ASIR of EC significantly decreased from 2000 to 2018 [average annual percent change (AAPC) = -3.5% in males, AAPC = -7.0% in females], possibly due to dietary improvements and economic progress [49]. However, these trends have become flattened or reversed in recent years (Figure 4), which may contribute to other prominent risk factors, such as hot beverage consumption in the region that outweigh the relatively lower association of smoking and alcohol consumption with EC compared to Western countries [50-52].
Notably, the increasing prevalence of obesity in Asia due to shifts toward Western lifestyle and dietary, predicts a gradual rise in EAC incidence [53]. However, the incidence of EAC is unlikely to surpass ESCC in Asia as it has in Western countries. Data from 2008 to 2012 highlights regions like the Taihang Mountains and Yanting County with unusually high incidences of EAC and cardia gastric cancer [4]. The rise in EAC here may be linked to common pathogenic factors shared with ESCC, such as nitrosamine exposure, genetic susceptibility, and nutritional deficiencies, illustrating the etiologic heterogeneity of both subtypes [54, 55].
These also represent the striking etiologic heterogeneity of both ESCC and EAC. Therefore, reducing the global incidence of EC requires multi-dimensional prevention strategies.
4 PREVENTION AND SCREENING
4.1 Prevention
Here, primary prevention focuses on etiological measures. Effective strategies for EAC and ESCC vary due to different risk factors and carcinogenic pathways.
4.1.1 Risk factors
Multiple complex risk factors, including environmental, genetic, and cultural, interact in the multi-stage development of EC, processing from localized damage, inflammation, cell proliferation, mutation, and eventually carcinogenesis. Smoking is a common risk factor for both ESCC and EAC, particularly for ESCC [56]. Other factors, however, pose risks specific to different pathological subtypes. While the carcinogenic risks of some factors, such as tobacco smoking, alcohol consumption, areca nut, achalasia, gastroesophageal reflux disease, BE, and helicobacter pylori infection, are relatively well-established and offer potential for prevention, others remain inconclusive. Table 1 outlines the major reported risk factors for ESCC and EAC.
| Esophageal squamous cell carcinoma (ESCC) | Esophageal adenocarcinoma (EAC) | |||||
|---|---|---|---|---|---|---|
| Risk factors | Risk coefficient | Type of study conducted | Preventability | Risk Coefficient | Type of study conducted | Preventability |
| Risk factors with consistent evidence for causation | ||||||
| Tobacco smoking | RR = 4.18 (Current smokers vs. Non-smokers) | Systematic review and meta-analysis [51] | Smoking cessation time-dependently decreases risk of ESCC | RR = 2.34 (Current Smokers vs. Non-Smokers) | Systematic Review and Meta-analysis [51] | Cessation did not reduce EAC risk over time |
| Alcohol consumption | RR = 5.38 (High Consumption) | Case-control and cohort studies [52, 57] | Continuous cessation or reduction of drinking habit decreased ESCC risk [58, 59] | RR = 0.96 (95% CI, 0.79 - 1.16) | Meta-analysis [60] | / |
| Areca nut | RR = 3.05 | Case-control study [57, 61] | Betel quid without tobacco (BQ-T) cessation did not show risk reversal. Risk reduction after BQ + T cessation was uncertain [59]. | / | / | / |
| Achalasia | RR = 72.65 | Meta-analysis [62] | Surgical intervention (surgical myotomy with or without a fundoplication) does not completely prevent tumor development. | RR = 6.63 | Meta-analysis [62] | Surgical intervention does not completely prevent tumor development. |
| Gastroesophageal reflux disease (GERD) | OR = 1.1 (95% CI, 0.7 - 1.9) | Case-control study [63] | / | OR = 7.7 (95% CI, 5.3 - 11.4) (Recurrent vs. no reflux); OR = 43.5(Long-standing and severe) | Case-control study [63] | Anti-reflux therapies have the potential in risk reduction of Barrett's esophagus (BE) but are controversial for EAC. |
| BE | / | / | / | RR = 11.3 (95% CI, 8.8 - 14.4) | Population-based, cohort study [64] | Potential to curtail dysplasia in BE with anti-reflux therapies, but surgery is not recommended [65]. |
| Helicobacter pylori infection | HR = 1.22 (95% CI, 0.42 - 3.53) | Prospective population-based cohort study [66] | / | HR = 0.35 (95% CI, 0.12 - 0.97) | Prospective population-based cohort study [66] | Not suitable as an intervention |
| Risk factors with repeatedly reported associations, but not confirmed | ||||||
| Fruit intake | RR = 0.51 (95% CI, 0.40 - 0.64) | Case-control and cohort studies [57] | Increase intake decreased risk (an increment of 100 g/day, RR = 0.61, 95% CI, 0.52 - 0.72) | RR = 0.98 (95% CI, 0.90 - 1.08), not consistent. | Cohort study [67], Meta-analysis [68] | Inconclusive |
| Vegetable intake | RR = 0.57 (95% CI, 0.43 - 0.75), not consistent | Case-control and cohort studies [57, 67] | Increased intake decreased risk (an increment of 100 g/day, RR = 0.84, 95% CI, 0.78 - 0.92) | SRRs = 0.76 (95% CI, 0.59 - 0.96), not consistent | Cohort study [67], Meta-analysis [68] | Increased intake of raw vegetables inverse EAC risk (RR per 25 g/day increment: 0.80, 95% CI, 0.68 - 0.98). |
| Nitrosamine exposure (Pickled vegetables) | OR = 2.75 (95%CI,1.62 - 4.70) | Case-control and cohort study [57] | Reduced intakes decrease risk | OR = 1.17 (95% CI, 1.00 - 1.36) | Population-based case-control study [69] | Not significant |
| Vitamin B6 | OR = 0.47 (95% CI, 0.33 - 0.67) | Meta-analysis [57,70] | For every 1 mg/day increase in vitamin B6 intake, the risk of EC was reduced by 16%. | OR = 0.58 (95% CI, 0.49 - 0.68) | Meta-analysis [57,70] | Reduced risk |
| Hot beverages/foods consumption | OR = 1.60 (95% CI,1.29 - 2.00) | Case-control studies [57, 71] | Avoidance may contribute to the prevention | OR = 0.79 (95% CI, 0.53 - 1.16) | Case-control study [71] | / |
| Human papillomavirus (HPV) infection | ORS = 6.4; but not consistent | Case-control study [72, 73] | No report yet | IRR = 2.87 (95% CI, 1.69 - 4.86), but not consistent. | Prospective PCR-based study [74-76] | Dysplasia or EAC might be eliminated after clearance of HPV. |
| Genetic factors (polymorphisms) | Protective genetic characteristics: ORs = 0.41 - 0.93; Susceptibility genetic traits: ORs = 1.09 -13 | Most were population-based case-control study [50] | No actionable targets yet | / | / | / |
| Diet high in red/processed meats | ORs = 2.33 (95% CI, 1.56 - 3.48) (Ever vs. Never intake) | Case-control study [77] | Reduced frequency and amount suggest decreased risk | RR = 1.41 (95% CI, 1.09 - 1.83), not consistent | Meta-analysis [78, 79] | Dose-response association suggest decreased risk by reducing intake. |
| Obesity | Inverse correlation (HR = 1.61 (95% CI, 1.14 - 2.28) in normal BMI individuals vs. obese) | Prospective cohort study [56] | / | HR = 2.20 (95% CI, 1.84 - 2.68) (obese vs. normal weight) | Prospective cohort study [56] | Bariatric surgery reduced EAC risk [80], but can worsen GERD or cause de novo reflux, esophagitis, and Barrett´s metaplasia [65]. |
- Abbreviations: HR, hazard ratio; IRR, incidence rate ratio; OR, odds ratio; RR, relative risk; SRR, summary relative risks.
The etiology of ESCC, while not fully understood, is associated with several lifestyle and environmental factors, such as tobacco smoking, alcohol consumption, and the chewing of betel nuts [50, 57, 61]. Combined risk factors like smoking and alcohol significantly elevate the risk [50]. These risk factors act directly on the esophagus and cause chronic irritation and inflammation of the esophageal mucosa, leading to DNA damage through oncogene activation and tumor suppressor genes inhibition, ultimately resulting in mutagenesis [81]. Diet-related factors constitute the majority of ESCC risk factors, such as fruit, citrus fruit, vegetables, pickled vegetables, maté tea, hot beverages and foods, hot tea, salt, folate, and vitamin B6 [57, 71, 77, 70, 82]. However, not all cases can be explained by diet alone. Chronic esophageal irritation can also occur when food is retained and broken down by bacteria, releasing various chemical irritants. Physiological conditions like achalasia significantly increase ESCC risk through food retention, chronic inflammation, and bacterial overgrowth [62, 82]. Biological infections like Human papillomavirus (HPV) promote malignant transformation of esophageal epithelium, but epidemiological and mechanistic findings remain inconsistent, even in high-incidence regions [72, 73]. Geographic variations in ESCC incidence, prevalent in China, Africa, and the Middle East, suggest a link to nutritional deficiencies, such as selenium [83].
EAC is influenced by a different combination of genetic, environmental, and lifestyle factors, characterized by prolonged esophageal exposure to acid reflux, leading to inflammation-induced hyperplasia and epithelial metaplasia [84]. Symptomatic GERD, achalasia, and the resulting BE are well-established high-risk factors and/or precancerous conditions for EAC [62-64]. Obesity links not only to GERD and its complications but also to distinct metabolic and immune features in the tumor and systemic microenvironment, promoting the development of EAC through pro-inflammatory adipokine release and insulin resistance [85]. The inverse relationship between Helicobacter pylori infection and EAC may result from reduced stomach acidity caused by chronic inflammation-induced glandular atrophy and chief cell loss, which decreases the likelihood of reflux esophagitis, BE, and EAC [66, 86]. However, epidemiological and mechanistic evidence of HPV infection for EAC risk require further clarification [74-76]. Neither alcohol consumption nor nitrosamine exposure has shown a significant risk for EAC [60, 69]. The association between the intake of vegetables, fruits and processed meats with EAC risk remains inconclusive [67, 68, 78, 79].
4.1.2 Preventive measures
Consequently, the primary prevention recommendations in ESCC are drawn from available public etiological data, emphasizing risk reduction through lifestyle modifications, such as alcohol and betel nut cessation, and increased fruit and vegetable intake [87]. Reducing or eliminating tobacco and alcohol use are the most effective strategies, with benefits increasing over time [51, 58, 59], although betel quid without tobacco (BQ-T) cessation did not show risk reversal [88]. However, in patients with achalasia, risk reduction with surgical intervention, such as surgical myotomy with or without a fundoplication, does not completely prevent tumor development [82]. Chemoprevention strategies, such as cyclooxygenase 2 (COX2) inhibitors, statins, metformin, and proton pump inhibitors (PPI), have demonstrated potential for preventing ESCC [89]. However, more robust and conclusive research is required to support formal guideline recommendations.
Risk reduction, such as smoking cessation, weight management, and reducing red meat intake, are also the basic prevention strategies for EAC. It is worth noting that bariatric surgery lowers EAC risk, but it may exacerbate GERD or cause new onset reflux, esophagitis, and BE [65, 80]. Anti-reflux therapies have the potential to curtail dysplasia in BE, subsequently reducing the EAC risk, but surgery with fundoplication did not show superiority over medication [90]. American College of Gastroenterology (ACG) current practice guidelines advise against using anti-reflux surgery for prevention in BE patients [91]. Anti-reflux medications are controversial because most EAC cases do not originate from either GERD or BE, indicating only a small proportion of the population benefits from anti-reflux therapies [92-94]. And genome-wide association studies (GWAS) data from the IEU Open GWAS project (mrcieu.ac.uk) suggested anti-reflux therapies primarily lower the risk of EAC by preventing BE formation rather than acting on existing BE [95]. While preclinical and case-control studies indicated aspirin/nonsteroidal anti-inflammatory drugs (NSAIDs) may reduce tumor progression (from metaplasia to dysplasia and cancer) more effectively than BE initiation [96-99]. Hence, the use of anti-reflux therapy might go beyond BE patients. The AspECT trial (EudraCT 2004-003836-77) provided strong evidence for EAC chemoprevention, showing that high-dose PPI (40 mg twice daily) was superior to low-dose PPI (20 mg once daily) in reducing all-cause mortality, EAC development, or high-grade dysplasia [time ratio (TR) = 1.27; 95% confidence interval (CI) = 1.01-1.58; p = 0.038]. High-dose PPIs combined with aspirin performed best (TR = 1.59; 95% CI = 1.14-2.23), though primarily for all-cause mortality reduction rather than cancer-related outcomes [100]. Thus, the ACG guidelines only endorse at least once-a-day PPI therapy in BE patients because of the result of an undouble-blind trial [91].
The most direct prevention is endoscopic eradication for the esophageal neoplasia. The SURF (Surveillance vs. Radiofrequency Ablation) trial (NTR1198) revealed radiofrequency ablation (RFA) in low-grade dysplasia (LGD) BE patients reduced the risks of progression to high-grade dysplasia (HGD) and to EAC by 25% and 7.4%, respectively [101]. Similarly, endoscopic mucosal resection (EMR) was proven to prevent or delay the progression of esophageal squamous from LGD to HGD (dysplasia downgrading of 82.5% in EMR group vs. 49.2% in the group without EMR, p < 0.001) by a randomized study (Chi-CTR-TRC-1200201) [102].
4.2 Screening and early detection
Screening serves as a secondary prevention strategy for malignant tumors. Given the uncertainty of most high-risk factors, the focus of EC prevention has shifted to screening and early diagnosis. High-sensitivity, accurate, and cost-effective screening strategies targeting high-risk populations and regions with high EC prevalence can facilitate early detection, and reduce EC mortality and disease burden (as suggested by NCT02094105) [103, 104].
4.2.1 Endoscopic screening
The current gold standard for EC screening is endoscopy with biopsies, allowing for the detection of precursor lesions.
Data from the Dutch National Histopathological Diagnostic Registry (PALGA) indicated that patients with LGD had a higher risk of progressing to EAC (2.51/100 person-years; 95% CI = 1.46-3.99/100) compared to those without LGD (1.01/100 person-years; 95% CI = 0.41-2.10/100), with a median progression interval of 2.93 years, emphasizing the importance of endoscopic surveillance within the first 2 to 3 years [105]. In ESCC, the only randomized controlled study (NCC1788) on endoscopic screening found that patients with mild-to-moderate dysplasia had a 1.04% overall annual risk of progression to advanced neoplasia, with critical surveillance needed within the first 2 to 3 years [106].
Endoscopic screening is recommended for high-risk EC populations. The definitions of people at risk for BE and EAC vary slightly in different guidelines [91, 107–109]. The ACG recommends screening should be performed for patients with chronic GERD symptoms and with at least three risk factors for BE (e.g., male, Caucasian, age over 50, obesity, smoking, and first-degree relatives with a family history of BE or EAC) [91]. While the latest American Gastroenterological Association (AGA) Clinical Practice Update considers GERD not an essential risk factor [108]. For ESCC, there is no uniform definition of high-risk. To maximize cost-effectiveness, the Chinese Expert Consensus on Early Diagnosis and Treatment of Esophageal Cancer recommends opportunistic screening for EC in high-incidence areas, targeting individuals ≥40 years of age with any high-risk conditions (e.g., a pre-cancerous disease in the upper gastrointestinal tract; first-degree relatives with a history of EC; squamous cell carcinoma of the head, neck, and/or respiratory tract; and other high-risk factors) [110]. In comparison, the European Society of Gastrointestinal Endoscopy (ESGE) defined high-risk individuals as those with a history of head/neck cancer, achalasia, or previous caustic injury [107].
White light endoscopy (WLE) with targeted biopsies is the primary technique for EC screening but has limitations in identifying early cancers and in adhering to the BE biopsy protocol, which requires four-quadrant biopsy at 1 cm to 2 cm intervals plus biopsy from any suspicious visual lesions [111-113]. High-resolution endoscopes (HRE) with advanced charge-coupled devices (CCD) and adjustable focus improve mucosal visualization and dysplasia detection [114]. Chromoendoscopy enhances lesion visualization using stains. Lugol chromoendoscopy (LCE) plus indicative biopsy has become the most practical and effective method to identify squamous dysplasia or cancer, with up to 98% sensitivity. The sensitivity for the detection of severe dysplasia could be 87.0% (NCT01688908) [115]. Methylene blue or acetic acid chromoendoscopy improves the sensitivity of BE and EAC diagnosis [116, 117], though the accuracy reported varies [118]. Digital chromoendoscopy, or virtual endoscopy, uses varied light wavelengths to highlight mucosal structures without dye-related risks. Techniques include narrow-band imaging (NBI), blue light imaging, autofluorescence imaging, optical enhancement, spectral fusion imaging, and photoelectric composite staining imaging. NBI is the most widely used technique, offering comparable accuracy to LCE for high-grade intraepithelial neoplasia (HGIN) detection but with reduced sensitivity for low-grade lesions (LGIN) (NCT04224896) [119]. The main advantage of NBI is its ability to enhance the visualization of intraepithelial papillary endocapillary loops (IPCL), facilitating lesion assessment and cancer infiltration depth evaluation using Japanese Esophageal Society (JES) or Inoue staging [120], and providing biopsy guidance for EAC screening. A randomized trial (NCT00576498) showed NBI-targeted biopsies matched the BE detection rate of Seattle biopsies with fewer biopsies per patient needed (3.6 in the NBI group vs. 7.6 in the high-definition-WLE group, p < 0.0001) and higher dysplasia detection (30% in the NBI group vs. 21% in the high-definition-WLE group, p = 0.01) [121]. Confocal laser microendoscope (CLE) provides a real-time, highly magnified cellular level view for histological differentiation without the need for biopsy (virtual histology). It demonstrates high sensitivity, specificity, and accuracy in detecting early ESCC (NCT01909518) [122]. A meta-analysis found that CLE-based targeted biopsies' sensitivity and specificity in detecting HGD/EAC are 68% and 88%, respectively [123]. However, CLE has not fully replaced traditional histopathology due to limited evidence of direct comparison with traditional histopathology. Optical coherence tomography (OCT)-based volumetric laser endomicroscopy (VLE) allows scanning up to 3 mm deep into mucosa and submucosa, enhancing early diagnosis of BE and EAC. A multicenter registry (NCT02215291) has shown that VLE-guided biopsy increased tumor detection rates seven-fold over random biopsy [124]. Furthermore, VLE showed higher tumor detection rates and diagnostic accuracy for superficial ESCC confined to the epithelium (EP) or lamina propria mucosa (LPM) compared to endoscopic ultrasound (EUS) [125]. However, more precise computer-aided assessments and additional prospective studies are necessary to fully validate VLE's value.
4.2.2 Non-endoscopic screening
Non-endoscopic methods are less invasive, potentially more cost-effective, and easier to administer compared to endoscopic screening in non-specialist settings such as primary care.
Cytology is an effective and more acceptable non-endoscopic screening method compared to endoscopic screening. Various tethered cell sampling devices have been developed, such as the Cytosponge. It consists of a capsule that dissolves in the stomach, releasing a spherical sponge that collects cells from the esophageal wall as it is pulled back through the mouth [126, 127]. The collected samples can be analyzed for specific immunomarkers to enhance diagnostic efficiency. The most well-established biomarker used by Cytosponge is trilobal factor family 3 (TFF3) performed with immunohistochemistry, which is a specific marker for BE [128]. The sensitivity and specificity of Cytosponge-TFF3 for BE diagnosis can reach 80% and >90%, respectively [129].
Current screening and surveillance for ESCC or EAC are recommended only for individuals with squamous epithelial hyperplasia or BE. However, 40%-50% of cases without hyperplasia at baseline eventually progress to ESCC [130]. Genomic studies show that Lugol unstained lesions already harbor genetic variants (including mutations, copy number variants, and clonal expansions) and are associated with an increased risk of developing ESCC [14]. Similarly, over 80% of EAC cases have no prior diagnosis of BE [131, 132]. This raises the question of how to screen for oncogenic initiation without dysplasia signs.
Whole-genome sequencing of BE biopsies demonstrated that genomic signals can predict the risk of progression up to 10 years before histopathologic changes [133]. Biomarkers, including multi-gene next-generation sequencing sets, differentially methylated genes, and microRNAs, were explored to identify Cytosponge cytological dysplasia [134-136]. TP53 mutations, key events in EC progression [17, 18], as well as other biomarkers, could help identify BE patients at high risk of malignant progression requiring endoscopic surveillance [137]. Preliminary results also suggested that combining Cytosponge with biomarkers, such as p53 protein staining in this study, could improve the accuracy of ESCC screening, as evidenced by a pilot study in Iran showing 100% sensitivity and 97% specificity for detecting HGIN [138].
With the advent of liquid biopsy technology and precision medicine, novel biomarkers show potential for EC screening. Genetic alterations in cell-free DNA (cfDNA) can detect the risk of BE progression [139]. Circulating microRNA (miRNA)-based signatures have been validated for early ESCC detection [140]. A Chinese study identified miRNA panels that distinguish ESCC from squamous dysplasia [141]. Circulating extracellular vehicles (EVs)-14 also displayed a high accuracy in ESCC screening [142]. Beyond genetic markers, circulating systemic inflammation biomarkers and serum metabolomics provide insights into the risk and early diagnosis of EC [143-146]. However, it remains challenging to identify malignant transformation risk early through one signature.
Integrating endoscopic and non-endoscopic modalities with a deep learning-driven artificial intelligence (AI) system can significantly enhance the identification of high-risk esophageal lesions, offering a promising adjunctive tool for EC and precancerous lesion screening (ChiCTR2100044126) [147].
5 TREATMENT
5.1 Early-stage EC
5.1.1 EAC
In mucosal EAC (T1a), lymph node metastasis (LNM) risk is accorded with clinicopathologic features such as lesion size ≥2 cm, poorly differentiated, and lymphovascular invasion [148, 149]. Low-risk T1a patients rarely develop LNMs, with rates ranging from 0% to 3% [150]. The National Comprehensive Cancer Network (NCCN) guideline recommends endoscopic resection (ER) for these patients, using techniques like EMR and endoscopic submucosal dissection (ESD), without additional interventions [151]. ESD is favored for larger lesions due to higher complete resection rates and lower recurrence rates [152]. A randomized controlled study showed that ESD had a higher R0 resection rate for BE with HGIN or for early EAC with ≤3 cm focal lesions compared to EMR. Both ESD and EMR were effective in terms of surgery necessity, tumor remission, and recurrence, while ESD was more time-consuming and prone to severe stenosis (NCT01871636) [153]. There is little literature on optimal tumor size cutoffs for the selection of endoscopic resection techniques. ASGE recommends ESD for patients with early-stage, highly differentiated, non-ulcerative lesions larger than 20 mm, while either ESD or EMR can be used for similar lesions ≤20 mm [154]. In addition, eradication of residual Barrett's mucosa post-curative resection to prevent metachronous neoplasia is standard practice [154-156], but for patients unable to undergo ablation, endoscopic surveillance is an alternative [157]. High-risk T1a patients with ≥10% risk of delayed extraesophageal and LNMs require complete staging and additional treatments such as esophagectomy [158].
Submucosal EAC (T1b) carries up to a 30% risk of LNMs [159]. Esophagectomy is its standard care, showing improved overall survival (OS) compared to ER [160], with five-year survival rates of 89% versus 59% in a multicenter study [161]. However, T1b patients with low-risk features (e.g., pT1b sm1, good to moderately differentiated, and no lymphovascular invasion [LVI]) have a 2%-4% risk of LNMs [149, 162]. For these low-risk T1b EAC, endoscopic eradication and recurrence rates are comparable to T1a patients [163]. Therefore, current AGA guidelines recommend endoscopic treatment as a reasonable alternative to esophagectomy despite limited direct comparative evidence [164]. Chemoradiotherapy (CRT) is another organ-preserving option, though it is less studied in T1b EAC. A small two-center retrospective study demonstrated CRT is a safe and effective curative treatment for selected T1 EAC, with a 3-year OS of 78% [165]. However, a Japanese retrospective study compared ESD followed by CRT with ESD followed by esophagectomy for clinical T1bN0M0 EAC patients, and found no significant difference in 2-year disease-free survival (DFS) rates between two groups (88% in the CRT group vs. 100% in the esophagectomy group, p = 0.43) [166].
The treatment of T1 EAC is evolving towards minimally invasive procedures. Therefore, histologic evaluation is essential to identify high-risk individuals and select appropriate treatment. However, significant inconsistencies exist in the histologic evaluation of ER specimens. The Amsterdam University Medical Center has proposed improvements in histopathological assessment, though further clinical validation and updates are imperative [167].
5.1.2 ESCC
ER is the primary treatment for superficial ESCC in various guidelines. cT1a- epithelium (EP, M1)/ lamina propria mucosae (LPM, M2) ESCC, evaluated according to the JES classification [168], is an absolute indication for ER [158], featuring a LNM rate of 0%-5% [169, 170], and a 5-year DFS of nearly 100% [171]. ESD is preferred for its superior curative resection and reduced local recurrence rate [172], especially for lesions >15 mm [154].
The LNM rate increases to 18% when the tumor invades the mucosal muscle (MM, M3) and exceeds 50% when it extends into the submucosa [173]. Preoperatively differentiating between cT1a-MM and cT1b-submucosa ≤ 200 µm (SM1) using endoscopic techniques is challenging. Consequently, most studies and guidelines categorize these as cMM/SM1 cancers. ESD without additional treatment is an appropriate option for T1a-MM/T1b-SM1 ESCCs [174, 175].
The current debate centers on the optimal treatment for high-risk T1b patients. It is widely accepted that ER alone is inadequate for patients with deep submucosal invasion (T1bSM2-3), lymphovascular invasion, or poor differentiation [176]. Esophagectomy remains the standard treatment of T1b ESCC [151]. However, assessing tumor depth before ER is challenging; thus, ESD serves as an initial diagnostic tool and potential cure [177]. Noncurative ER followed by esophagectomy significantly improves OS and recurrence-free survival (RFS) of cT1N0M0 ESCC patients compared to esophagectomy alone [178]. Definitive CRT (dCRT) offers OS comparable to radical surgery while preserving the organ [179, 180], making it a guideline-recommended option [181]. However, it carries a higher risk of recurrence. Additional CRT post-ER improved local control and reduced radiation dose to minimize toxicity compared to dCRT [182, 183]. Compared to ESD alone, a multicenter, cross-sectional study demonstrated adjuvant radiotherapy after ESD could improve survival in pT1b ESCC with LVI+, with a 5-year OS of 91.7% versus 59.5% [184]. Therefore, the first question is what the best adjuvant therapy after ER is. Reported 5-year OS rates ranged from 90% to 100% for esophagectomy and from 75% to 85% for CRT after ER, though the CRT dose was low in most studies [176]. Esophagectomy is preferred for extensive submucosal tumor invasion due to the lower risk of recurrence [185]. The ongoing phase III Ad-ESD trial (NCT04135664), which compared esophagectomy and dCRT for patients with cN0-pT1b ESCC after ESD will answer the question [186]. Secondly, JCOG 0508 (UMIN000000553) showed that the 5-year OS of pT1b or of high-risk pT1a (LVI) ESCC patients receiving additional CRT post-ER are comparable to those receiving surgery alone [187, 188]. Further exploration is needed to answer the question of the value and optimal candidates for the organ-preserving ER-CRT approach as an alternative to surgery.
5.2 Locally advanced resectable EC
5.2.1 Surgical approaches
Esophagectomy remains the cornerstone of curative treatment for localized EC. Common surgical procedures encompass left thoracic (Sweet), transhiatal esophagectomy (THE), and right thoracic esophagectomy [three-field (McKeown or modified McKeown) and two-field (Ivor Lewis)]. The right thoracic approach is preferred for better lymphadenectomy as supported by studies such as NST1501 (NCT02448979) [189-191]. Despite a higher complication rate than the left thoracic approach, it offers better safety than THE [189-191], regardless of neoadjuvant therapy, as evidenced by the SAKK trial 75/08 (NCT01107639) [192]. Procedure choice depends on the individual circumstances, such as tumor location, the patient's overall health, surgical risks, and the expected quality of life post-surgery. McKeown or Ivor Lewis esophagectomies are suitable for middle and lower esophageal tumors, while tumors at or above the carina benefit from three-field lymphadenectomy for sufficient proximal resection margins [193, 194]. Compared to Ivor-Lewis esophagectomy, McKeown has a comparable 5-year OS and perioperative mortality [195], but with a higher complication rate [196]. This also pertains to another debate, namely the question of two-field or three-field lymph node (LN) dissection. A national ESCC study suggested approximately one-third of patients had undetected laryngeal and/or cervical lymph node metastases, underscoring the benefit of three-field LN dissection in enhancing 5-year OS [197]. High cervical and recurrent laryngeal cervicothoracic LNM rates were also discovered in EAC [198]. However, a randomized clinical trial (NCT01807936) demonstrated no improvement in OS or DFS with three-field versus two-field lymphadenectomy [199]. It is important to note that both groups underwent dissection of the laryngeal recurrent nerve lymph nodes, introducing a confounding factor.
The trend toward improved overall mortality from EC is attributed to early detection and the development of minimally invasive esophagectomy (MIE) [200]. MIE has a low postoperative mortality rate (1.7% at 30 days) and fewer pulmonary complications compared to open surgery [201]. Randomized controlled trials (such as the TIME Trial (NTR TC 2452) and NCT00937456) suggested comparable or superior 3-year OS and progression-free survival (PFS) for MIE compared to open esophagectomy (OE) [202-204]. However, MIE had a longer operative time, a higher 30-day reoperation rate, and an increased risk of esophageal hiatal hernia [205, 206].
Despite challenges like limited visualization, restricted instrument movement, and a steep learning curve, MIE is recommended in institutions with the necessary expertise. Robotic-assisted minimally invasive esophagectomy (RAMIE) addresses these challenges with better three-dimensional visualization and flexible instruments. A randomized controlled trial (NCT01544790) has shown that RAMIE offers outcomes comparable to OE but with fewer surgery-related and cardiopulmonary complications, reduced postoperative pain, improved short-term quality of life, and faster short-term functional recovery [207]. Though no breakthroughs in perioperative outcomes and OS than conventional MIE [208], RAMIE improves LN clearance, especially along the left recurrent laryngeal nerve, enhancing PFS [208]. The RAMIE trial (NCT03094351) found shorter operative times and better lymph node clearance with similar complication rates compared to MIE, even after neoadjuvant therapy [209].
5.2.2 Neoadjuvant treatment
Neoadjuvant CT vs. CRT
However, surgical resection alone is insufficient for satisfactory outcomes, and multidisciplinary treatment is essential. The prestigious phase III randomized CROSS (NTR487) and NEOCRTEC5010 (NCT01216527) trials established neoadjuvant chemoradiotherapy (nCRT) followed by esophagectomy as the preferred standard of care for EC [210-212]. Alternatively, JCOG 9907 (NCT00190554) established neoadjuvant chemotherapy (nCT) with the DCF regimen (docetaxel + cisplatin + fluorouracil) as standard for ESCC in Japan [213]. For adenocarcinoma of the lower esophagus and esophagogastric junction, nCT with PF or CF (fluorouracil + cisplatin) plus surgical resection is also supported by high-level evidence like the MAGIC (ISRCTN93793971), FFCD9703 (NCT00002883), FLOT4 (NCT01216644) and UK MRC OE05 (NCT00041262) trials [214-217].
A Japanese ESCC database analysis showed greater pathologic downstaging and response in the nCRT group compared to nCT but no significant difference in 5-year survival [218]. Phase III JCOG 1109 NExT (jRCTs031180202), initially presented at the American Society of Clinical Oncology (ASCO) GI meeting in 2022, indicated the highest pathological complete response (pCR) in nCRT group (36.7% vs. 2.2% in CF group and vs. 18.6% in DCF group), but nCT with DCF may offer better survival benefits, challenging current views on optimal neoadjuvant treatment [219, 220]. However, intriguingly, the patients in the CRT group had lower EC-induced mortality (63.2% vs. 80.8%), and lower treatment-related mortality (2.6% vs. 5.3%) compared to those in the DCF group. To minimize the impact of surgical trauma of open esophagectomy after CRT, the CMISG1701 (NCT03001596) study compared nCRT and nCT followed by MIE for ESCC [221, 222]. Similarly, higher pCR, and lower recurrence risk without survival difference were essentially consistent with previous studies after open esophagectomy. These findings underscore the pending issue of the best strategy for neoadjuvant therapy. The HCHTOG1903 study (NCT04138212) is expected to provide further clarity.
In EAC, the Neo-Res trial (NCT01362127) demonstrated that adding radiotherapy to nCT (platin/5-fluorouracil) yielded results similar to those observed in ESCC [223]. Following the confirmation by the II/III clinical study FLOT4 that perioperative FLOT (fluorouracil, leucovorin, oxaliplatin, and docetaxel) improved OS compared to ECF/ECX (epirubicin, cisplatin plus either fluorouracil or capecitabine) in locally advanced, resectable G/GEJ adenocarcinoma [216], the Neo-AEGIS (NCT01726452) study directly compared the nCRT CROSS regimen (41·4 Gy in 23 fractions on days 1-5, 8-12, 15-19, 22-26, and 29-31 with intravenous area under the curve 2 mg/mL per min carboplatin plus intravenous 50 mg/m2 paclitaxel on days 1, 8, 15, 22, and 29) to perioperative chemotherapy using FLOT or the modified MAGIC regimen (three preoperative and three postoperative 3-week cycles of intravenous 50 mg/m2 epirubicin on day 1 plus intravenous 60 mg/m2 cisplatin ). The results were consistent with previous findings: while nCRT increased the pCR rates, major pathological response (MPR), and R0 resection, there were no differences in OS, DFS, or patterns of recurrence [224]. Notably, only 15% of patients completed the FLOT regimen, raising concerns about uncertainty in tumor control. New reported ESOPEC study (NCT02509286) in ASCO has added further compelling evidence for nCT in EAC [225]. Median OS (mOS) and 3-yearOS rate were 66 months and 57.4% in the FLOT group versus 37 months and 50.7% in the CROSS group (HR 0.70; 95% CI, 0.53-0.92; p = 0.012). It is of concern that more patients completed all planned neoadjuvant therapy in the FLOT group (87.3% vs. 67.7%) and achieved pCR (19.3% vs. 13.5%) compared to the CROSS group. Discrepancies in pCR rates with the CROSS study and regimen completion rate with the Neo-AEGIS complicate the interpretation of these findings, thus, the possibility of revising the guidelines necessitates additional details to elucidate the results. Furthermore, integrating treatments like adjuvant immunotherapy from the Checkmate 577 trial profoundly shapes the discourse and determines the consensus.
For both ESCC and EAC, higher pCR rates with nCRT have not consistently translated to improved survival, casting doubt on pCR as a reliable oncological marker. One interpretation is the potential micrometastases and circulating tumor cells, which increase the likelihood of recurrence [226]. Even so, it remains to be confirmed whether nCT, the paradigm that prioritizes systemic impact over the primary tumor, truly delivers on its projected outcomes. Given nCRT's well tolerability and fewer post-surgical uncertainties, it remains the preferred approach. Furthermore, distant recurrence remains the most common failure pattern in both nCRT and nCT groups [224], suggesting adjuvant immunotherapy might surpass the frequently incomplete high-intensity nCT (NCT02743494) [227]. A retrospective analysis found comparable 3-year survival rates between CROSS and FLOT regimens. However, the CROSS regimen increased postoperative respiratory failure and atrial fibrillation, while only 40% of patients completed the full FLOT regimen compared to 92% for CROSS [228]. Therefore, selecting the appropriate regimen—such as avoiding nCRT for patients with poor cardiopulmonary function and nCT for those with lower physical scores—is essential for optimal outcomes.
Neoadjuvant ICT vs. CT
The introduction of immunotherapy has revolutionized neoadjuvant therapy for EC. Neoadjuvant immunotherapy combined with chemo/radiotherapy (ICT/RT) is a current focus of interest. Multiple immune checkpoint inhibitors (ICIs) have catalyzed a series of single-arm phase I/II studies, especially the studies in ESCC [229]. Recent findings from the phase III ESCORT-NEO/NCCES01 study (ChiCTR2000040034) showed significantly higher pCR rates in the group receiving camrelizumab with albumin paclitaxel and cisplatin (28.0%) compared to those receiving camrelizumab with paclitaxel and cisplatin (15.4%), and to those receiving paclitaxel and cisplatin alone (4.7%) in ESCC [230-232]. Interim results from the phase III KEYNOTE-585 study (NCT03221426) demonstrated neoadjuvant pembrolizumab plus chemotherapy had a superior pCR (13% vs. 2%) and event-free survival (EFS) (median, 44.4 months vs. 25.3 months) compared to chemotherapy plus placebo in G/GEJ adenocarcinoma [233]. However, the survival outcomes have not yet matured. The 2-year outcomes were reported from the NICE study (ChiCTR1900026240) [234]. This is a phase II study investigating neoadjuvant carilizumab combined with chemotherapy for ESCC staged as T1b-4a, N2-3 (≥ 3 stations), and M0 or M1 lymph node metastasis. A recurrence rate of 37.3% and a two-year survival rate of 78.1% in this study are comparable to historical controls for neoadjuvant chemoradiotherapy.
Neoadjuvant ICT vs. CRT
The inclusion of immunotherapy has also complicated the issue of the optimal neoadjuvant treatment for EC. Adding immunotherapy to nCT has increased pCR and MPR rates [235]. A retrospective analysis comparing neoadjuvant immunotherapy combined with chemotherapy (nICT) to nCRT in ESCC patients found that nICT matched nCRT in R0 resection and in pCR rates, and showed superior 3-year OS and DFS [236]. However, the conflicting results were questioned because the hazard ratio (HR) indicated a higher risk of nICT [236]. In contrast, another retrospective analysis showed that nCRT significantly outperformed nICT in both pathological response and prognosis [237]. However, there are no prospective controlled study results comparing nICT with standard nCRT yet. The SCCH-TS2201 (NCT05244798) and KEYSTONE 002 (NCT04807673) trials are ongoing for this comparison.
Neoadjuvant ICRT vs. CRT
The PALACE-1 (NCT04435197) and the SCALE-1 (ChiCTR2100045104) studies incorporated immunotherapy into nCRT (nICRT) for ESCC, achieving the highest pCR rates in history [238, 239]. A network meta-analysis confirmed that the highest pCR and MPR rates remain in radiotherapy groups [neoadjuvant immunotherapy combined with CRT (ICRT) and nCRT] [240]. However, the long-term survival impact of pCR achieved through immunotherapy requires further follow-up. Several phase III trials in ESCC, including iCROSS (NCT04973306) and NEOCRTEC2101 (NCT05357846) based on the standard nCRT regimen, are currently underway and expected to yield practice-changing data [241].
However, a pooled analysis of 17 non-randomized prospective trials revealed that adding immunotherapy to nCRT does not improve pCR in resectable esophageal or gastroesophageal junction (GEJ) cancers but significantly increases severe adverse events (AEs) [242]. The recent ECOG-ACRIN EA 2174 study (NCT03604991) presented at the 2024 ASCO meeting is poised to provide critical insights into neoadjuvant therapy for EAC [243]. This phase II/III randomized trial evaluated immunotherapy in a neoadjuvant setting in combination with nCRT, and in an adjuvant setting with either nivolumab or ipilimumab/nivolumab. This report spotlights the outcomes of the neoadjuvant treatment phase. Unfortunately, the addition of nivolumab to nCRT has not improved the pCR rate in E/GEJ adenocarcinoma (24.8% in nICRT vs. 21.0% in nCRT). Though the adjuvant element is still pending, the neoadjuvant approach for EAC does not endorse immuno-enhancement with nCRT.
To date, neoadjuvant or perioperative immunotherapy is only recommended by the NCCN guidelines for resectable, locally advanced dMMR/MSI-H G/GEJ patients, based on the GERCOR NEONIPIGA (NCT04006262) and INFINITY (NCT04817826) [151, 244, 245]. In these ICIs-sensitive tumors, the pCR reached 60%.
The quest for the most effective neoadjuvant treatment strategies in the era of immunotherapy continues, with ongoing phase III clinical trials depicted in Figure 5. Limited data from retrospective comparisons indicated that nICT may be more beneficial for metastatic lymph nodes [246], while nCRT is more valuable for the primary tumor and metastatic lymph nodes at levels 1 and 2R [247]. Thus, personalizing treatment strategies for different patient groups is likely the future direction in oncology care.
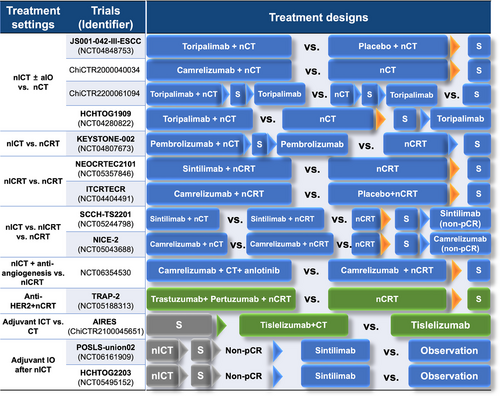
Neoadjuvant targeted therapy
Targeted therapies have shown limited efficacy in enhancing neoadjuvant therapy. EGFR is overexpressed in 50%-80% of ESCC and 10%-45.5% in EAC patients [248-250], with amplification in over 20% of ESCC and 5% in EAC patients [248, 251]. The phase III SAKK 75/08 trial (NCT01107639) demonstrated the integration of neoadjuvant and adjuvant anti-EGFR antibody cetuximab to a regimen of induction chemotherapy plus nCRT did not improve survival in patients with locally advanced EC [252].
EAC exhibited higher expression of human epidermal growth receptor 2 (HER2)/neu compared to ESCC (13% for EAC vs. 1% for ESCC) [253]. The TRAP study assessed the feasibility of trastuzumab and pertuzumab added to nCRT in HER2-positive EAC and achieved a pCR of 34% and an improved 3y-OS of 71% compared to nCRT [254]. However, the phase III NRG Oncology/RTOG 1010 trial (NCT01196390) suggested no benefit from the addition of trastuzumab to nCRT for HER2-overexpression EAC (median PFS: 19.6 months in the trastuzumab group vs. 14.2 months in the nCRT alone group) [31]. The phase III TRAP-2 study (NCT05188313) is ongoing to gather more conclusive evidence.
5.2.3 Adjuvant treatment
Another potential integrative treatment strategy is postoperative adjuvant therapy. Previous studies on perioperative chemotherapy for G/GECJ adenocarcinoma above advocate continuing the same regimens for adjuvant chemotherapy who responded to nCT [216, 224]. However, postoperative adjuvant chemotherapy for ESCC failed to provide additional survival benefits in JCOG studies [255, 256]. Despite the absence of prospective studies, adjuvant therapy after nCRT and surgery is not generally recommended in ESCC [257, 258]. Retrospective studies indicate that high-risk ESCC patients, such as those with poor responses to nCRT or those with advanced pathological stages, may benefit from adjuvant chemotherapy [259, 260].
Nivolumab represents a most significant advancement in adjuvant therapy for EC. In the Checkmate 577 trial (NCT02743494), it prolonged DFS regardless of histology (22.4 months vs. 11.0 months with placebo) and is approved for EC patients with residual pathological disease following CRT [227]. Figure 5 presents ongoing phase III studies investigating the integration of immunotherapies and novel targeted agents in the perioperative treatment of EC.
5.2.4 Organ preservation
Given the approximately half of pCR after nCRT, organ preservation based on active surveillance is an important direction for resectable EC, especially ESCC. The FFCD 9102 study (NCT00416858) indicated no survival benefit for surgery after response to CRT compared to continued dCRT for ESCC patients [261]. A phase II randomized study (NCT02959385) in ESCC patients achieving clinical complete response (cCR) reached the same conclusion [262]. A multicenter study showed that active surveillance for EC patients achieving cCR from nCRT yielded equivalent PFS and OS outcomes compared to immediate esophagectomy [263]. However, the ESOPRESSO study (NCT01740375) on ESCC patients suggested a trend toward better DFS, PFS, and time to progress in the surgery group and a higher local recurrence in the observation group, despite no survival differences in only 37 cCR patients due to slow enrollment [264]. The main challenge of ESCC active surveillance post-CRT is the subtlety of residual tumors, characterized by sparse viable cancer cells, low lymph node pCR rates, and varied regression patterns [265]. The PreSANO study (NTR6803), utilizing a multimodal approach including repeated endoscopic ultrasound examinations, bite-on-bite biopsies, and fine needle aspiration of suspicious lymph nodes, reported a 10% miss rate for tumor regression grades 3 and 4 tumors [266]. Innovative strategies like liquid biopsy for circulating tumor DNA and the Cytosponge™ device are emerging to enhance the detection of residual tumors [267, 268]. However, it is noteworthy that the negative predictive value is low [266]. Ongoing trials, such as SANO (NCT05953181), SANO-2 (NCT04886635), Esostrate-Prodige 32 (NCT02551458), preSINO (NCT03937362), and NEEDS (NCT04460352), are further evaluating surveillance strategies, as shown in Figure 6 [269-272]. Additionally, the PALACE3 study (NCT06339060) aims to explore the value of immunotherapy combined with chemoradiotherapy as an alternative to surgery.
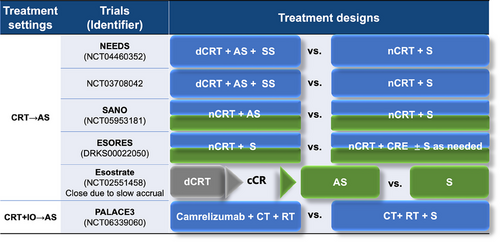
To clearly outline the key clinical research landscape for locally advanced resectable EC, Table 2 provides a systematic summary.
| Histologic types | Trials | Year | Phase | Treatment settings | Treatment regimens | PFS/DFS/EFS | p | OS | p | pCR, % | R0, % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neoadjuvant CT vs. CRT | |||||||||||
| ESCC | JCOG 9907 [213] | 2012 | III | nCT | CF →S | 5y: 39% | 0.22 | 5y: 43% | 0.04 | 2.52 | 95.5 |
| aCT | S →CF | 5y: 44% | 5y: 55% | - | 90.7 | ||||||
| ESCC | CROSSa [211] | 2012 | III | nCRT | PC + RT (41.4 Gy) → S | mPFS: 74.7 months | 0.006 | mOS: 81.6 months | 0.008 | 49 | 92 |
| S alone | S | mPFS: 11.6 months | mOS:21.1 months | - | 69 | ||||||
| ESCC | Neo-Resa [223] | 2016 | II | nCT | CF →S | 3y: 52% | 0.78 | 3y: 52% | 0.78 | 16 | 84 |
| nCRT | CF + RT (40Gy) → S | 3y: 56% | 3y: 56% | 42 | 83 | ||||||
| ESCC | NEOCRTEC5010 [212] | 2018 | III | nCRT | VP + RT (40Gy) → S | mDFS:100 months |
0.001 |
mOS:100 months | 0.025 | 43.2 | 98.4 |
| S alone | S | mDFS:42 months | mOS: 67 months | - | 91.2 | ||||||
| ESCC | JCOG 1109 NExT [219] | 2022 | III | nCT | CF → S | mPFS: 2.7 years | HR 0.67, (95% CI, 0.51-0.88) | mOS: 5.6 years | 0.006 | 2.1 | 84.4 |
| nCT | DCF → S | mPFS: NR | mOS: NR | 19.8 | 85.6 | ||||||
| nCRT | CF + RT (41.4 Gy) → S | mPFS: 5.3 years | HR 0.77, (95% CI, 0.59-1.01) | mOS: 7.0 years | vs.CF:0.12 | 38.5 | 87.5 | ||||
| ESCC | CMISG1701 [221] | 2023 | III | nCT | PC → MIE | mPFS: 34.1 months | 0.27 | mOS: 43.2 months | 0.28 | 2.9 | 96.2 |
| nCRT | PC + RT (40Gy) → MIE | mPFS: 46.5 months | mOS: NR | 27.7 | 97.3 | ||||||
| EAC | MAGIC [214] | 2006 | III | nCT | ECF →S | HR 0.66 (95% CI, 0.53 to 0.81) | < 0.001 | 5y: 36.3% | 0.009 | 31 | 79.3 |
| S alone | S | 5y: 23.0% | - | 70.3 | |||||||
| EAC | FFCD9703/ACCORD07 [215] | 2011 | III | nCT | CF →S | 5y: 34% | 0.003 | 5y: 38% | 0.02 | - | 84 |
| S alone | S | 5y: 19% | 5y: 24% | - | 73 | ||||||
| EAC | CROSSa [211] | 2012 | III | nCRT | PC + RT (41.4 Gy) →S | mPFS: 29.9 months | 0.01 | mOS: 43.2 months | 0.038 | 23 | 92 |
| S alone | S | mPFS: 17.7 months | mOS: 27.1 months | - | 69 | ||||||
| EAC | Neo-Resa [223] | 2016 | II | nCT | CF →S | 3y: 41% | 0.92 | 3y: 48% | 0.54 | 7 | 71 |
| nCRT | CF + RT (40Gy) →S | 3y: 40% | 3y: 43% | 22 | 89 | ||||||
| EAC | UK MRC OE05 [217] | 2017 | III | nCT | ECX →S | mDFS: 14.4 months | 0·051 | mOS: 26.1 months | 0.19 | 7 | 70 |
| nCT | CF →S | mDFS: 11.6 months | mOS: 23.4 months | 1 | 62 | ||||||
| EAC | FLOT4 [216] | 2019 | II/III | nCT | ECF/ECX →S | mPFS: 18 months | 0.0036 | mOS: 35 months | 0.012 | - | 78 |
| nCT | FLOT →S | mPFS: 30 months | mOS: 50 months | - | 85 | ||||||
| EAC | Neo-AEGIS [224] | 2023 | III | nCT | modified MAGIC/FLOT CT→S | mDFS: 32.4 months | 0.41 | mOS: 48 months | 0.82 | 4 | 82 |
| nCRT | PC + RT (41.4 Gy) (CROSS) →S | mDFS: 24.0 months | mOS: 49.2 months | 12 | 96 | ||||||
| EAC | ESOPEC [225] | 2024 | III | nCT | FLOT →S | mPFS: 38 months | 0.001 | mOS: 66 months | 0.012 | 19.3 | 94.2 |
| nCRT | PC + RT (41.4 Gy) (CROSS) →S | mPFS: 16 months | mOS: 37 months | 13.5 | 95 | ||||||
| Neoadjuvant ICT vs. CT | |||||||||||
| ESCC | NICE [234] | 2022 | II | nCT | Camre + nab-PC →S | 2y: 67.9% | - | 2y: 78.1% | - | 39.2 | 98.0 |
| ESCC | ESCORT-NEO/NCCES01 [231] | 2024 | III | nICT | Camre + nab-TP →S | Unmatured | Unmatured | 28.0 | 99.1 | ||
| nICT | Camre + TP →S | 15.4 | 95.7 | ||||||||
| nCT | TP →S | 4.7 | 92.2 | ||||||||
| EAC | KEYNOTE-585 [233] | 2024 | III | nCT + aIO | Pembro + Cisplatin-based CT →S →ICT → Pembro | mEFS: 44.4 months | 0.0198 (unmet) | mOS: 60.7 months | 0·174 | 12.9 | 80 |
| nICT + aICT | PBO + Cisplatin-based CT→S →PBO + CT → Pembro | mEFS: 25.3 months | mOS: 58.0 months | 10.9 | 75 | ||||||
| nCT + aIO | Pembro + FLOT →S →ICT →Pembro | mEFS: 45.8 months | HR: 0·81 (95% CI, 0·68 - 0·97) | mOS: 60.7 months | HR: 0·93(95%CI, 0·76 - 1·12) | 13.0 | - | ||||
| nICT + aICT | PBO + FLOT → S → PBO + CT → Pembro | mEFS: 25.7 months | mOS: NR | 2.4 | - | ||||||
| Neoadjuvant ICRT vs. CRT | |||||||||||
| ESCC | PALACE-1 [238] | 2021 | II | nICRT | Pembro + PC + RT (41.4Gy) → S | - | - | - | - | 55.6 | 94 |
| ESCC | SCALE-1 [239] | 2024 | II | nICRT | Toripalimab + PC + RT (30Gy/12f) → S | 2y:63.8% | - | 2y: 78% | 55 | 95 | |
| EAC | ECOG-ACRIN EA 2174 [243] | 2024 | II/III | nICRT | PC + RT(41.4-50.4 Gy) + Nivo → S → Nivo ± ipi or not (randomized) | - | - | - | - | 24.8 | - |
| nCRT | PC + RT(41.4-50.4 Gy) → S→ Nivo ± ipi or not (randomized) | - | - | - | - | 21.0 | - | ||||
| Neoadjuvant targeted therapy | |||||||||||
| ESCC | SAKK 75/08 [252] | 2018 | III | nCRT+TT | DP + RT (45Gy) + Cetuximab→ S→Cetuximab | mPFS:2.9 years | 0.13 | mOS:NR | 0.11 | 51 | 95 |
| nCRT | DP + RT (45Gy)→ S | mPFS:2 years | mOS: 2.5 years | 48 | 97 | ||||||
| EAC | TRAP [254] | 2020 | II | nCRT + TT | Trastu + pertu +PC+RT (41.4Gy) → S | 3y:57% | - | 3y: 71% | - | 34 | 100 |
| EAC | RTOG 1010 [31] | 2022 | III | nCRT + TT | Trastu +PC+RT (50.4Gy) → S→ Trastu | mDFS: 19.6 months | 0.97 | mOS: 38.5 months | 0.85 | 27 | 98 |
| nCRT | PC + RT (50.4Gy) → S | mDFS: 14.2 months | mOS: 38.9 months | 29 | 100 | ||||||
| Adjuvant treatment | |||||||||||
| EAC | Checkmate 577 [227] | 2021 | III | aIO | CRT→ S → Nivo | mDFS: 22.4 months | < 0.001 | - | - | - | - |
| Obs | CRT→ S | mDFS: 11.0 months | - | - | - | ||||||
| Organ preservation | |||||||||||
| ESCC | FFCD 9102 [261] | 2007 | III | CRT + S | CF + RT (46Gy or 15Gy split-course) → S | - | - | mOS: 17.7 months | 0.44 | - | - |
| CRT | CF + RT (46Gy or 15Gy split-course) → CF + RT (20Gy or 15Gy) | - | mOS: 19.3 months | - | - | ||||||
| ESCC | ESOPRESSO [264] | 2019 | III | S for cCR | XP→CRT (50.4Gy) →cCR → S | mDFS: NR | 0.282 | mOS: NR | 0.560 | 61.5 | 92.3 |
| Obs | cCR → Obs | mDFS: 25.6 months | mOS: NR | - | - | ||||||
- Abbreviations: aCT, adjuvant Chemotherapy; aIO, adjuvant Immunotherapy; Camre, Camrelizumab; CF, Cisplatin + 5-Fluorouracil; CRT, Chemoradiotherapy; CT, Chemotherapy; cCR, Clinical Complete Response; DCF, Docetaxel + Cisplatin + Fluorouracil; DFS, Disease Free Survival; DP, Docetaxel + Cisplatin; ECF, Epirubicin + Cisplatin + Infused Fluorouracil; ECX, Epirubicin + Cisplatin + Capecitabine; EFS, event-free survival; ESCC, Esophageal Squamous Cell Carcinoma; FLOT, Fluorouracil + Leucovorin + Oxaliplatin + Docetaxel; ipi, Ipilimumab; MIE, Minimally Invasive Esophagectomy; nab-PC, Albumin-Bound Paclitaxel + Carboplatin; nab-TP, Albumin-Bound Paclitaxel + Cisplatin; nCT, Neoadjuvant Chemotherapy; nCRT, Neoadjuvant Chemoradiotherapy; nICT, Neoadjuvant Immunochemotherapy; nICRT, Neoadjuvant Immune-Chemoradiotherapy; Nivo, Nivolumab; Obs, Observation; OS, Overall Survival; PBO, Placebo; PC, Paclitaxel + Carboplatin; pCR, Pathological Complete Response; Pembro, Pembrolizumab; PFS, Progression-Free Survival; Pertu, Pertuzumab; RT, Radiotherapy; S, Surgery; Trastu, Trastuzumab; VP, Vinorelbine + Cisplatin; XP, Capecitabine + Cisplatin.
- aIndicates that the study included data from both ESCC and EAC.
5.3 Locally advanced inoperative EC
5.3.1 cCRT: standard regimen
Concurrent chemoradiotherapy (cCRT) remains the standard treatment for locally advanced inoperative EC based on the result of RTOG 8501 [273]. Even in elderly ESCC patients, phase III studies have confirmed that cCRT with S-1 offers superior response rates and survival compared to radiotherapy alone [274, 275]. From RTOG 9405 to recent studies, including CONCORDE (PRODIGE-26, NCT01348217), ARTDECO (NCT01457677), and two Chinese randomized studies (NCT01937208, NCT02850991), have established the recommended dose regimen for dCRT in EAC and ESCC [276-280]. No significant local control benefit with radiation doses escalated to 60 Gy over 50 Gy [278-280]. However, local recurrence remains the predominant failure following cCRT, particularly in ESCC [281]. The latest phase III randomized study (ChiCTR-IPR-15007172), which compared both dose and irradiation field, suggested that a high dose of 60 Gy to the involved field was most effective in improving local control and survival [282]. The ESO-Shanghai 12 study (NCT03790553) is ongoing to explore positron emission computed tomography-guided dose escalation in ESCC [283].
About the optimal concurrent chemotherapy regimen, no differences in PFS and in 2-year OS were shown in both ESCC and EAC patients among various fluorouracil-based regimens, including capecitabine in the CRTCOESC study (NCT02025036) and PRODIGE5 study (NCT00861094) [284, 285]. Similarly, an ESCC phase III trial (NCT02459457) found no survival advantage for paclitaxel-based regimens [286]. Direct comparisons between these regimens are lacking.
5.3.2 Immunotherapy combination
Another strategy involves intensifying drug combinations. The benefits of induction or consolidation chemotherapy with dCRT are disputed, and meta-analysis showed no significant long-term survival benefits [287]. Inspired by the PACIFIC study (NCT02125461) in locally advanced non-small cell lung cancer (NSCLC) [288], the incorporation of immunotherapy offers new therapeutic insights for locally advanced EC.
TENERGY (EPOC1802) study (UMIN000034373) is the first reported single-arm phase II trial on atezolizumab post-dCRT for ESCC, reported a 40% cCR rate but disappointing median PFS (3.2 months) and 12-month PFS rate (29.6%), which fell short of expectations [289]. Conversely, a Korean phase II study (NCT03377400) of consolidation dual immunotherapy with durvalumab and tremelimumab in ESCC showed 24-month PFS and OS rates of 57.5% and 75%, markedly better than historical controls [290].
Induction immuno-chemotherapy followed by cCRT was also evaluated in a phase II single-arm ESCC trial (NCT03985046), and it showed improved radiotherapy sensitivity and local control rates via vascular normalization and hypoxia alleviation [291]. These findings were validated by the ImpactCRT trial (ChiCTR2000034304) and a real-world study [292, 293].
The EC-CRT-001 study (NCT04005170), employing concurrent CRT and immunotherapy in ESCC patients, achieved a 95% completion for planned chemoradiotherapy, with 62% attaining cCR and a 1-year PFS of 54.5%, demonstrating encouraging activity [294]. These studies highlight the potential of combining immunotherapy with CRT in locally advanced ESCC, though few studies report on combining dCRT with immunotherapy in EAC. Ongoing phase III trials (Figure 7) like KEYNOTE-975 (NCT04210115) and KUNLUN (NCT04550260) are expected to provide further insights. Concurrently, the immunotherapy combination has introduced a cascade of additional queries regarding the optimal combination regimens, such as optimal timing, radiation dose, radiation volume, and immunotherapy course, requiring further investigation.
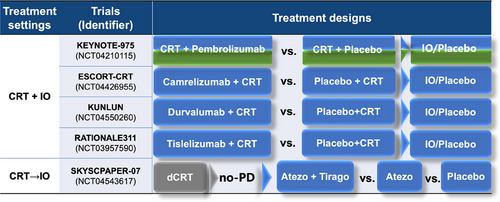
5.3.3 Targeted therapy combination
Despite limited success in neoadjuvant therapy, targeted therapy holds promise for locally advanced unresectable ESCC. A phase II study (NCT02375581) in elderly ESCC patients showed that combining erlotinib with radiotherapy significantly improved OS (mOS, 24.0 months vs. 16.3 months) compared to radiotherapy alone, particularly in those with EGFR-overexpression, offering options for patients intolerant to cCRT [295]. However, another phase II study (NCT02577341) found that cCRT combined with nimotuzumab failed to prolong PFS or OS, although it reduced distant metastasis, possibly due to unselected EGFR expression status of the included subjects [296]. Further phase III NXCEL1311 study (NCT02409186) demonstrated that nimotuzumab combined with CRT increased response rate, though long-term follow-up is warranted for survival outcomes [297]. Another phase III study has revealed that cCRT combined with erlotinib improved long-term survival in locally advanced ESCC, particularly the patients with EGFR-overexpression [27]. However, RTOG 0436 (NCT00655876) and SCOPE1 (ISRCTN47718479) studies demonstrated no benefit of cetuximab to cCRT for EC [298, 299]. Therefore, the role of EGFR-targeted agents requires further validation.
5.3.4 Conversion surgery
Contrary to the debate on organ preservation in resectable EC, recent advances in improved regression by adding immunotherapy to CRT and the adoption of a lower radical dose of 50 Gy have reinvigorated the discussion on conversion surgery for initially unresectable diseases, particularly in ESCC. A phase II Japanese study (UMIN000011089) on unresectable T4b and supraclavicular lymph node metastatic ESCC patients using DCF chemotherapy ± radiotherapy for conversion surgery showed a 41.7% conversion rate, 95% R0 resection rate, and 20% pCR rate. Postoperative complications occurred in 25% of patients, with a 3-year OS of 46.6% and significantly better OS in R0 resection patients (71.4% for R0 patients vs. 30.1% for patients who did not) [300, 301]. A multicenter real-world study from China employing an immunotherapy combination for induction treatment of ESCC achieved a 74.8% conversion rate, 94% R0 resection rate, and 22.3% pCR rate, surpassing chemotherapy alone with fewer postoperative complications [302]. Conversion CRT in a phase II study (NCT04278287) in China reported a pCR rate of 47.6% in 46.7% of ESCC patients undergoing surgery and a significantly improved OS compared with dCRT (2y-OS: 84.2% vs. 54.4%; HR = 3.41; 95% CI = 1.10-10.61) [303]. Furthermore, they conducted another phase II NEXUS-1 study (ChiCTR2100054327) incorporating immunotherapy into the conversion CRT and achieved an R0 resection rate of 100% and pCR rate of 61.5% in ESCC patients [304, 305]. Despite the promising prospects of conversion surgery, it is crucial to note that selecting cT4b candidates for surgery after induction therapy is a considerable challenge. Surgery isn't advisable for patients unable to achieve R0 resection, as palliative procedures have higher complication rates and offer no long-term survival benefits [306].
Similarly, Table 3 summarizes the key clinical research landscape for locally advanced unresectable EC.
| Histologic types | Trials | Year | Phase | Treatment settings | Treatment arms | PFS | p | OS | p | ORR, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Definitive cCRT | ||||||||||
| ESCC/EAC | RTOG 8501 [273] | 1999 | III | cCRT | CF + RT (50Gy) | - | - |
5y: 21%(ESCC) 13%(EAC) |
- | - |
| RT | RT (64Gy) | - | 5y: 0 | - | ||||||
| ESCC/EAC | RTOG 9405 [276] | 2002 | III | HD-cCRT | CF + RT (64.8Gy) | - | - | mOS: 13 m | No significant | - |
| SD-cCRT | CF + RT (50.4Gy) | - | mOS: 18.1m | - | ||||||
| ESCC/EAC | CONCORDE (PRODIGE-26) [277] | 2021 | II/III | HD-cCRT | FOLFOX-4 + RT (66Gy) | mLRPFS: 18.4m | 0.88 | mOS: 23.5m | 0.44 | - |
| SD-cCRT | FOLFOX-4 + RT (50Gy) | mLRPFS: 16.2m | mOS: 25.2m | - | ||||||
| ESCC/EAC | ARTDECO [278] | 2021 | III | HD-cCRT | PC + RT (61.6Gy) | 3y-LRPFS: 64% (ESCC), 49% (EAC) | 0.26 | 3y: 39% | 0.22 | - |
| SD-cCRT | PC + RT (50.4Gy) | 3y-LRPFS: 58% (ESCC), 42% (EAC) | 3y: 42% | - | ||||||
| ESCC/EAC | PRODIGE5 [285] | 2014 | II/III | cCRT | FOLFOX + RT (50Gy) | mPFS: 9.7m | 0.64 | - | - | - |
| cCRT | CF + RT (50Gy) | mPFS: 9.4m | - | - | ||||||
| ESCC | NCT01937208 [279] | 2022 | III | HD-cCRT | DP + RT (61.6Gy) | mPFS: 27.7m | 0.86 | mOS: 45.3m | 0.96 | 90.6 |
| SD-cCRT | DP + RT (50.4Gy) | mPFS: 25.5m | mOS: 41.2m | 93.4 | ||||||
| ESCC | NCT02850991 [280] | 2023 | III | HD-cCRT | PC + RT (59.4Gy) | 3y: 36.7% | 0.28 | mOS: 28.1m | 0.54 | - |
| SD-cCRT | PC + RT (50.4Gy) | 3y: 32.2% | mOS: 23.6m | - | ||||||
| ESCC | ChiCTR-IPR-15007172 [282] | 2024 | III | HD-ENI | SP + RT (59.4Gy+ENI) | mPFS: 21.2m |
Dose: 0.012; Field: 0.888 |
mOS: 27.2m | Dose: 0.318; Field: 0.93 | 80.0 |
| HD-IFI | SP + RT (59.4Gy+IFI) | mPFS: 30.8m | mOS: 46.3m | 73.3 | ||||||
| SD-ENI | SP + RT (50.4Gy+ENI) | mPFS: 21.0m | mOS: 30.9m | 80.6 | ||||||
| SD-IFI | SP + RT (50.4Gy+IFI) | mPFS: 16.9m | mOS: 28.3m | 74.6 | ||||||
| ESCC | CRTCOESC [284] | 2024 | III | cCRT | CAP + RT (50Gy) (C) | mPFS: 26.1m |
C vs. P: 0.45 X vs. P: 0.615 |
mOS: 40.9m |
C vs. P: 0.637 X vs. P: 0.444 |
- |
| cCRT | XELOX + RT (50Gy) (X) | mPFS: 30.2m | mOS: 41.9m | - | ||||||
| cCRT | CF + RT (50Gy) (P) | mPFS: 28.2m | mOS: 35.4m | - | ||||||
| dCRT + immunotherapy | ||||||||||
| ESCC | TENERGY (EPOC1802) [289] | 2022 | II | dCRT→IO | dCRT (60Gy) →Atezo | mPFS: 3.2m | - | mOS: 31.0m | - | cCR: 40.0% |
| ESCC | NCT03985046 [291] | 2024 | II |
IO + CT →cCRT |
Sintilimab + PC →CRT (50.4Gy) |
2y: 61.3% | - | 2y: 77.3% | - | - |
| ESCC | ImpactCRT [292] | 2023 | II | IO + CT→cCRT |
Camre + nab-PC →dCRT (50-66Gy) |
1y: 74.2% | - | 1y: 87.6% | - | 97.6 |
| ESCC | EC-CRT-001 [294] | 2023 | II | cCRT + IO | Toripalimab+ CRT (50.4Gy) | 1y: 54.5% | - | 1y: 78.4% | - | cCR: 62% |
| ESCC | NCT03377400 [290] | 2022 | II |
cCRT + IO →IO |
CRT + Durva + Tremeli →Durva + Tremeli → Durva | 24m:57.5% | - | 24m: 75% | - | - |
| CRT+ targeted therapy | ||||||||||
| ESCC | NXCEL1311 [297] | 2023 | III | cCRT + anti-EGFR | cCRT + nimotuzumab | - | - | - | - | 93.8 |
| cCRT | cCRT + Placebo | - | - | 72.0 | ||||||
| ESCC | NCT00686114 [27] | 2018 | III | ENI-cCRT | ENI-RT (60Gy) + TP | 5y: 35.5% | 0.007 | 5y: 34.8% | 0.013 | - |
| ENI + anti-EGFR | ENI-cCRT + erlotinib | 5y: 41.8% | 5y: 44.9% | - | ||||||
| CFI-cCRT | CFI-RT (60Gy) + TP | 5y: 19.2% | 5y: 19.6% | - | ||||||
| CFI + anti-EGFR | CFI-cCRT + erlotinib | 5y: 32.1% | 5y: 33.8% | - | ||||||
| ESCC | RTOG 0436 [298] | 2017 | III | cCRT + anti-EGFR | cCRT + Cetuximab | - | - | 36m: 33.8% | 0.47 | cCR: 59.3% (ESCC), 52.7% (EAC) |
| cCRT | cCRT | - | 36m: 27.9% | cCR: 64.4% (ESCC), 53.9% (EAC) | ||||||
| ESCC | SCOPE1 [299] | 2013 | II/III | cCRT + anti-EGFR | cCRT + Cetuximab | mPFS: 15.9m | 0.18 | mOS: 22.1m | 0.035 | - |
| cCRT | cCRT | mPFS: 21.6m | mOS: 25.4m | - | ||||||
| Conversion surgery | ||||||||||
| ESCC | NEXUS-1 [304] | 2022 | II |
IO + cCRT →CS |
Tisle + PC + RT (50Gy) →CS |
CS rate: 61.9% | - | - - |
- | pCR: 61.5% |
- Abbreviations: Atezo, Atezolizumab; CAP, Capecitabine; Camre, Camrelizumab; cCRT, Concurrent Chemoradiotherapy; CF, Cisplatin + Fluorouracil; CRT, Chemoradiotherapy; CS, Conversion surgery; CT, Chemotherapy; dCRT, Definitive chemoradiotherapy; DP, Docetaxel + Cisplatin; Durva, Durvalumab; ENI, Elective nodes irradiation; ESCC, Esophageal Squamous Cell Carcinoma; EAC, Esophageal Adenocarcinoma; FOLFOX, Infusional fluorouracil + leucovorin + bolus fluorouracil + oxaliplatin; HD, High dose; IFI, Involved-field irradiation; IO, Immunotherapy; mLRPFS, Median locoregional progression-free survival; mOS, Median overall survival; nab-PC, Albumin-bound paclitaxel + carboplatin; ORR, Objective response rate; OS, Overall survival; PC, Paclitaxel + Carboplatin; PFS, Progression-free survival; RT, Radiotherapy; SD, Standard dose; SP, S-1 + Cisplatin; Tisle, Tisilizumab; Tremeli, Tremelimumab; XELOX, Capecitabine + oxaliplatin.
5.4 Advanced or metastasis EC
5.4.1 First-line treatment for advanced ESCC and HER2 negative EAC
Immunotherapy combined with chemotherapy
The key clinical research landscapes for advanced or metastatic EC are displayed in Table 4. The most significant breakthrough in the advanced esophageal cancer treatment landscape has emanated from immunotherapy. ICIs combined with chemotherapy have become the standard first-line treatment for recurrent and distant metastatic ESCC and HER2 negative EAC, based on the results of seven randomized controlled phase III clinical studies in ESCC [CheckMate-648 (NCT03143153), ESCORT-1st (NCT03691090), ORIENT-15 (NCT03748134), JUPITER-06 (NCT03829969), ASTRUM-007 (NCT03958890), RATIONALE-306 (NCT03783442), GEMSTONE-304 (NCT04187352)], five pivotal trials in EAC [ChekMate-649 (NCT02872116), ORIENT-16 (NCT0374517), RATIONALE-305 (NCT03777657), KEYNOTE-859 (NCT03675737), GEMSTONE-303 (NCT03802591)], and one trial for both ESCC and EAC [KEYNOTE-590 (NCT03189719)] [307-319]. Despite this, a meta-analysis of randomized clinical trials suggested a lack of survival benefit from immunochemotherapy for patients with PD-L1 combined positive score (CPS) <1 [320]. Furthermore, the conventional ≥1% tumor proportion score (TPS) and ≥10 CPS cutoffs do not accurately predict the benefits of immunochemotherapy by meta-analysis [321]. This indicates a need for better predictive tools and individualized treatment strategies, especially for patients with low PD-L1 expression. Challenges and controversies in drug treatment for advanced EC persist, necessitating further research to refine therapeutic strategy.
| Histologic types | Strategy | Trials | Year | Phase | Treatment settings | Treatment arms (N) | Median PFS, months | p | Median OS, months | p | ORR, % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| First-line | |||||||||||
| ESCC | Immunotherapy + CT | CheckMate-648 [307] | 2022 | III | IO + CT | Nivo + CF | 5.8 |
0.04 |
13.2 | - | 47.0 |
| CT | CF | 5.6 | 10.7 | 27.0 | |||||||
| IO + IO | Nivo + Ipi | 2.9 | NR | 12.7 | 28.0 | ||||||
| ESCORT-1st [308] | 2021 | III | IO + CT | Camre + TP | 6.9 | 0.56 | 15.3 | 0.70 | 72.1 | ||
| CT | TP | 5.9 | 12.0 | 62.1 | |||||||
| ORIENT-15 [309] | 2022 | III | IO + CT | Sintilimab + TP/CF | 7.2 | < 0.001 | 16.7 | < 0.001 | 66.1 | ||
| CT | TP/CF | 5.7 | 12.5 | 45.5 | |||||||
| JUPITER-06 [310] | 2022 | III | IO + CT | Toripalimab + TP | 5.7 | < 0.001 | 17.0 | 0.0004 | 69.3 | ||
| CT | TP | 5.5 | 11.0 | 52.1 | |||||||
| ASTRUM-007 [311] | 2023 | III | IO + CT | Serplulimab + CF | 5.8 | 0.6 | 15.3 | 0.002 | 57.6 | ||
| CT | CF | 5.3 | 11.8 | 42.1 | |||||||
| RATIONALE-306 [312] | 2023 | III | IO + CT | Tislelizumab + TP/CF | 7.3 | < 0.001 | 17.2 | < 0.001 | 63.5 | ||
| CT | TP/CF | 5.6 | 10.6 | 42.2 | |||||||
| GEMSTONE-304 [313] | 2024 | III | IO + CT | Sugemalimab + CF | 6.2 | 0.0002 | 15.3 | 0.0076 | 60.1 | ||
| CT | CF | 5.4 | 11.5 | 45.2 | |||||||
| KEYNOTE-590 [319] | 2021 | III | IO + CT | Pembro + CF | 6.3 | < 0.001 | 12.4 | < 0.001 | 45.0 | ||
| CT | CF | 5.8 | 9.8 | 29.3 | |||||||
| SKYSCRAPER-08 [322] | 2024 | III | IO + IO + CT →IO | Tira + Atezo + TP →Tira + Atezo | 6.2 | < 0.001 | 15.7 | 0.0024 | 59.7 | ||
| CT | TP → Placebo | 5.4 | 11.1 | 45.5 | |||||||
| HER2- EAC | Immunotherapy + CT | KEYNOTE-590 [319] | 2021 | III | IO + CT | Pembro + CF | 6.3 | < 0.001 | 12.4 | < 0.001 | 45.0 |
| CT | CF | 5.8 | 9.8 | 29.3 | |||||||
| ChekMate-649 [314] | 2021 | III | IO + CT |
Nivo + XELOX / FOLFOX |
7.7 | 0.77 | 13.8 | 0.0002 | 58.0 | ||
| CT | XELOX / FOLFOX | 6.9 | 11.6 | 46.0 | |||||||
| ORIENT-16 [315] | 2023 | III | IO + CT | Sintilimab + XELOX | 7.7 | < 0.001 | 15.2 | 0.009 | 65.1 | ||
| CT | XELOX | 5.8 | 12.3 | 58.7 | |||||||
| RATIONALE-305 [316] | 2024 | III | IO + CT | Tislelizumab + XELOX/ CF | 7.2 | 0.67 | 17.2 | 0.0056 | 50.4 | ||
| CT | XELOX/ CF | 5.9 | 12.6 | 43.0 | |||||||
| KEYNOTE-859 [317] | 2023 | III | IO + CT | Pembro + CF/CAPOX | 6.9 | <0·0001 | 12.9 | <0·0001 | 51.3 | ||
| CT | CF/CAPOX | 5.6 | 11.5 | 42.0 | |||||||
| GEMSTONE-303 [318] | 2023 | III | IO + CT | Sugemalimab + CAPOX | 7.6 | <0.0001 | 15.6 | 0.006 | 68.6 | ||
| CT | PBO + CAPOX | 6.1 | 12.6 | 52.7 | |||||||
| HER2- EAC | Target therapy | SPOTLIGHT [323] | 2023 | III | TT + CT | Zolbetuximab + mFOLFOX6 | 10.6 | 0.0066 | 18.2 | 0.0053 | 60.7 |
| CT | PBO + mFOLFOX6 | 8.7 | 15.5 | 62.1 | |||||||
| GLOW [324] | 2023 | III | TT + CT | Zolbetuximab + CAPOX | 8.2 | 0.0007 | 14.4 | 0.0118 | 42.5 | ||
| CT | PBO + CAPOX | 6.8 | 12.2 | 40.3 | |||||||
| HER2+ EAC | Target therapy | ToGA [29] | 2010 | III | TT + CT | Trastu + CF/XP | 6.7 | 0.0002 | 13.8 | 0∙0046 | 47.0 |
| CT | CF/XP | 5.5 | 11.1 | 35.0 | |||||||
| HER2+ EAC | Immunotherapy + target therapy | KEYNOTE-811 [325] | 2021 | III | IO + CT + TT | Pembro + CF/XELOX+ Trastu | 10 | 0.0002 | 20.0 | 0.084 | 74.4 |
| CT + TT | PBO + CF/XELOX + Trastu | 8.1 | 16.9 | 51.9 | |||||||
| HER2+ EAC | AIO INTEGA [326] | 2022 | II | TT + IO | Trastu + Nivo + ipi | 3.2 | - | 16.4 | - | 32.0 | |
| CT + IO | Trastu + Nivo + mFOLFOX6 | 10.7 | 21.8 | 56.0 | |||||||
| Second- or later-lines | |||||||||||
| HER2- EAC | Anti-angiogenic targeted therapy | RAINBOW [327] | 2014 | III | TT + CT | Ramucirumab + paclitaxel | 4.4 | <0·0001 | 9.6 | 0.017 | 28.0 |
| CT | PBO + paclitaxel | 2.9 | 7.4 | 16.0 | |||||||
| HER2- EAC | FRUTIGA [328] | 2024 | III | TT + CT | Fruquintinib + paclitaxel | 5.6 | < 0.0001 | 9.6 | 0.6064 | 42.5 | |
| CT | PBO + paclitaxel | 2.7 | 8.4 | 22.4 | |||||||
| HER2+ EAC | Anti-angiogenic targeted therapy | DESTINY-Gastric01 [329] |
2020 |
II |
TT | T-DXd | 5.6 | - | 12.5 | 0.01 | 51.3 |
| CT | Irinotecan or paclitaxel | 3.5 | 8.4 | 14.3 | |||||||
| HER2+ EAC | DESTINY-Gastric02 [330] | 2023 | II | TT | T-DXd | 5.6 | - | 12.1 | - | 42.0 | |
| Local treatment | |||||||||||
| ESCC | LT | ESO-Shanghai 13 [331] | 2023 | II | ST alone | CT/IO/CT + IO | 6.4 | < 0.0001 | 18.6 | 0.002 | - |
| ST + LT | CT/IO/CT + IO + RT/S/Ablation | 15.3 | NR | - | |||||||
| EAC | LT | AIO-FLOT3 [332] | 2017 | II | CT + S | FLOT→S→FLOT | NR | - | NR | - | - |
| CT± S | FLOT→ ± S→FLOT | 10.7 | 22.9 | 60.0 | |||||||
| CT± palliation S | FLOT→ ± palliation S | 6.3 | 10.7 | 43.3 | |||||||
- Abbreviations: Atezo, Atezolizumab; Camre, Camrelizumab; CAPOX, capecitabine + oxaliplatin; CF, cisplatin + fluorouracil; CT, chemotherapy; EAC, esophageal adenocarcinoma; ESCC, esophageal squamous cell carcinoma; FLOT, fluorouracil + leucovorin + oxaliplatin + docetaxel; IO, immunotherapy; ipi, ipilimumab; LT, local therapy; mFOLFOX6, oxaliplatin + fluorouracil bolus + folinic acid + fluorouracil; MST, median survival time; Nivo, nivolumab; NR, not reached; OS, overall survival; ORR, objective response rate; PBO, placebo; Pembro, pembrolizumab; PFS, progression-free survival; RT, radiation therapy; S, surgery; ST, systemic therapy; T-DXd, Trastuzumab deruxtecan; Tira, Tiragolumab; TP, paclitaxel + cisplatin; Trastu, Trastuzumab; TT, target therapy; XELOX, capecitabine + oxaliplatin; XP, capecitabine + cisplatin.
Novel agents in ESCC
Various approaches to bolster the standard regimen with novel agents have been explored in ESCC, including anti-angiogenic small molecule tyrosine kinase inhibitors (TKIs) (NCT03603756) [333], anti-EGFR antibodies (NCT05221658) [334], and T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domain (TIGIT) antibodies (NCT04540211) [322], or substituting PD-1/PD-L1 antibodies with PD-1/ cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) bispecific antibodies (NCT05522894) [335]. Most of these studies, predominantly single-arm phase II trials, achieved encouraging objective response rates (ORRs) ranging from 55.2% to 86.7%. The combination of TIGIT antibodies has prolonged the OS compared with chemotherapy alone in the phase III study [322]. Yet, it remains to be seen whether these combinations can outperform standard therapy in head-to-head trials. The LEAP-014 phase III study is underway to assess the role of lenvatinib, a multi-targeted TKI, as a potential new standard in first-line ESCC treatment (NCT04949256). Another optimization strategy focuses on omitting chemotherapy to improve treatment tolerability. For instance, studies that have reported results include the combination of a PD-1 antibody with a CTLA-4 antibody (CheckMate-648) [307] and a PD-1 antibody with an anti-angiogenic TKI (ALTER-E003, NCT05038813) [336]. The CheckMate-648 trial's evaluation of the nivolumab and ipilimumab combination without chemotherapy has shown significant OS benefits, particularly in patients with PD-L1 TPS ≥ 1% [307]. While not yet approved for first-line ESCC treatment, it is undoubtedly an option for patients who cannot tolerate or refuse chemotherapy. The combination of a PD-1 antibody with an anti-angiogenic TKI has also shown early promise. Anlotinib combined with PD-L1 antibody benmelstobart achieved an ORR of 69.6% in a prospective single-arm, phase II study, with an mPFS of 15.44 months [336]. Further validation in phase III trials is required to confirm these findings.
Novel agents in HER2 negative EAC
In HER2 negative EAC, optimizing the first-line therapeutic interventions is a principal research inquiry. Novel immunotherapeutic approaches have been explored. The phase Ib/II COMPASSION-04 trial (CTR20182027) of PD-1/CTLA-4 bispecific antibody cadonilimab combined with chemotherapy showed promising clinical activity with an ORR of 52.1% and a mOS of 17.48 months, regardless of PD-L1 expression [337]. Unfortunately, adding anti-Lymphocyte activation gene 3 (LAG-3) to anti-PD-1 plus chemotherapy failed in the primary endpoint of improved ORR in G/GEJ adenocarcinoma patients with LAG-3 expression ≥ 1%, as showed in the phase II RELATIVITY-060 study (NCT03662659) [338]. The combination of regorafenib with nivolumab and chemotherapy achieved 71% of PFS in a phase II trial (NCT04757363), indicating the synergistic potential of combining targeted therapies and the necessity for additional phase III study [339]. There is also controversy regarding the efficacy of ICIs for patients with CPS < 5. In the ChekMate-649 and ORIENT-16 studies, the HR for OS in the CPS < 5 subgroup was 0.94 (95% CI = 0.78-1.13) and 0.90 (95% CI = 0.66-1.21), respectively [314-316], indicating a potentially limited benefit.
Optimizing treatment strategies for patients exhibiting low PD-L1 expression has become a focal point of research. The COMPASSION-15 study (NCT05008783) demonstrated a promising benefit of OS in the low PD-L1 expression subgroup with the strategy of PD-1/CTLA-4 bispecific antibody cadonilimab combined with chemotherapy [340]. However, in the CheckMate-649 study, the combination of nivolumab and ipilimumab failed to prolong OS and PFS over chemotherapy alone in the overall population regardless of PD-L1 status, except for patients with high microsatellite instability (MSI-H) or mismatch repair protein deficiency (dMMR) [341]. It is worth noting that, despite the generally negative outcomes, patients responding to nivolumab plus ipilimumab had the longest response duration in the study [342]. Increased toxicity and early mortality are a significant concern, and identifying patients’ benefits safely requires further research. dMMR/MSI-H status has emerged as a more potent predictor of response to ICI-containing regimens than PD-L1 expression levels [343]. However, silico analysis indicated the immunological distinctions between MSI-H and MSI-L samples may be less pronounced in GAC and EAC, highlighting the need for further evaluation of biomarkers [344].
For HER2-negative EAC, the transmembrane protein Claudin 18.2 has emerged as a significant novel target. With the favorable PFS and OS of Zolbetuximab combined with mFOLFOX6 or CAPOX in the SPOTLIGHT study (NCT03504397) and the GLOW study (NCT03653507), respectively, Zolbetuximab combined with chemotherapy has become a new standard first-line treatment for Claudin 18.2-positive (defined as ≥ 75% of tumor cells had moderate or strong membrane staining), HER-2-negative advanced G/GEJ adenocarcinoma [323, 324]. A preliminary study (NCT05632939) combining Claudin 18.2 antibodies with chemotherapy and immunotherapy has demonstrated encouraging outcomes with an ORR of 80%, raising expectations for subsequent research [345]. Whether incorporating immunotherapy can outperform the combination of anti-Claudin 18.2 with chemotherapy, and whether a lower threshold for Claudin 18.2 positivity may be adopted with the new strategy require further investigation (like NCT06206733). Other targets, like FGFR2b, which is highly co-expressed with PD-L1, have shown the potential to prolong PFS and OS in the phase II FIGHT trial (NCT03694522) [346]. The combination of bemarituzumab and mFOLFOX6 has notably prolonged both mPFS and OS [347]. Confirmatory phase III trial FORTITUDE-101 (NCT05052801) and the subsequent trial combined with immunotherapy FORTITUDE-102 (NCT05111626) are ongoing.
First-line treatment for HER-2 positive EAC
In advance of ESCC, EAC has multiple targeted drugs developed. Since 2010, trastuzumab combined with chemotherapy has become the first-line standard treatment for patients with HER-2-positive advanced G/GEJ adenocarcinoma based on the multi-center phase III ToGA study, enhancing mOS to 13.8 months over chemotherapy's 11.1 months [29]. Dual-targeted anti-HER2 therapy has been further explored. However, trastuzumab combined with pertuzumab failed to improve efficacy [348]. The ongoing HERIZON-GEA-01 trial (NCT05152147) is investigating the HER2-targeted bispecific antibody zanidatamab compared to trastuzumab in the first-line combined regimen.
Immunotherapy has also been explored in HER-2-positive patients. Pembrolizumab has been recommended to be added to the first-line standard regimen of trastuzumab and chemotherapy for advanced HER2 positive EAC with PD-L1 CPS ≥ 1 based on the interim result of the KEYNOTE-811 study (NCT03615326), though OS has not met the prespecified criteria for significance yet [325, 349]. The chemotherapy-free regimen of combined Fc-engineered anti-HER2 antibody margetuximab and anti-PD-1 antibody retifanlimab has also demonstrated a promising efficacy with ORR of 53% in HER2+ / PD-L1 + GEJ adenocarcinoma in the MAHOGANY study (NCT04082364) [350]. However, patients with PD-L1 expression CPS < 1 derived limited benefit from pembrolizumab in the KEYNOTE-811 study, with OS potentially worsening [349]. Dual anti-CTLA-4 and anti-PD-1 antibodies did not further improve OS in the AIO INTEGA trial (NCT03409848) [326]. Pan-HER inhibitor pyrotinib was investigated to improve synergistic activity with immunochemotherapy, and a phase Ib study (ChiCTR2000029717) showed a favorable ORR of 77.8% and mOS of 22.1 months despite not distinguishing by PD-L1 expression levels [351]. These indicate other new research avenues.
5.4.2 Second- or later-line treatment
Advanced ESCC
In the second-line treatment landscape, immunotherapy monotherapy was approved to be the standard second-line treatment for advanced ESCC after first-line chemotherapy by the ATTRACTION-3 (NCT02569242), KEYNOTE-181 (NCT02564263) and RATIONALE-302 (NCT03430843) studies [352-355]. However, after the failure of the current first-line chemoimmunotherapy strategy, there is an absence of standardized second-line treatments.
Single-agent chemotherapy remains the primary second-line option in clinical practice. Retrospective analysis of data from a randomized phase III clinical study (NCT03958890) has demonstrated that nab-paclitaxel-based chemotherapy is an efficacious second-line and later-line treatment option regardless of first-line immunotherapy, with an ORR of 33.3% [356]. The retrospective analysis demonstrates that second-line therapy with irinotecan combined with 5-fluorouracil (5-FU) following first-line chemoimmunotherapy elicits a similar therapeutic response as it does after first-line chemotherapy alone [357].
The cross-line use of immune checkpoint inhibitors with anti-angiogenic drugs represents a novel treatment strategy. The ALTER-E-006 study reported an ORR of 29.6%, an mPFS of 6.31 months, and a mOS of 10.97 months for the combination of anlotinib with immunotherapy [358]. The CAP-02 study (NCT03736863) showed an ORR of 13.2%, a mPFS of 4.6 months, and a mOS of 7.5 months with apatinib plus camrelizumab [359]. An upcoming Phase III trial (NCT05049681) will compare the efficacy and safety of SHR-1210 combined with apatinib versus SHR-1210 alone in the second-line treatment of ESCC, potentially validating this approach.
Drugs for other targets like EGFR are being explored. A phase Ib study (NCT02779699) of AL2846, an antiangiogenic TKI with multiple targets [cellular-mesenchymal epithelial transition factor (c-MET), vascular endothelial growth factor receptor 1 (VEGFR1), c-KIT, Axl, RET, kinase insert domain receptor (KDR), and VEGFR3], in combination with an anti-PD-L1 antibody (TQB2450) exhibited a favorable safety profile in immunotherapy-refractory advanced ESCC [360]. Phase III study evaluating the EGFR-HER3 Antibody-drug conjugate (ADC) drug BL-B01D1(NCT06304974) as the second-line treatment and multi-targeted TKI KC1036(NCT06194734) as the third-line treatment compared to single-agent chemotherapy are ongoing.
Advanced EAC
HER-2 negative EAC
Chemotherapy remains the cornerstone of second-line treatment of advanced EAC. Docetaxel, irinotecan, and paclitaxel have been established as standard second-line options through phase III clinical trials [COUGAR-02 (ISRCTN13366390), WJOG 4007 (NCT01224652) and AIO (NCT00144378)], with ORRs of 15%-25%, a mPFS of 2-3 months, and a mOS of 8-9 months [361-363].
Anti-angiogenic targeted drugs have a place in the second-line and subsequent treatment for EAC. Ramucirumab, a VEGFR antibody, combined with paclitaxel, improved OS compared to paclitaxel alone in the second-line treatment of advanced GEJ/G adenocarcinoma after first-line chemotherapy in the RAINBOW study (NCT01170663), and has been recommended for patients regardless of HER2 status by the NCCN guideline [151, 327]. The FRUTIGA study (NCT03223376) showed that the combination of fruquintinib and paclitaxel prolonged mPFS compared to paclitaxel monotherapy (5.6 months vs. 2.7 months; HR = 0.57; 95% CI = 0.48-0.68; p < 0.0001) [328]. Phase III trial (NCT01512745) of apatinib for subsequent antitumor therapies in patients with chemotherapy-refractory GEJ adenocarcinoma indicated a co-endpoint OS benefit [364]. The combination of camrelizumab with apatinib and chemotherapy showed promising anti-tumor activity regardless of PD-L1 expression, with a mPFS of 6.5 months in a phase II study (NCT04345783) [365]. Phase III trials combining anti-angiogenic targeted drugs with immunotherapy are ongoing (NCT04385550, NCT06341335). However, the combination of ICIs, such as cabozantinib combined with atezolizumab (COSMIC-021 study, NCT03170960), and the addition of tremelimumab to durvalumab plus chemotherapy (PRODIGE 59-FFCD 1707-DURIGAST trial, NCT03959293), has shown minimal activity preliminarily [366, 367].
These findings above highlight a significant unmet clinical need in later-line treatment of advanced EAC refractory to chemotherapy. Most importantly, the optimal approach for EAC that progresses after ICI treatment remains unknown, representing a critical gap in scientific and clinical knowledge. A retrospective analysis reveals promising response and survival with ramucirumab plus chemotherapy in patients with post-first-line checkpoint inhibitors and chemotherapy [368]. Further research is needed. Figure 8 presents the ongoing phase III studies of various lines of novel targeted agents in advanced EC.
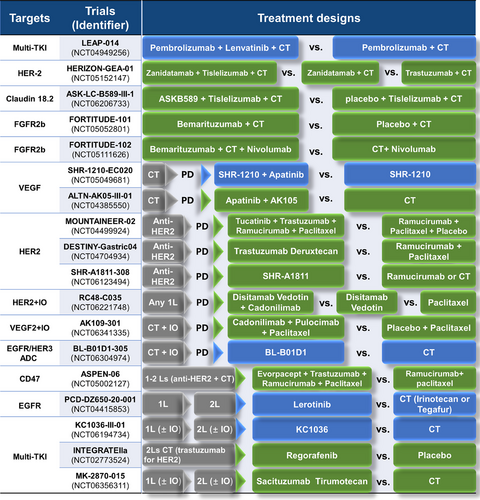
HER2 positive EAC
ADC drugs have also achieved breakthroughs. The DESTINY-Gastric01 study (NCT03329690) led to the approval of trastuzumab deruxtecan for second-line or later treatment of HER-2-positive GEJ/G adenocarcinoma, with an ORR of 51% and a mOS of 12.5 months [329]. Disitamab vedotin, developed in China, was approved based on the RC48-C008 study (NCT03556345) results, showing an ORR of 24.8% and a mOS of 7.9 months, even for patients with HER2 immunohistochemistry 2+ without Fluorescence In Situ Hybridization (FISH) testing, achieving an ORR of 23.0% and a mOS of 7.1 months at third-line [369]. Upcoming studies are set to investigate the synergy of HER-2 ADCs with immunotherapy (NCT06221748) and to determine the potential benefits of ADCs for patients with low HER-2 expression after progression on anti-HER2 therapy [DESTINY-Gastric04(NCT04704934), NCT06123494]. Besides, trophoblastic cell-surface antigen 2 (TROP2) ADCs are investigated for third-line or later treatment regardless of HER-2 status (NCT06356311).
The optimal approaches after progression on targeted drug regimens are actively being explored. In HER2-expressing patients who progressed after first-line trastuzumab-containing chemotherapy, DESTINY-Gastric02 (NCT04014075) supports the use of trastuzumab deruxtecan as second-line therapy with an ORR of 42% [330]. Other intensified anti-HER2 regimens, including ramucirumab plus trastuzumab and paclitaxel, as well as anti-HER2 bispecific antibody KN026, have also demonstrated substantial efficacy in HER-2 positive G/GEJ cancer by multicenter studies (NCT04888663, NCT03925974) [370, 371].
5.4.3 Local treatment for advanced EC
Although systemic therapy remains the standard of care for patients with advanced disease, the integration of local treatments, such as radiotherapy, rather than concurrent chemoradiotherapy, has significantly improved the management of local swallowing symptoms [372, 373]. In phase III clinical trials, despite the administration of efficacious chemoimmunotherapy, the mPFS for these patients without local radiotherapy remains approximately seven months [319]. Radiotherapy has been demonstrated to enhance survival in patients with advanced ESCC [374, 375]. The ESO-Shanghai 10 trial (NCT03000816) assessed the efficacy of stereotactic body radiotherapy (SBRT) in treating oligometastatic ESCC, revealing an mPFS of 13.3 months and a 2-year OS of 58.0% [376]. Subsequently, The ESO-Shanghai 13 trial (NCT03904927), employing a randomized controlled design, has conclusively demonstrated that the addition of local therapy for metastatic foci markedly improves the PFS in patients with oligometastatic ESCC undergoing systemic treatments, including immunotherapy [331]. Cohort study has demonstrated that cCRT for both primary and oligometastatic ESCC lesions as a first-line treatment had a superior survival to chemotherapy alone (mPFS: 9.7 months vs. 7.6 months) [377]. The ESO-Shanghai 20 study (NCT06190782) evaluates the combination of anti-PD-1 antibodies with radiotherapy for primary and metastatic sites in oligometastatic ESCC.
The AIO-FLOT3 trial (NCT00849615) also demonstrated a combination of chemotherapy followed by surgery yields favorable OS for oligometastatic G/GEJ adenocarcinoma [332]. The Delphi consensus advocates that the management of the primary tumor in oligometastatic EC should adhere to the guidelines for locally advanced EC [378]. The phase III AIO-FLOT5 (RENAISSANCE, NCT02578368) trial evaluating perioperative FLOT therapy followed by surgery of the primary tumor and metastatic lesions against palliative chemotherapy for EAC or GAC, and the ECOG-ACRIN EA2183 study (NCT04248452), which evaluating chemotherapy with consolidative radiotherapy to all sites of disease in oligometastatic EAC, are ongoing [379].
6 CONCLUSIONS
The landscape of esophageal cancer research is rapidly evolving, with significant advancements in understanding the disease's biology, improving diagnostic techniques, and developing more effective treatments. The shifting disease spectrum indicates preventive initiatives for EC have achieved certain success but also necessitate a reorientation of strategies for EC prevention. The advancement of endoscopic technology has enhanced the detection and diagnosis rates, however, non-invasive methods with more reliable biomarkers represent an increasingly popular focus. Liquid biopsy (circulating tumor DNA) and breath analysis for volatile organic compounds are under investigation. The optimal therapeutic strategy will be an individualized approach that combines multidisciplinary treatments, including the adaptive optimization of surgical strategies and novel radiotherapy approaches in both localized and advanced diseases. Novel strategies to further enhance anti-tumor immune responses, such as chimeric antigen receptor-based approaches, are under active development. AI is being employed to improve early detection through imaging analysis, predict treatment responses, and personalize patient care. With these endeavors, the path to conquering esophageal cancer advances further with each step.
AUTHOR CONTRIBUTIONS
Wei Jiang conceived the presented idea, investigated the literature, collected the data, and drafted the manuscript. Bo Zhang contributed to the literature collection and data extraction. Jiaqi Xu contributed to the creation of the figures. Liyan Xue contributed to the literature collection and data extraction. Luhua Wang supervised the findings of this work. All authors discussed the results and contributed to the final manuscript.
ACKNOWLEDGMENTS
This review is supported by the Key Project of In-hospital Research Subject at Shenzhen Hospital of Cancer Hospital, Chinese Academy of Medical Sciences (SZ2020ZD002), and the Young Scientists Fund of the National Natural Science Foundation of China (Grant No. 82202938).
CONFLICT OF INTEREST STATEMENT
We have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.
Not applicable.




