IL2-mediated modulation of small extracellular vesicles secretion and PD-L1 expression: a novel perspective for neutralizing immune suppression within cancer cells
List of Abbreviations
-
- Con
-
- control
-
- cPD-L1
-
- cellular programmed death-ligand 1
-
- ePD-L1
-
- exosomal programmed death-ligand 1
-
- IL2
-
- Interleukin-2
-
- IL2R
-
- Interleukin-2 receptor complex
-
- IL2-T
-
- Interleukin-2-tethered
-
- PD-1
-
- programmed cell death-1
-
- sEV
-
- small extracellular vesicle
-
- WT
-
- wide type
Cancer cells secrete small extracellular vesicles (sEVs) to regulate various cellular functions, like tumor growth and metastasis, by promoting epithelial-mesenchymal transition and angiogenesis [1, 2]. Additionally, cancer cells evade immune surveillance by upregulating the surface expression of cellular programmed death-ligand 1 (cPD-L1), which interacts with programmed cell death-1 (PD-1) on cytotoxic T cells, suppressing immune responses [3]. Moreover, cancer cells release sEVs displaying PD-L1 on their surface [4]. Cancer-derived exosomal PD-L1 (ePD-L1), similar to cPD-L1 in cancer cells, can hinder immune cell activation, inducing an immunosuppressive tumor microenvironment [5]. Therefore, modulating sEV secretion or PD-L1 expression in cancer cells may be a crucial strategy for altering the tumor microenvironment.
Herein, we conducted a screening to identify natural factors regulating sEV secretion from cancer cells and discovered that Interleukin-2 (IL2) predominantly reduces sEV secretion in B16F10 cells (Supplementary Figure S1). IL2, a commonly used FDA-approved therapy for melanoma, typically exerts anticancer effects by activating immune cells expressing the IL2 receptor complex (IL2R), consisting of IL2RA, IL2RB, and IL2RG [6-8]. Remarkably, IL2R is also expressed in certain types of cancer cells, including melanoma cells. However, the potential impact of IL2 on IL2R-expressing cancer cells has not been thoroughly investigated.
To explore the effects of IL2 on cancer cells, we utilized mouse and human melanoma cells expressing IL2R (Supplementary Figure S2A). We treated these melanoma cells with IL2 and subsequently isolated sEVs to assess the impact of IL2 on sEV secretion (Supplementary Figure S2B-D). IL2 reduced the number of secreted sEVs compared to the PBS control (Figure 1A) and significantly decreased the expression of Rab GTPases, which regulate sEV biogenesis and secretion in melanoma cells (Figure 1B and Supplementary Figure S2E). This effect presents a different pattern from the previously known effects of IL2 on sEV regulation in immune cells [9].
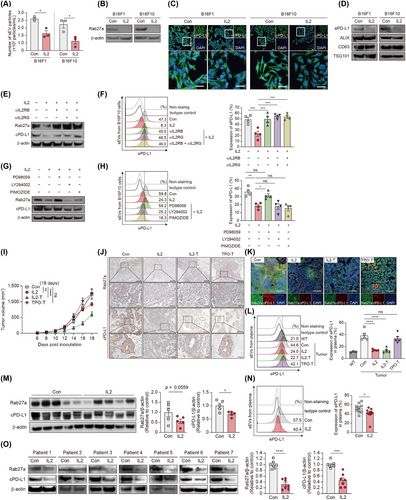
In addition to examining the effects of IL2 on cancer cell-secreted sEVs, we also investigated its impact on immune checkpoints, which are crucial regulators of immune surveillance [5]. Interestingly, IL2 treatment significantly reduced cPD-L1 levels among immune checkpoints in melanoma cells without affecting cell proliferation (Figure 1C and Supplementary Figure S3). IL2 treatment significantly reduced ePD-L1 expression in melanoma cell-derived sEVs, with equal sEV amounts confirmed by sEV markers (Figure 1D and Supplementary Figure S4A-B). Next, we assessed whether regulation of ePD-L1 by IL2 affects CD8+ T cell activity. sEVs from PBS-treated B16F10 cells significantly reduced Granzyme B levels in CD8+ T cells compared to control, whereas sEVs from IL2-treated B16F10 cells did not. The inhibitory effects of sEVs on CD8+ T cell activation were abolished by anti-PD-L1 antibodies (Supplementary Figure S4C). Furthermore, we observed that IL2-mediated reduction of ePD-L1 derived from melanoma cells enhanced the cytotoxicity of CD8+ T cells in co-culture with cancer cells (Supplementary Figure S4D-E). These data suggest that the decrease in ePD-L1 caused by IL2 promotes CD8+ T cell cytotoxicity and cancer cell death.
Subsequently, we analyzed whether the effects of IL2 in cancer cells depend on IL2R signaling to uncover the molecular mechanism of IL2 action. The formation of the IL2RB-IL2RG complex, regardless of IL2RA, is crucial for IL2 signal transduction [7]. Neutralizing antibodies against IL2RB and IL2RG restored IL2-mediated reduction in Rab27a expression, sEV secretion, and expression of both cPD-L1 and ePD-L1 (Figure 1E-F, Supplementary Figure S5A-C). Similarly, IL2RB knockdown abolished IL2-mediated regulation in cancer cells (Supplementary Figure S5D-F). Next, we explored the intracellular signaling pathways through which IL2 exerts its effects in melanoma cells [6-8]. IL2 potently increased ERK phosphorylation in melanoma cells, while STAT5 and AKT phosphorylation were unaffected (Supplementary Figure S6A). Co-treatment of melanoma cells with IL2 and a MEK inhibitor abrogated the inhibitory effects of IL2 on sEV secretion and PD-L1 expression, unlike treatment with a STAT5 inhibitor or a PI3K inhibitor (Figure 1G-H and Supplementary Figure S6B-C). Melanoma cells expressing dominant-negative ERK1 showed no alteration in phosphorylated ERK, Rab27a and PD-L1 levels upon IL2 treatment (Supplementary Figure S6D-F). Furthermore, the increased sensitivity of melanoma cells to T cell-mediated cytotoxicity induced by IL2 was attenuated by the MEK inhibitor (Supplementary Figure S6G). Taken together, these results suggest that IL2 regulates melanoma cell function through an IL2R-MEK/ERK axis.
Next, we examined whether the effects of IL2 on cancer cells observed at the cellular level extended to mouse tumor models. Conventional intravenous or intratumoral injection of IL2 in cancer-grafted mouse models fails to induce selective activation of IL2R signaling in transplanted cancer cells. To address this limitation, we developed a novel method to analyze the cancer cell-specific effects of IL2 in vivo by anchoring IL2 to the cancer cell plasma membrane using a flexible linker, enabling self-stimulation without diffusion [10] (Supplementary Figure S7). Applying this method, we observed a remarkable reduction in tumor growth without changes in body weight in both IL2-treated and IL2-tethered (IL2-T) mice, but not in control mice (Figure 1I and Supplementary Figure S8A-D). Compared to control mice, IL2-T mice, like soluble IL2-treated mice, showed reduced expression of Rab27a and cPD-L1 in tumor tissue and decreased levels of ePD-L1 in plasma (Figure 1J-L and Supplementary Figure S8E-F). This indicates that cancer cell-specific stimulation by IL2 can limit cancer cell activity, such as sEVs secretion and PD-L1 expression, similar to systemic IL2 administration.
In the previous syngeneic mouse tumor model, PD-L1 could be expressed in both cancerous and non-cancerous cells, which led to uncertainty about the origin of plasma ePD-L1 (Figure 1L). To specifically analyze cancer cell-derived ePD-L1, human cancer cells were transplanted into nude mice, and human ePD-L1 levels were measured. As expected, IL2 significantly suppressed Rab27a and PD-L1 expression in tumor tissues (Figure 1M and Supplementary Figure S9). Consistent with the syngeneic mouse results, IL2 treatment significantly reduced plasma levels of human melanoma cell-derived ePD-L1 (Figure 1N and Supplementary Figure S10). These data indicate that IL2 can decrease cancer cell-derived ePD-L1 in vivo.
To extend these findings to other cancer types, we investigated IL2's effects on human lung cancer cells. IL2 treatment downregulated Rab27a and PD-L1 levels in IL2R-expressing lung cancer cells, but not in IL2R-non-expressing lung cancer cells (Supplementary Figure S11). Additionally, we examined patient-derived lung cancer cells and found that IL2 significantly decreased Rab27a and cPD-L1 levels in IL2R-expressing lung cancer samples from seven donors (Figure 1O and Supplementary Figure S12). Our data suggest that IL2's effects on cancer cells are conserved across human, mouse, and heterogeneous cell types, providing new clinical insights into IL2 mechanisms.
Current studies on IL2 have predominantly focused on immune cells; however, its key functions in non-immune cells, particularly cancer cells, remain poorly understood. This study demonstrates that IL2 acts directly on cancer cells, reducing sEV secretion and PD-L1 expression through the IL2R-MEK/ERK signaling. In mouse tumor model, melanoma cell-specific IL2 stimulation strongly inhibited tumor growth and decreased Rab27a and cPD-L1 expression in tumor tissues, along with ePD-L1 levels in plasma. Furthermore, the efficacy of IL2 was confirmed in patient-derived lung tumor cells, suggesting potential clinical implications for IL2 in patients with IL2R-expressing tumors. These findings provide insights that may broaden the clinical applicability of IL2 by suggesting novel immuno-oncology mechanisms. Further investigations into the direct effects of IL2 on IL2R-expressing cancers may warrant reconsideration of IL2 as a significant treatment option for various cancers.
AUTHOR CONTRIBUTIONS
Soojeong Noh: Experimental design; performance; investigation and writing manuscript. Suyeon Ryu: Conceptualization; Experimental design; performance and investigation. Dokyung Jung: Formal analysis and investigation. Sanghee Shin: Investigation and writing manuscript. Inseong Jung: Formal analysis and writing manuscript. Sung-Min Kang: Investigation and writing manuscript. Christine Seulki Kim: Formal analysis and writing manuscript. Sung-Jin Choi: Investigation. Hanchae Cho: Investigation and writing manuscript. Melanie Schwämmle: Writing manuscript. Youngtae Jeong: Writing manuscript. Felicitas Bucher: Writing manuscript. Il-Kyu Choi: Funding and writing manuscript. Shin Yup Lee: Experimental design and writing manuscript. Sin-Hyeog Im: Experimental design and writing manuscript. Kyungmoo Yea: Conceptualization; experimental design; funding and writing manuscript. Moon-Chang Baek: Conceptualization; experimental design; funding and writing manuscript.
ACKNOWLEDGMENTS
We thank A. Becker and J.-I Park at Medical College of Wisconsin for sharing the pCEP4-ERK1-K71R vectors.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
FUNDING INFORMATION
This study was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) of Korea funded by the Korean government (MSIT) (2022R1A2C109214511 and 2020M3A9I4039539), the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2021R1A5A2021614, 2023R1A2C3005553), and the DGIST Program of the Ministry of Science and ICT (21-DGRIP-01).
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All animal experiments were conducted according to protocols approved by the Daegu Gyeongbuk Institute of Science and Technology (DGIST) Institutional Animal Care and Use Committee (Approval number, 20071501-010100-0000). Human cell line sample analysis was also approved by the DGIST Institutional Review Board (Approval number, DGIST-20210715-HR-121-01), and the applicable ethical guidelines were strictly followed. Lung tumor resections of seven patients were obtained from Kyungpook National University Hospital (KNUH). The biospecimens and data used for this study were provided by the Biobank of Korea-KNUH, a member of the Korea Biobank Network. All materials derived from the National Biobank of Korea-KNUH were obtained (with informed consent) under institutional review board (Approval number, 2022-02-011) -approved protocols (project No.2024-ER0506-00).
Open Research
DATA AVAILABILITY STATEMENT
pCEP4-ERK1-K71R vectors were obtained under an MTA from Medical College of Wisconsin. All other data are available in the manuscript and Supplementary materials.




