Ex vivo STAT3 phosphorylation in circulating immune cells: a novel biomarker for early cancer diagnosis and response to anti-PD-1 therapy
Sung-Woo Lee and Young Ju Kim contributed equally to this work.
Abbreviations
-
- IL
-
- Interleukin
-
- NK
-
- Natural killer
-
- NSCLC
-
- Non-small cell lung cancer
-
- PBMC
-
- Peripheral blood mononuclear cell
-
- PR
-
- Partial response
-
- pSTAT3ex vivo
-
- ex vivo phosphorylated signal transducer and activator of transcription 3
-
- TIL
-
- Tumor-infiltrating lymphocyte
-
- TME
-
- Tumor microenvironment
Basal signal transducer and activator of transcription 3 (STAT3) activation is well-documented in the tumor microenvironment (TME) due to its association with cancer prognosis [1]. However, its presence and clinical relevance in the bloodstream remain unexplored. Given that STAT3-inducing cytokines, such as interleukin-6 (IL-6), are often elevated in the bloodstream of various cancer patients [2, 3], we aimed to investigate basal STAT3 activation in blood by developing a methodology to assess ex vivo phosphorylation of STAT3 (pSTAT3ex vivo) in circulating immune cells.
Since phosphorylation is a transient process prone to dephosphorylation, we sought to minimize the time between blood collection and the experiment. Specifically, 1) we limited the use of peripheral blood mononuclear cell (PBMC) samples to those processed within 1 hour of blood collection, and 2) immediately fixed the samples after thawing (Figure 1A). Notably, 135 non-small cell lung cancer (NSCLC) patient samples processed in this way exhibited significantly higher levels of pSTAT3ex vivo-positive cells compared to healthy controls (Figure 1B and Supplementary Table S1). Prolonged handling and extended experimental steps significantly decreased pSTAT3ex vivo expression (Supplementary Figure S1), underscoring the importance of our novel approach in controlling the time between blood collection and the experiment.
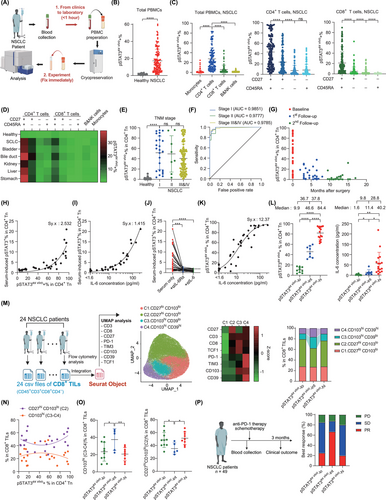
We next investigated the cell types within PBMCs that express pSTAT3ex vivo. CD4+ T cells exhibited the highest pSTAT3ex vivo expression, followed by CD8+ T cells, whereas monocytes, B cells, and natural killer (NK) cells showed minimal pSTAT3ex vivo expression (Figure 1C). Within both CD4+ and CD8+ T cells, pSTAT3ex vivo expression was highest in the least differentiated CD27+ CD45RA+ naïve subset (Figure 1C) [4]. A similar pattern was observed across multiple other cancer types (Figure 1D and Supplementary Figure S2).
Focusing on CD4+ naïve T cells, pSTAT3ex vivo expression showed a stark contrast between NSCLC patients and healthy donors, even at stage I (Figure 1E). The area under the receiver operating characteristic curve for distinguishing stage I NSCLC patients from healthy donors was 0.9851, with a sensitivity of 0.92 at 95% specificity (Figure 1F). No tumor-specific or patient-specific clinical variables correlated with pSTAT3ex vivo expression in NSCLC patients (Supplementary Figure S3), while surgical removal of the tumor decreased pSTAT3ex vivo expression (Figure 1G), supporting a direct association between pSTAT3ex vivo and tumor burden. These findings underscore the potential of pSTAT3ex vivo as a blood-based diagnostic biomarker for early cancer detection, particularly useful in screening for cancer before proceeding to more invasive standard confirmation methods.
Next, we investigated the inducer of pSTAT3ex vivo. Among the various cytokines tested, in vitro IL-6 stimulation of STAT3 best matched the pSTAT3ex vivo expression profiles in NSCLC patients (Supplementary Figure S4A-C). To confirm the involvement of IL-6 in pSTAT3ex vivo expression, we cultured healthy PBMCs with patient serum and assessed pSTAT3 expression. Serum-induced pSTAT3 expression tightly correlated with both the pSTAT3ex vivo levels in the serum donors and the IL-6 concentration in the corresponding serum (Figure 1H-I). Importantly, the addition of anti-IL-6Rα or anti-IL-6 antibodies to the serum completely abrogated pSTAT3 induction (Figure 1J and Supplementary Figure S4D). These findings strongly suggest that IL-6 is the predominant inducer of pSTAT3ex vivo expression.
Given the role of IL-6 in inducing pSTAT3ex vivo, we further investigated their relationship. Notably, the two parameters closely followed a dose-response curve, with a half-maximal effective concentration of 11.63 pg/mL (Figure 1K) [5]. Interestingly, this value is 1000-fold lower than previous in vitro estimates [6, 7] providing strong evidence that serum IL-6 can actively induce the signaling cascade. Moreover, the dose-response curve suggests that biological activity correlates with the logarithmic form of IL-6 concentration. Accordingly, while patients with low (0%-20%), intermediate (21%-60%), and high (61%-100%) pSTAT3ex vivo (pSTAT3ex vivo-lo, pSTAT3ex vivo-int, and pSTAT3ex vivo-hi, respectively) showed a stepwise increase in pSTAT3ex vivo expression, IL-6 levels in the pSTAT3ex vivo-hi group were markedly higher than in the other two groups, rendering them relatively similar (Figure 1L). These results suggest that measuring pSTAT3ex vivo expression provides a more accurate representation of systemic IL-6 activity—its capacity to trigger intracellular signaling cascades—than direct IL-6 measurements.
Given the potential of pSTAT3ex vivo as a marker of systemic IL-6 activity, we investigated the relationship between pSTAT3ex vivo and immune cell populations. Initially, we compared pSTAT3ex vivo expression with the distribution of immune cell populations in peripheral blood. However, we did not observe any gross alterations (Supplementary Figure S5). We then explored immune cell populations within the TME. Notably, the frequency of M2-like CD206hi tumor-associated macrophages and CD39hi regulatory T cells correlated with pSTAT3ex vivo and were most abundant in the pSTAT3ex vivo-hi group (Supplementary Figure S6A-G), suggesting a link between systemic IL-6 activity and the formation of immunosuppressive TMEs.
Interestingly, the distribution of CD8+ tumor-infiltrating lymphocytes (TILs) showed a unique relationship with pSTAT3ex vivo expression. We performed uniform manifold approximation and projection analysis on CD8+ TILs by integrating flow cytometry data from 24 NSCLC patients (Figure 1M). Among the four CD8+ TIL clusters, those with high CD103 expression (C3.CD103hiCD39lo and C4.CD103hiCD39hi) were specifically elevated in the pSTAT3ex vivo-int group, whereas the C2.CD27hiCD103lo cluster was reduced (Figure 1N-O and Supplementary Figure S6H). Given that CD103+CD8+ TILs include tumor-reactive cells (Supplementary Figure S7) [8, 9], these results suggest that the accumulation of tumor-reactive CD8+ T cells within tumors increases at a specific range of systemic IL-6 activity but diminishes at higher levels.
Given the strong correlation between pSTAT3ex vivo expression and key elements of anti-tumor immunity, we hypothesized that pSTAT3ex vivo expression could serve as a prognostic marker for cancer immunotherapy. We analyzed pSTAT3ex vivo expression in pre-therapy PBMCs from 49 NSCLC patients who received anti-PD-1 therapy (± chemotherapy) (Supplementary Table S2). The pSTAT3ex vivo-hi group showed the worst response (partial response (PR) 20%; Figure 1P), consistent with their highly immunosuppressive TMEs. Notably, the pSTAT3ex vivo-int group exhibited significantly better outcomes than pSTAT3ex vivo-lo (PR 66.7% versus 25%; Figure 1P), suggesting a potential connection with CD103+CD8+ T cells. Moreover, the biomarker performance of pSTAT3ex vivo was significantly greater than tumor PD-L1 expression (Supplementary Figure S8). These findings suggest an unappreciated non-linear relationship between systemic IL-6 activity and anti-PD-1 responsiveness.
Collectively, these findings suggest that systemic IL-6, despite its extremely low concentration (median ∼10 pg/mL) [3], can actively induce the STAT3 signaling cascade in vivo and modulate anti-tumor immunity. Our refined methodology enabled quantification of systemic IL-6 activity as pSTAT3ex vivo, which could serve as a biomarker for cancer diagnosis and a predictor of responsiveness to anti-PD-1 therapy. Overall, our study offers new avenues for exploring systemic cytokines in various disease models, as demonstrated in our cancer patient cohort.
AUTHOR CONTRIBUTIONS
Sung-Woo Lee and Jae-Ho Cho administered this project; Sung-Woo Lee, Young Ju Kim, and Jae-Ho Cho conceptualized, designed methodologies, performed formal analysis, curated data, and wrote the original draft; Sung-Woo Lee, In-Jae Oh, and Jae-Ho Cho supervised, acquired funding, reviewed and edited the manuscript; Saei Jeong, Kyung Na Rho, Jeong Eun Noh, Hee-Ok Kim, Hyun-Ju Cho, Yoo Duk Choi, Deok Hwan Yang, Eu Chang Hwang, Woo Kyun Bae, Sook Jung Yun, Ju Sik Yun, Cheol-Kyu Park, and In-Jae Oh curated resources.
ACKNOWLEDGEMENTS
We thank Biobank of Chonnam National University, Hwasun Hospital for providing patient biospecimens; and the Korean Red Cross for providing healthy blood samples.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
FUNDING INFORMATION
This work is funded by National Research Foundation of Korea (2020R1A5A2031185, 2020M3A9G3080281, 2022R1A2C2009385, 2020M3A9G3080330, and 2022R1A6A3A01086438).
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the Institutional Review Boards of Chonnam National University Medical School and Hwasun Hospital (CNUHH-2022-021 and CNUHH-2024-034). All cancer patients provided written informed consent. Written informed consent from healthy donors from the Korean Red Cross was formally waived in accordance with the Korean Bioethics and Safety Act.
Open Research
DATA AVAILABILITY STATEMENT
All data are included within the article and its supplemental information.