Dysfunction of dendritic cells in tumor microenvironment and immunotherapy
Jie Chen and Yuhang Duan equally contribute to this work.
Abstract
Dendritic cells (DCs) comprise diverse cell populations that play critical roles in antigen presentation and triggering immune responses in the body. However, several factors impair the immune function of DCs and may promote immune evasion in cancer. Understanding the mechanism of DC dysfunction and the diverse functions of heterogeneous DCs in the tumor microenvironment (TME) is critical for designing effective strategies for cancer immunotherapy. Clinical applications targeting DCs summarized in this report aim to improve immune infiltration and enhance the biological function of DCs to modulate the TME to prevent cancer cells from evading the immune system. Herein, factors in the TME that induce DC dysfunction, such as cytokines, hypoxic environment, tumor exosomes and metabolites, and co-inhibitory molecules, have been described. Furthermore, several key signaling pathways involved in DC dysfunction and signal-relevant drugs evaluated in clinical trials were identified. Finally, this review provides an overview of current clinical immunotherapies targeting DCs, especially therapies with proven clinical outcomes, and explores future developments in DC immunotherapies.
List of abbreviations
-
- DCs
-
- Dendritic cells
-
- TME
-
- tumor microenvironment
-
- MHC
-
- major histocompatibility complex
-
- PPARα
-
- peroxisome proliferator-activated receptor α
-
- VEGF
-
- vascular endothelial-derived growth factor
-
- PGE2
-
- prostaglandin E2
-
- NK
-
- natural killer
-
- HIF-1
-
- hypoxia-inducible factor 1
-
- ER
-
- endoplasmic reticulum
-
- ROS
-
- reactive oxygen species
-
- TEXs
-
- tumor exosomes
-
- EOC
-
- epithelial ovarian cancer
-
- m6A
-
- N6-methyadenosine
-
- YTHDF1
-
- YT521-B homology (YTH) domain-containing family protein 1
-
- LRP5/6
-
- lipoprotein receptor-related protein 5/6
-
- UM
-
- uveal melanoma
-
- TLR
-
- toll-like receptor
-
- CAR-T
-
- chimeric antigen receptor T
-
- MDA5
-
- melanoma differentiation-associated protein 5
-
- XCR1
-
- X-C chemokine receptor 1
-
- TRP2
-
- tyrosinase-related protein 2
-
- BsAb
-
- bispecific antibody
-
- EGFR
-
- epidermal growth factor receptor
-
- T-BsAbs
-
- T cell-engaging BsAbs
-
- MIP-β
-
- macrophage inflammatory protein-1 beta
-
- HIV
-
- human immunodeficiency virus
-
- TAAs
-
- tumor-associated antigens
-
- ORR
-
- objective response rate
-
- DCR
-
- disease control rate
-
- HNSCC
-
- head and neck squamous cell carcinoma
-
- ISG
-
- interferon-stimulated gene
-
- PORCN
-
- porcupine O-acyltransferase
-
- imDCs
-
- immature dendritic cells
-
- cDC1
-
- conventional dendritic cell 1
-
- cDC2
-
- conventional dendritic cell 2
-
- PD-1
-
- programmed cell death 1
-
- CTL
-
- cytotoxic T lymphocytes
-
- IFN-γ
-
- interferon-γ
-
- IL
-
- interleukin
-
- CTLA-4
-
- cytotoxic T-lymphocyte antigen-4
-
- CD
-
- cluster of differentiation
-
- IDO
-
- indoleamine 2,3-dioxygenase
-
- MDSCs
-
- myeloid-derived suppressor cells
-
- Treg
-
- regulatory T cell
-
- TAMs
-
- tumor-associated macrophages
-
- CD40L
-
- CD40 ligand
-
- CCR7
-
- CC-chemokine receptor 7
-
- PD-L1
-
- programmed cell death 1 ligand 1
-
- GM-CSF
-
- granulocyte-macrophage colony-stimulating factor
-
- Poly (I:C)
-
- polyinosinic-polycytidylic acid
-
- ICBs
-
- immune checkpoint blockers
-
- MoDC
-
- monocyte-derived DC
-
- mRNA
-
- messenger ribonucleic acids
-
- NCT
-
- national clinical trial
-
- BRAF
-
- v-raf murine sarcoma viral oncogene homolog B
-
- DDK1
-
- Dickkopf-1
-
- Poly-ICLC
-
- polyinosinic-polycytidylic acid-poly-l-lysine carboxymethylcellulose
-
- MUC1
-
- mucin 1
-
- p53
-
- protein 53
-
- TCR
-
- T cell receptor
-
- CMV
-
- cytomegalovirus
-
- Her2
-
- human epidermal growth factor receptor 2
-
- ASA
-
- acetylsalicylic acid
-
- OCDC
-
- ovarian cancer dendritic cell vaccine (a personalized whole-tumor lysate-pulsed dendritic cell vaccine)
-
- Bev
-
- bevacizumab
-
- Cy
-
- cyclophosphamide
-
- CBP
-
- cAMP response element-binding protein-binding protein
-
- Bcl-2
-
- B cell lymphoma 2
-
- iDCs
-
- immature DCs
-
- HIF-α
-
- hypoxia-inducible factor-α
-
- PI3K
-
- phosphoinositide 3-kinase
-
- Akt
-
- protein kinase B
-
- Wnt/β-catenin
-
- wingless-related integration site/β-catenin
1 BACKGROUND
Dendritic cells (DCs) are derived from CD34+ hematopoietic stem cells of the bone marrow and are widely distributed; however, they exist as a rare population in most organs except the brain [1, 2]. DCs play important roles in bridging innate and adaptive immunity and maintaining tolerance [3], and are involved in different biological functions, such as antigen presentation, immune cells migration, cytokine release, anti-viral and anti-tumor activities, immune modulation, and phenotypic changes by expressing different surface markers [4, 5]. Therefore, cancer therapies that targeting DCs have gained considerable attention. Antigen recognition is essential for DC priming. Apoptotic cells, which are recognized as immunogens by the DCs, have been developed as antigens for DC vaccines for many years, with potential clinical efficacy [6]. In addition, tumor antigens, including antigen peptides and tumor lysates, were generated and co-cultured with DCs to activate the immune system. Numerous clinical trials focusing on DC therapy, exploring their role as an adjuvant, vaccine, or agonist, are currently underway to evaluate the efficiency of DCs in inhibiting cancer. DC vaccines pulsed with personalized neoantigens for advanced lung cancer have demonstrated high disease control rates (DCR) in clinical trials (75%) [7]. Moreover, clinical trials on glioblastoma therapy have confirmed the safety and clinical efficiency of DC vaccines [8, 9]. However, DCs often appear dysfunctional in the tumor microenvironment (TME), which affects T-cell function in certain situations. For example, DCs in the central and peripheral lymphoid organs induced antigen-specific tolerance or unresponsiveness and generated tolerance by eliminating self-reactive T cells in the thymus [10]. In gliomas, mutations resulted in specifically altered and dysfunctional DCs that limited antigen-specific T cell responses [11]. Antigen-loaded immature DCs silenced T cells either by eliminating them or expanding regulatory T cells [12], which impaired the anti-tumor activity of some immunotherapies. Therefore, the dysfunction of DCs in the TME inhibited the immune system against cancer.
Various factors can inhibit DCs’ dysfunction in the TME, including co-suppressor molecules, oncometabolites, hypoxic environments, and tumor exosomes (TEXs) [13, 14]. Diverse mechanisms of DC dysfunction from different aspects have been previously reported. A 2005 review enumerated tumor-derived factors and revealed that immune suppression in cancer could be attributed to alterations in DC differentiation, maturation, and longevity [15]. Another review published in 2017 summarized the molecular mechanisms of DC dysfunction and discussed the effects of DC ontogeny and DC subset heterogeneity on DC recruitment, differentiation, and function [16]. The role of different DC subsets in cancer therapy has been summarized, and DCs’ dysfunction induced by major histocompatibility complex-1 (MHC-I) and MHC-II pathways has been discussed [17]. A strategy to overcome antigen presentation defects was also proposed in this review [17]. This summary and analysis of DC dysfunction in cancer therapy may provide potential insights for drug discovery. Peroxisome proliferator-activated receptor α (PPARα) inhibition could overcome DC dysfunction induced by tumor-derived exosomal lipid [18]. Therefore, recent advancements in understanding and addressing DC dysfunction have facilitated the development of novel approaches to cancer immunotherapy. This review comprehensively analyzed the recent advancements in DC dysfunction in the TME and described the crucial inhibitory signal transmission that impairs antigen presentation ability of DCs. Based on this, the current DC therapies in clinical trials have been summarized, and the possible combination approaches with other immunotherapeutic reagents have been discussed. This review aims to offer valuable insights into the research and development of novel and efficient therapies targeting DCs for refractory and relapsed cancers.
2 DYSFUNCTION OF DCS IN THE TME
The TME, which is associated with tumor growth, differentiation, and proliferation, has become a popular topic in cancer research. The TME can cause dysfunction in various immune cells [19]. DCs in the TME can activate T cells via antigen presentation (Figure 1, Pathway A). However, the inhibition of anti-tumor activity by the TME includes diverse perspectives [15], such as inducing the expression of large amounts of immunosuppressive ligands (Figure 1, Pathway B), inhibiting the maturation of DCs, causing abnormal differentiation of DCs (Figure 1, Pathway C), and producing tumor-associated metabolites (Figure 1, Pathway D) [15, 20, 21]. The different perspectives on DC dysfunction have been elaborated in the following subsections.
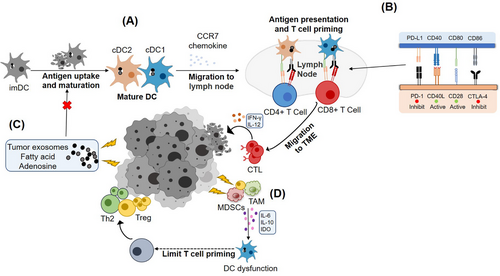
Immune regulation of DCs in the TME. (A) Under normal conditions, cDC1and cDC2 presented tumor antigens to CD8+ and CD4+ T cells, respectively, and activated cytotoxic T cells released IFN-γ and IL-12 to promote tumor lysis. (B) T cells activation was positively and negatively regulated by co-stimulatory molecules on DCs, and the upregulation of CTLA-4 receptors and downregulation of CD40L in DCs in the TME inhibited T cells activation. (C) The hypoxic environment in the TME promotes the production of tumor-associated metabolites, which inhibits the maturation of DCs and impairs antigen-presenting function of DCs to tumor cells. (D) Anti-inflammatory cytokines such as IDO, IL-6, IL-10, released by related pro-oncogenic Treg, TAM, and MDSCs impair the function of DCs.
Abbreviations: imDCs, immature dendritic cells; cDC1, conventional dendritic cell 1; cDC2, conventional dendritic cell 2; PD-1, programmed cell death 1; CTL, cytotoxic T lymphocytes; IFN-γ, interferon-γ; IL, interleukin; CTLA-4, cytotoxic T-lymphocyte antigen-4; CD, cluster of differentiation; IDO, indoleamine 2,3-dioxygenase; DC, dendritic cell; MDSCs, myeloid-derived suppressor cells; Treg, regulatory T cell; TAM, tumor associated macrophage; CD40L, CD40 ligand; CCR7, CC-chemokine receptor 7; PD-L1, programmed cell death 1 ligand 1; TME, tumor microenvironment; Th2, type 2 T helper cells.
2.1 DC dysfunction influenced by cytokines
Cancer cells, myeloid-derived suppressor cells, tumor-associated macrophages (TAMs), regulatory T cell (Treg) and other cells produce various interleukins (ILs) (such as IL-6, IL-10, and IL-13), indoleamine 2,3-dioxygenase (IDO), growth factors (vascular endothelial growth factor [VEGF], granulocyte-macrophage colony-stimulating factor [GM-CSF]), and other factors (ganglioside and prostaglandin E2 [PGE2]) in the TME may influence or even damage the function of DCs (Figure 1, Pathway D) [22-24].
In some immunological reactions, such as inflammation stimulated by lipopolysaccharide, Treg cells secrete a large amount of the cytokine IL-10 in the TME [25], which not only reduces the expression of B cell lymphoma 2 (Bcl-2) in DCs but also inhibits the differentiation of monocytes into DCs [26]. In addition to IL-10, the abnormal expression of IL-6 by tumor-infiltrating DCs affects the differentiation of T helper cells to type 2 T helper cells, which impairs immune responses against pathogens [27]. Tumor-derived versican is a chondroitin sulfate proteoglycan that binds to toll-like receptor 2 to enhance the autocrine function of IL-6 and IL-10, and the upregulation of their respective cell surface receptors can lead to DC dysfunction, such as IL-10-producing conventional dendritic cells (cDC) [28-31]. Moreover, massive abnormally secreted bioactive substances in the TME, such as GM-CSF, gangliosides, and interferon-γ (IFN-γ), dysregulated DC maturation, inhibited the antigen presentation ability of DCs, and subsequently suppressed the immune response [32-34]. VEGF, another growth factor, inhibited DC differentiation and maturation [35], and treatment with anti-VEGF antibodies alleviated DC dysfunction in the TME [22, 36]. In addition, PGE2, an inflammatory mediator that accumulates in the TME, inhibited the antigen presentation function of DCs and attenuated the activation of natural killer (NK) cells, cytotoxic T lymphocytes (CTL), and other immune cells [37, 38]. Cytokines, as crucial regulators of the immune system, also play critical roles in the function of DCs, especially negative regulatory cytokines that induce DC dysfunction.
2.2 DC dysfunction mediated by hypoxic environment
As tumors demonstrate rapid growth, they require an increased amount of nutrients and oxygen; however, the blood vessels within the tumor failed to transport sufficient nutrients to the inner region of the tumor, leading to the development of a hypoxic environment and the production of numerous metabolic byproducts within the tumor cells [39-41]. Immune cell function was limited by several factors in the TME, such as oxygen, pH, glycogen, and metabolic byproducts (Figure 1, Pathway C) [14, 24, 39, 40, 42, 43].
Hypoxia-inducible factor 1 (HIF-1), a heterodimeric DNA-binding transcription factor, negatively regulates DC function under hypoxic conditions [39, 44]. In the hypoxic environment, chemokine receptors (CC-chemokine receptor 2/3/5, CX3C chemokine receptor 1, C5a receptor gene 1, and formyl peptide receptor 3) were up-regulated to polarize immature DCs (iDCs) into a migrating phenotype [45], which was mediated by the hypoxia-inducible factor-α (HIF-α) to enhance the migration ability of hypoxic iDCs via phosphoinositide 3-kinase/ protein kinase B signaling [46-48]. Hypoxic DCs down-regulated the expression of RhoA/Ezrin-Radixin-Moiesin and lectin receptor cluster of differentiation 206 (CD206), which impaired the ability of DCs to capture antigens [46]. Moreover, the secretion of VEGF and IL-10 mediated by HIF-αinhibited DC function [48-50].
Moreover, the hypoxic TME continuously activated the endoplasmic reticulum (ER) stress factor X-box binding protein l due to mutations in oncogenes and tumor metabolism [45, 51]. This induced the ER stress response of the unfolded protein response, and ultimately damaged the antigen processing and presentation functions of DCs [52]. Under normal conditions, low levels of reactive oxygen species (ROS) mediated DNA oxidation enhanced the immune recognition of DCs [53]. However, the secretion of nicotinamide adenine dinucleotide phosphate oxidase in DCs induced by high ROS level contributed to proton depletion in the phagosome and eventually increased the intracellular pH, which inhibited antigen presentation by DCs [54, 55]. Therefore, further studies on the regulatory mechanisms of ROS and hypoxia in the TME are warranted to strategize effective therapies [45].
2.3 Immune tolerance of the DCs mediated by TEXs
TEXs are extracellular membrane vesicles secreted by tumors [56] that contain various soluble components such as micro ribonucleic acids, proteins, enzymes, lipids, and cytokines [56, 57]. Hypoxia increased exosome secretion from epithelial ovarian cancer (EOC) cells. These exosomes were engulfed by unpolarized macrophages, and the TAM phenotype was adjusted to promote the progression of EOC [58]. Moreover, TEXs secreted during ferroptosis of cancer cells inhibited the maturation of DCs and impaired antigen cross-presentation [59]. In addition, fatty acid-carrying tumor-derived exosomes induced dysfunctional DCs to facilitate immune evasion, which was reversed by PPARα inhibition [18]. Based on the mechanisms of TEXs, cancer-derived TEXs as antigens in DC vaccines may exert more potential effects on DC maturation and cross-presentation of MHC molecules than those of conventional tumor-associated antigen (TAA) lysates [60]. In summary, although TEXs exerted a negative effect on DCs, they were potential targets used not only as drug delivery materials but also as anti-tumor DC vaccines [61].
2.4 Suppression of the immune system by other metabolites in the TME
As previously described, the hypoxic environment in the TME promoted the production of large amount of lactic acid in the tumor cells [62], which induced a low pH in the TME. Low pH further attenuated antigen presentation by DCs and inhibited their maturation [62]. Moreover, hypoxia led to glycogen accumulation in the tumor cells and altered the metabolism of tumor-infiltrating lymphocytes and other immune cells, which eventually promoted the growth of the tumor cells [63]. Aberrant cyclooxygenase activity associated with hypoxia in tumors induced the production of prostaglandin E2, which mediated cDC1 dysfunction through the cDC loss of interferon regulatory factor 8 [64]. In addition, tumor-derived α-fetoprotein downregulated the transcriptional expression of the enzymes, resulting in lipid disorders and inhibiting DC maturation and development [65]. Abnormal lipid metabolism in the TME resulted in ER stress and functional damage to DCs [52]. Adenosine produced in the TME also affected the immune function of DCs [66]. Abnormal regulation of N6-methyadenosine (m6A) RNA levels in the TME partly impaired antigen presentation by DCs via the YT521-B homology (YTH) domain-containing family protein 1 (YTHDF1) signaling pathway [67]. Therefore, the metabolites produced in the TME mediated DC dysfunction and promoted tumor growth.
2.5 DC dysfunction mediated by co-inhibitory molecules
A few molecules displayed co-inhibitory functions in DCs (Figure 1, Pathway D). Cytotoxic T-lymphocyte antigen-4 (CTLA-4) expressed on tumors blocked the co-stimulatory signal of DCs to T cells by binding to CD28, which competed with CD86 expressed on DCs [14, 68]. T cell immunoglobulin and mucin domain 3 expressed on tumor-infiltrating DCs inhibited the tumor nucleic acid monitoring system to prevent DCs from cleaning abnormal nucleic acids and impeded DC-mediated immunogenic cell death [69, 70]. CD73, an immunoinhibitory protein that plays a key role in tumor growth and metastasis, suppressed the anti-tumor immunity of DCs [71]. Blocking CD73 enhanced the recruitment of cDC1 to tumor sites after radiotherapy and improved anti-tumor efficiency [72]. Therefore, analyzing these co-inhibitory molecules in DC dysfunction may contribute to the discovery of novel immunotherapies targeting DCs.
These findings indicate that DC dysfunction is induced by several external factors, such as cytokines, hypoxia, TEXs, tumor metabolites, and co-inhibitory molecules. Therefore, focusing on these factors may facilitate the development of targeted therapies to overcome DC dysfunction. Besides the external factors, intracellular signal transduction plays an important role in DC dysfunction, which needs to be considered.
3 IMPORTANT SIGNALS OF DC DYSFUNCTION
Many bioactive substances in the TME may induce DC dysfunction. These active substances either directly enter DCs to regulate relevant signals or bind to receptors on the surface of DCs to alter their functional activity and sequentially regulate their downstream signals [15]. Among many signals in DCs, the wingless-related integration site/β-catenin (Wnt/β-catenin) signal and YTHDF1 signal are essential signaling pathways involved in regulating the biological functions of DCs, and the related therapies have been summarized.
3.1 Wnt/β-catenin signaling and relevant therapies
In abnormal cDC1, the dysregulated Wnt/β-catenin signaling pathway has been identified as a key factor promoting tumor progression [73]. The over-activated Wnt signal in the TME elevated β-catenin levels, which in turn reduced the downstream gene transcriptional level of chemokines (or ligands), which subsequently prevented intra-tumoral migration of CD103+ DCs and impaired the infiltration of T cells into the TME [74, 75]. This was also confirmed by a study on anti-programmed cell death 1 (anti-PD-1) immunotherapy, in which elevated β-catenin levels impeded the recruitment of CD103+ DCs and triggered the tolerance of PD-1 therapy in mice with hepatocellular carcinoma [76]. These studies suggested that DC dysfunction was mediated by Wnt/β-catenin signaling [77, 78]. Targeting Wnt/β-catenin signaling may be a promising approach to overcome DC dysfunction and improve the efficacy of current cancer immunotherapies [73, 79], such as therapies through PORCN (porcupine O-acyltransferase) inhibitors, Dickkopf-1 (DKK1) antibodies, and cAMP response element-binding protein-binding protein/β-catenin (CBP/β-catenin) inhibitors.
3.1.1 PORCN inhibitors
PORCN inhibitors blocked the binding of Wnts to their cognate receptors, such as lipoprotein receptor-related protein 5/6 (LRP5/6), by blocking the palmitoylation of Wnt, thereby enhancing the antigen presentation of DCs and inhibiting the proliferation of cancer cells [80]. WNT974, a PORCN inhibitor, has been extensively investigated in several ongoing clinical trials and has demonstrated promising efficacy against different tumors (NCT01351103) [79] (Table 1). WNT974 effectively reduced the levels of axis inhibition protein 2 in patients and increased the level of T cell-related chemokine in early trials [81]. However, a subsequent clinical trial with a combination of encorafenib and cetuximab, has demonstrated concerns regarding the safety of WNT974 and lacked preliminary evidence of promising anti-tumor activity (NCT02278133) [82]. Other PORCN inhibitors have also been used in clinical trials to evaluate their efficacy in cancer therapy (Table 1). Among them, RXC004 was safe and well tolerated and was used to evaluate the preliminary efficacy alone or in combination therapy (NCT04907539).
| Drug | NCT Number | Status | Phase | Condition |
|---|---|---|---|---|
| LGK974 or WNT974 | NCT01351103 | Active, not recruiting | Phase I |
Pancreatic cancer BRAF mutant colorectal cancer Melanoma Triple negative breast cancer Head and neck squamous cell Cancer Cervical squamous cell cancer Esophageal squamous cell cancer Lung squamous cell cancer |
| NCT02649530 | Withdrawn | Phase II | Squamous cell carcinoma, head and neck | |
| NCT02278133 | Completed | Phase I, II | Metastatic colorectal cancer | |
| ETC-159 or ETC-1922159 | NCT02521844 | Recruiting | Phase I | Solid tumors |
| CGX1321 | NCT03507998 | Unknown† | Phase I |
Colorectal adenocarcinoma Gastric adenocarcinoma Pancreatic adenocarcinoma Bile duct carcinoma Hepatocellular carcinoma Esophageal carcinoma Gastrointestinal cancer |
| NCT02675946 | Recruiting | Phase I |
Solid tumors Gastrointestinal cancer |
|
| RXC004 | NCT03447470 | Active, not recruiting | Phase I | Cancer; solid tumor |
| NCT04907539 | Recruiting | Phase II | Colorectal cancer | |
| NCT04907851 | Recruiting | Phase II | Advanced solid tumors | |
| AZD5055 | NCT05134727 | Completed | Phase I | Idiopathic pulmonary fibrosis |
| NCT05644600 | Not yet recruiting | Phase I | Healthy subjects | |
| NCT05630677 | Completed | Phase I | Healthy volunteers |
- Abbreviations: PORCN, porcupine O-acyltransferase; NCT, national clinical trial; BRAF, v-raf murine sarcoma viral oncogene homolog B.
- a The information was collected from the website ClinicalTrials.gov (https://clinicaltrials.gov/).
3.1.2 DKK1 antibodies
Although DKK1 inhibited the β-catenin-dependent Wnt signal by binding to LRP5/6 and competing with Wnts, recent research has revealed that DKK1 activated β-catenin-independent Wnt signal and promoted the proliferation, invasion, and growth of tumor cells [83]. As shown in Table 2, a humanized IgG4 neutralizing antibody (DKN-01) targeting DKK1 decreased the concentration of serum DKK1 and enhanced the ability of immune cells to suppress tumor growth [79, 84]. One clinical trial showed that DKN-01 was well tolerated but did not exhibit potent activity, suggesting the need for a higher dose (NCT02375880) [85]. Other trials demonstrated the safety and tolerability of DKN-01 and showed that it effectively promoted the recovery of immune cell function and improved survival rate (NCT02013154, NCT03395080) [86, 87]. In addition to DKN-01, BHQ880 is a human neutralizing IgG1 anti-DKK1 monoclonal antibody (mAb) that has been proven safe and tolerable in a phase I trial (NCT00741377). The trial showed that BHQ880 increased bone mineral density and strength in the spine and hip [88]. Phase II trials are currently underway to evaluate the efficacy of BHQ880 in smoldering multiple myeloma and untreated multiple myeloma (NCT01302886, NCT01337752). In addition to biomacromolecular inhibitors, researchers have focused on small molecules and nucleic acid inhibitors, as reviewed by Jiang et al. [89].
| Drug | NCT Number | Status | Phase | Condition |
|---|---|---|---|---|
| BHQ880 | NCT01302886 | Completed | Phase II | Smoldering multiple myeloma |
| NCT00741377 | Completed | Phase I | Multiple myeloma bone disease | |
| NCT01337752 | Completed | Phase II |
Multiple myeloma Renal insufficiency |
|
| DKN-01 | NCT03645980 | Unknown | Phase I, II | Hepatocellular carcinoma |
| NCT05480306 | Recruiting | Phase II |
Colorectal cancer Colorectal Adenocarcinoma Colo-rectal cancer Colorectal cancer metastatic |
|
| NCT04681248 | Available |
Esophageal neoplasm Adenocarcinoma of the gastroesophageal junction gastroesophageal cancer (and 7 more…) |
||
| NCT04363801 | Recruiting | Phase II |
Gastric cancer Gastric adenocarcinoma Gastroesophageal cancer |
|
| NCT04057365 | Recruiting | Phase II | Biliary tract cancer | |
| NCT02013154 | Completed | Phase I |
Esophageal neoplasms Adenocarcinoma of the gastroesophageal junction Gastroesophageal cancer (and 2 more…) |
|
| NCT05761951 | Not yet recruiting | Phase II | Endometrial cancer | |
| NCT03837353 | Terminated | Phase I, II | Prostate cancer | |
| NCT01711671 | Completed | Phase I | Multiple myeloma | |
| NCT02375880 | Completed | Phase I |
Carcinoma of intrahepatic and extra-hepatic biliary system Carcinoma of gallbladder Bile duct cancer Cholangiocarcinoma |
|
| NCT03395080 | Completed | Phase II |
Endometrial cancer Uterine cancer Ovarian cancer Carcinosarcoma |
|
| NCT01457417 | Completed | Phase I |
Multiple myeloma Solid tumors Non-small cell lung cancer |
|
| NCT03818997 | Withdrawn | Phase II |
Esophageal cancer Biliary tract cancer Gastro esophageal cancer Hepatobiliary neoplasm |
|
| NCT04166721 | Recruiting | Phase I, II |
Metastatic esophageal cancer Metastatic gastric cancer |
- Abbreviations: DDK1, Dickkopf-1; NCT, national clinical trial.
- a The information was collected from the website ClinicalTrials.gov https://clinicaltrials.gov/.
3.1.3 CBP/β-catenin inhibitors
ICG-001 was the first specific small-molecule CBP/β-catenin inhibitor that inhibited the transcription of β-catenin-dependent genes [79]. Although an in vitro study on sarcoma cell lines have showed remarkable efficacy, ICG-001 enhanced the migration of osteosarcoma cells [90]. In a clinical trial, PRI-724 (an active enantiomer of ICG-001) was combined with gemcitabine to explore its tolerance, safety, and antineoplastic activity (NCT01764477). Two other clinical trials confirmed safety and tolerability at low doses, but showed limited efficiency and relevant serious adverse events (NCT02195440, NCT03620474) [91, 92]. The migration and invasion of uveal melanoma (UM) cells and the growth of subcutaneous tumors in a UM mouse model were inhibited when ICG-001 was co-cultured with UM cells [93]. In addition, the E7449 inhibitor showed good tolerability, promising anti-tumor activity, and substantial concentration-dependent polyadenosine-diphosphate-ribose polymerase inhibition in a phase I trial (NCT01618136) [94]. Phase II trials were also conducted to assess the anti-tumor effects and tolerability in patients with solid tumors (NCT03562832 and NCT03878849). In a recent review by Zhang et al. [95], the authors comprehensively summarized the Wnt/β-catenin signaling pathway and its relevant inhibitors. To date, only ICG-001 and E7449 have progressed to clinical trials (Table 3), and others are still in the preclinical study phase.
| Drug | NCT Number | Status | Phase | Condition |
|---|---|---|---|---|
|
E7449/ 2X-121 |
NCT02396433 | Withdrawn | Phase I, II | Cancer of the breast |
| NCT01618136 | Completed | Phase I, II |
Malignant solid tumor Ovarian cancer Triple negative breast cancer (and 2 more…) |
|
| NCT03878849 | Recruiting | Phase II | Advanced ovarian cancer | |
| NCT05571969 | Recruiting | Phase I | Advanced solid tumors | |
| NCT03562832 | Active, not recruiting | Phase II | Metastatic breast cancer | |
| ICG-001 | NCT02828254 | Completed | Hepatitis C virus-infected cirrhosis | |
| NCT03620474 | Completed | Phase I, II |
Hepatitis C Hepatitis B Liver cirrhoses |
|
| NCT01302405 | Terminated | Phase I | Advanced solid tumors | |
| NCT01606579 | Completed | Phase I, II |
Acute myeloid leukemia Chronic myeloid leukemia |
|
| NCT02195440 | Completed | Phase I | Hepatitis C virus-infected cirrhosis | |
| NCT01764477 | Completed | Phase I |
Advanced pancreatic cancer Metastatic pancreatic cancer Pancreatic adenocarcinoma |
|
| NCT02413853 | Withdrawn | Phase II |
Colorectal adenocarcinoma Stage IVA colorectal cancer Stage IVB colorectal cancer |
- Abbreviations: CBP, cAMP response element-binding protein-binding protein; NCT, national clinical trial.
- a The information was collected from the website ClinicalTrials.gov https://clinicaltrials.gov.
3.2 YTHDF1 drives the tumor evasion from DC surveillance
YTHDF1 regulated the translation of cancer-associated genes via m6A methylation and impaired the antigen presentation ability of DCs, thereby affecting the cytotoxic CD8+ T cells’ function [96]. Furthermore, YTHDF1 promoted the synthesis of lysosomal cathepsin in DCs and prevented tumor antigens from being processed and loaded onto MHC I molecules by DCs [97]. When treated with PD-1 inhibitors in YTHDF1 knockout mice, the therapeutic efficacy of the PD-L1 blockade was enhanced [97]. This underscores the potential synergistic effects of combining PD-L1 blockade with YTHDF1 inhibitors. Therefore, YTHDF1 plays a crucial role in the cross-presentation function of DCs, and therapies targeting YTHDF1 may contribute to the activity of DCs against cancer. However, because YTHDF1 is also expressed in normal cells, further verification is needed to avoid toxicity and autoimmunity caused by off-target effects [97, 98].
Despite the substantial role of Wnt/β-catenin and YTHDF1 signaling pathways in DC dysfunction, therapeutic strategies targeting these pathways have not shown notable progress. Considerable efforts are needed to explore the mechanism of DC dysfunction in basic research and develop novel and more efficient therapies that leverage these two signaling pathways.
4 CURRENT DC-BASED THERAPIES
With the development of immunology and antibody engineering technologies, researchers have better understood the immunological function of DCs, which has led to the development of different therapies based on DCs. The latest advances in cancer therapy (Figure 2), including adjuvant and autologous DC vaccines, messenger RNA (mRNA)-encoding neoantigens, and combination therapy with immune checkpoint blockers (ICBs), have been listed in this review, and currently available DC therapies have been discussed in the following subsections.
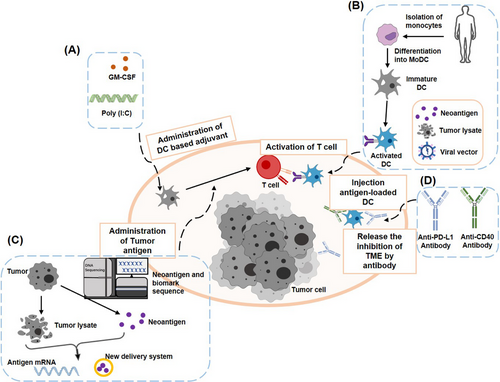
Scheme of DC-based immunotherapies. (A) Immune adjuvants and cytokines that can directly enhance DCs activation, such as Poly (I:C), GM-CSF, and IL-12. (B) Patients’ monocytes are isolated and differentiated into immature DCs. Tumor-specific DCs are collected by co-culturing immature DCs with tumor antigens or transfecting with lentivirus. Finally, those antigen-specific DCs will be transfused back into the body. (C) Tumor antigens are produced by lysing tumor cells or neo-antigen screening. These tumor antigens are delivered to DCs in vivo by mRNA technology and nanoparticles. (D) ICBs or DC-activating antibodies alleviate the inhibition of immune cells in the TME, such as anti-PD-L1 antibodies, CD40-activating antibodies. This figure is obtained from the article by Kim et al. [99]. Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; Poly (I:C), polyinosinic-polycytidylic acid; ICBs, immune checkpoint blockers; PD-L1, programmed cell death-1 receptor ligand 1; DC, dendritic cell; MoDC, monocyte-derived DC; IL, interleukin; CD, cluster of differentiation; TME, tumor microenvironment; mRNA, messenger ribonucleic acids.
4.1 DC-based adjuvant
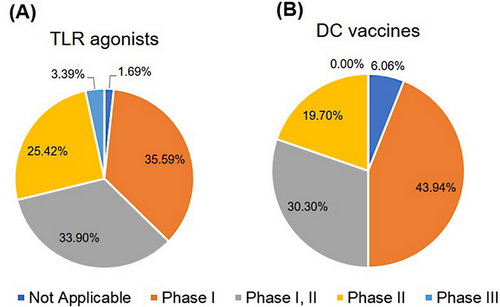
The early development of DC therapy was most associated with some immune adjuvants inducing inflammation factors (Figure 2A), such as aluminum salts, oils, cytokines (IFN-γ, IL-12, and GM-CSF), and synthetic compounds such as polyinosinic-polycytidylic acid (poly [I:C]) [100, 101]. The administration of these adjuvants boosted the body's immune reaction and exerted a specific anti-tumor effect [14, 101]. Among them, TLR agonists are highly promising adjuvants for vaccines against complex and life-threatening diseases such as malaria, acquired immunodeficiency syndrome, and cancer. More than 50 clinical trials have been conducted using TLR agonists such as TLR3, TLR4, TLR 7, TLR 8, and TLR 9 agonists (Supplementary Table S1). Most were in phase I and II clinical trials (94.91%) (Figure 3A). These agonists elicited a “danger” signal to induce an effective immune response, which may offer durable protection [102]. The results are summarized in Table 4. Most studies reported that TLR agonists were safe, well tolerated in patients and induced an immune response. The TLR-3 receptor agonist (poly [I:C]), the most commonly used anti-tumor adjuvant, enhanced the function of CD8+ and CD4+ T cells [103]. The combination treatment of poly (I:C) and chimeric antigen receptor T (CAR-T) cell therapy enhanced the activity of CAR-T and increased the secretion of IFN-γ [104]. TLR-3 receptor agonists prolonged the survival of glioblastoma patients [105]. Another synthetic-specific TLR-3 adjuvant, ARNAX, has attracted considerable attention. The TLR-3 adjuvant activated only the TLR-3 signal, which reduced systemic inflammatory side effects without activating melanoma differentiation-associated protein 5 (MDA5) [106]. In addition, ARNAX mitigated immunosuppression in the TME. The combination of anti-PD-1/PD-L1 antibodies with ARNAX further improved the therapeutic effects of monoclonal antibodies [107]. Despite this progress, the therapeutic effect of adjuvant treatment on cancer cells remains limited and sometimes triggered autoimmunity [101, 108]. Therefore, developing safe and more effective adjuvants is highly desirable for anti-tumor immunotherapy.
| TLR agonists | Drug | NCT Number | Status | Phase | Results |
|---|---|---|---|---|---|
| TLR3 agonists | Poly-ICLC | NCT01079741 | Completed | Phase I, II | On clinical trial |
| Poly-ICLC | NCT02643303 | Completed | Phase I, II | ||
| TLR4 agonists | GSK1795091 | NCT02798978 | Completed | Phase I | GSK1795091 was acceptably tolerated in healthy volunteers, demonstrated favorable pharmacokinetic properties, and stimulated immune cell changes in a dose-dependent manner, providing evidence of target engagement and downstream pharmacology. These results support the design in combination with other immunotherapies in patients with advanced cancer [109]. |
| GSK1795091 | NCT03447314 | Completed | Phase I | On clinical trial | |
| TLR7 agonists | GSK2245035 | NCT01607372 | Completed | Phase II | Intranasal GSK2245035 (<100 ng) has an acceptable safety profile at doses that induce local TLR7-mediated immune responses, such as IFN-stimulated immune changes [110]. |
| GSK2245035 | NCT01480271 | Completed | Phase I | ||
| GSK2245035 | NCT02833974 | Completed | Phase II | Although target engagement was observed, GSK2245035 did not substantially attenuate the late asthmatic response in participants with mild allergic asthma. Overall, treatment was well tolerated [111]. | |
| Imiquimod | NCT01421017 | Completed | Phase I, II | On clinical trial | |
| Vesatolimod | NCT02166047 | Completed | Phase II | GS-9620 had no significant effect on serum hepatitis B surface antigen levels, but increased T-cell and NK-cell responses and reduce the ability of NK to suppress T cells [112]. Vesatolimod demonstrated consistent dose-dependent pharmacodynamic induction of ISG15 without significant systemic induction of IFNα expression or related symptoms, which proved its safety and well-tolerability. However, no significant HBsAg declines were observed [113]. | |
| TLR8 agonists | VTX-2337/Motolimod | NCT01334177 | Completed | Phase I | Following VTX-2337 treatment, patient NK cells become more responsive to stimulation by NKG2D or FcγRIII, which indicated that TLR8 stimulation could complement FcγRIII engagement and may augment clinical responses in SCCHN patients treated with cetuximab [114]. |
| VTX-2337 /Motolimod | NCT01289210 | Terminated | Phase I, II | On clinical trial | |
| VTX-2337 /Motolimod | NCT02431559 | Completed | Phase I, II | ||
| TLR9 agonists | Tilsotolimod | NCT03445533 | Terminated | Phase III | On clinical trial |
| AZD1419 | NCT02898662 | Completed | Phase II | ||
| CMP-001 | NCT03618641 | Active, not recruiting | Phase II | ||
| CPG 7909 | NCT00226993 | Withdrawn | Phase I, II | In situ vaccination strategy is feasible and well tolerated, and the clinical responses that occurred in a subset of patients warrant further study with modifications to augment these therapeutic effects [115]. In situ tumor vaccination with a TLR9 agonist induces systemic anti-lymphoma clinical responses. This maneuver is clinically feasible and does not require the production of a customized vaccine product [116]. | |
| NCT00562939 | Completed | Phase I, II | The CPG 7909 had significantly higher relative cytokine responses (IL-1β, IL-2R, IL-6, IFN-γ and MIP-β), which suggested that CPG 7909 induced cellular memory to pneumococcal polysaccharides in HIV-patients, independently of the humoral response [117]. TLR9 agonist significantly enhanced the proportion of vaccine high responders [118]. | ||
| MGN1703 | NCT02443935 | Completed | Phase I, II | MGN1703 treatment was safe and improved innate and HIV-1-specific adaptive immunity in HIV-1+ individuals, which supported the incorporation of TLR9 agonism into combination HIV-1 cure strategies [119]. TLR9 agonist treatment in HIV infection inhibits a dual potential by increasing HIV-1 transcription and enhancing cytotoxic NK cell activation [120]. |
- Abbreviations: TLR, toll-like receptor; HIV, human immunodeficiency virus; NCT, national clinical trial; Poly-ICLC, polyinosinic-polycytidylic acid-poly-l-lysine carboxymethylcellulose; IL, interleukin; NK, natural killer; IFN-γ, interferon-γ; SCCHN, squamous cell carcinoma of the head and neck; MIP-β, macrophage inflammatory protein-1 beta; NKG2D, natural killer group 2, member D; FcγRIII, IgG-Fc gamma receptor III; ISG15, IFN-stimulated gene 15.
- a The information was collected from the website ClinicalTrials.gov https://clinicaltrials.gov/.
4.2 DC-based cancer vaccine
As shown in Figure 2B, the design principle of DC vaccines involves isolating monocytes from the human body. Monocytes are induced to become precursor DCs [121]. The precursor DCs were then incubated with TAA or tumor cell lysate and cytokines (such as GM-CSF, and IL-12) to differentiate into antigen-specific DCs, which were subsequently injected into the body to enhance the antigen presentation function [122-125]. In the past decades, Sipuleucel-T (Provenge®) was the only prostate cancer vaccine based on autologous DCs approved by the Food and Drug Administration in 2010 [126]. Sipuleucel-T isolates precursor DCs from the human body. These precursor cells were then stimulated to differentiate into mature DCs by GM-CSF and prostatic acid phosphatase. Ultimately, mature DCs were injected back into the patients to elicit an anti-tumor immune response [127]. However, Sipuleucel-T demonstrated limited clinical benefits, with only a 4.1-month improvement in median survival and no improvement in progression-free survival [128]. Low proportion of stimulated mature DCs and the complexity of administration and dosage were the potential disadvantages of first-generation DC vaccines [129, 130]. These problems have led to low clinical response rates to DC vaccines [130], warranting further exploration of diverse approaches to improve the efficacy of available vaccines. To date, many DC vaccines have been evaluated in clinical trials (Supplementary Table S2), and most vaccines are in phase I and II trials (93.94%) (Figure 3B). In these clinical trials, different approaches, such as exploring DC subsets and neoantigens, combining mRNA and DC vaccines, and combination therapy with ICBs, have been explored to identify the most promising candidates. Based on the results, these clinical trials have been summarized in Table 5. Most trials confirmed the safety and tolerability of DC-based vaccines and demonstrated clinical benefits for different clinical indications. Novel strategies for developing DC vaccines, including the development of new DC subsets, neo-antigen identification, mRNA vaccines, and immunomodulatory molecules, have been discussed in the subsequent sections.
| Drug | NCT Number | Status | Phase | Results |
|---|---|---|---|---|
| Alpha-type 1 DC-based vaccines loaded with allogeneic prostate cell lines | NCT00970203 | Completed | Phase II | On clinical trial |
| Adenovirus-p53 transduced DC vaccine | NCT01042535 | Completed | Phase I, II | On clinical trial |
| Autologous dendritic cell-adenovirus p53 vaccine | NCT00082641 | Completed | Phase I, II | On clinical trial |
| Autologous DC loaded with autologous tumor lysate | NCT00085436 | Completed | Phase II | Overall objective clinical response rate was 50% with three complete responses. Median time to progression for all patients was 8 months, and median survival has not been reached. Treatment-related changes in correlative immunologic end points were noted and the level of circulating CD4+ T regulatory cells had a strong association with outcome [131]. |
| Autologous DCs pulsed with apoptotic tumor cells (DC/PC3) | NCT00345293 | Completed | Phase I, II | Patients who received dendritic cell vaccines generated by the adherence method mounted increased T cell proliferation, which was due to the amount of 10% lymphocytes in the cultures. These lymphocytes were proliferating and producing IFN-γ in response to antigen in vitro at the time of administration. The presence of lymphocytes enhanced immunogenicity of adherence dendritic cell vaccinations [132]. |
| Autologous TriMix-DC therapeutic vaccine | NCT01302496 | Completed | Phase II | The combination treatment resulted in robust CD8+ T-cell responses in a meaningful portion of stage III or IV melanoma patients, and obviously with a clinical response. The levels of polyfunctional and multiantigen T-cell responses may provide a benchmark for the level of immune stimulation needed to achieve a durable clinical remission [133]. |
| Autologous tumor lysate-DC-vaccine | NCT00913913 | Terminated | Phase II | Mature DC vaccine, coupled with continuous infusion of IL-2 and IFN-α2a, resulted in a clinical objective response in 6 of 13 patients with metastatic RCC. Encouraging preliminary results raised the possibility of enhancing the objective response rate and, particularly, the durable clinical responses with therapy that took advantage of enhancing inflammatory and limiting regulatory pathways [134]. |
| Autologous vaccine comprised of autologous DC loaded in vitro with lysate from autologous oxidized tumor cells | NCT01132014 | Completed | Early Phase I | Adding ASA and low-dose IL-2 to the OCDC-Bev-Cy combinatorial regimen could elicit vaccine-specific T-cell responses that positively correlated with patients' prolonged time-to-progression and overall survival [135]. |
| HER-2 pulsed DC1 vaccine | NCT02063724 | Active, not recruiting | Phase I | Vaccination against HER-2/neu was safe and well tolerated and induced decline and/or eradication of HER-2/neu expression. These findings warrant further exploration of HER-2/neu vaccination in estrogen-independent breast cancer and highlight the need to target additional tumor-associated antigens and pathways [136, 137]. |
| HER-2 pulsed DC1 vaccine | NCT02061423 | Active, not recruiting | Phase I | |
| HER-2 pulsed dendritic cell vaccine | NCT02061332 | Completed | Phase I, II | |
| Human CMV pp65-LAMP mRNA-pulsed autologous DCs | NCT02529072 | Completed | Phase I | On clinical trial |
| mRNA tumor antigen pulsed autologous DCs | NCT02808364 | Completed | Phase I | Most TAAs induced antigen-specific CD4+ and/or CD8+ T cell responses, regardless of their expression levels in the tumor tissues. Personalized TAA immunization-induced-specific CD4+ and CD8+ T cell responses without obvious autoimmune adverse events and was associated with favorable overall survival [138]. |
| mRNA-TAA pulsed autologous DC cellular vaccine | NCT02709616 | Completed | Phase I | |
| Tumor antigen mRNA pulsed DC cellular vaccines | NCT02808416 | Completed | Phase I | |
| Multiple antigen-engineered DC vaccine for melanoma | NCT01622933 | Completed | Phase I | The clinical outcomes were 2 partial responses, 8 stable disease and 14 progressive diseases among patients. The majority of vaccinated patients showed an increase in vaccine antigen-specific CD8+ and CD4+ T cell responses. Although DC vaccines are a safe and reliable platform for promoting antitumor immunity, the combination with high dose IFNα did not improve outcomes [139]. |
| Peptide-pulsed vs. RNA-transfected DC vaccines | NCT00243529 | Completed | Phase I, II | Mature DC are superior to immature DC in the induction of immunological responses in melanoma patients [140]. A direct correlation between the presence of DC vaccine-related T cells and a positive clinical outcome was confirmed (P = 0.0012) [141]. |
| Recombinant adenovirus-transfected DC, which engineered to express MUC1 and survivin | NCT01924156 | Unknown | Phase I, II | This result showed an ORR of 39% and a DCR of 75%, with no clinically significant side effects. Only DCR was significantly related with cycles of treatment (P < 0.05), not ORR [142]. |
| Tumor neoantigen primed DC vaccines | NCT02956551 | Unknown | Phase II | The objective effectiveness rate was 25%; the DCR was 75%; the median progression-free survival was 5.5 months and the median overall survival was 7.9 months. All treatment-related adverse events were grade 1-2 and there were no delays in dosing due to toxic effects [7]. |
| Tumor specific antigen-loaded DCs | NCT03185429 | Unknown | Not Applicable | Adjuvant p53-specific vaccination of patients with HNSCC was safe and associated with promising clinical outcome. Two-year disease-free survival achieved 88%. p53-specific T-cell frequencies were increased (69%), and IFN-γ secretion was detected in four of 16 patients, as well as Treg levels were consistently decreased [143]. Vaccination promoted a diverse neoantigen-specific TCR repertoire, which demonstrated that vaccination directed at tumor-encoded amino acid substitutions broadened the antigenic breadth and clonal diversity of antitumor immunity [144]. |
| Tumor-specific intranodal autologous ALPHA-DC1 vaccines | NCT02432378 | Suspended | Phase I, II | The chemokine-modulating intraperitoneal-CITC was safe, tolerable, and associated with the local upregulation of ISG that favor CTL chemoattraction and function. Median progression-free survival and overall survival were 8.4 and 30 months, respectively. This combination (plus DC vaccine) would be tested in a phase II trial [145]. |
- Abbreviations; DC, dendritic cell; NCT, national clinical trial; p53, protein 53; CD, cluster of differentiation; IL, interleukin; IFN, interferon; TCR, T cell receptor; CMV, cytomegalovirus; Her2, human epidermal growth factor receptor 2; TAA, target-associated antigen; RNA, ribonucleic acids; ISG, interferon-stimulated gene; CTL, cytotoxic T lymphocytes; MUC1, mucin 1; ORR, objective response rate; DCR, disease control rate; HNSCC, head and neck squamous cell carcinoma; ASA, acetylsalicylic acid; OCDC, ovarian cancer dendritic cell vaccine (a personalized whole-tumor lysate-pulsed dendritic cell vaccine); Bev, bevacizumab; Cy, cyclophosphamide.
- a The information was collected from the website ClinicalTrials.gov https://clinicaltrials.gov/.
Suitable DC subsets with better antigen presentation capabilities should be identified by cell surface markers [122, 146]. A promising subset of DCs, the basic leucine zipper ATF-like transcription factor 3-dependent X-C chemokine receptor 1+ (XCR1+) CD8+ DCs, exhibited potent stimulating effects in CD4+ T cells, CD8+ T cells, and NK cells [147-149]. The chemokine receptor XCR1 was specifically expressed on DCs, and its ligand XCL1 promoted cross-presentation [149]. Moreover, the technique used to separate XCR1+ DCs was much more straight forward than that for other subsets [149, 150]. These results confirmed that XCR1+ DCs may be a suitable subset for DC therapy.
Another strategy for improving the efficacy of DC vaccines was neo-antigen identification to overcome refractory and relapsed tumors [151]. An antigen loaded with DC vaccines often showed tolerance to immunity in refractory patients. Therefore, DC vaccines loaded with new TAA may inhibit tumor escape. Bioinformatics and genomic technological advances have made it possible to anatomize the immune response to personalized neo-antigens encoded by tumor-specific mutations. Ideal neo-antigens are tumor-specific peptides that are absent in normal human tissues [152]. In a study focusing on the genome analysis of tumors in melanoma patients, researchers obtained an individualized neo-antigen, which was loaded into DCs as a vaccine. This neo-antigen-loaded DC vaccine elicited vital T cell responses [153] and showed that neo-antigen identification may be beneficial for improving the anti-tumor activity of DC vaccines.
The mRNA vaccine, a new type of cancer vaccine, stimulated innate immune responses and provided antigens by cell transfection in vivo [154]. In a recent clinical study, an mRNA vaccine (mRNA-4650) comprised mRNA backbones encoding up to 20 different antigens, including autologous tumor antigens and neo-antigens [155]. Although the mRNA vaccine demonstrated a less significant anti-tumor effect, it increased the population of CD8+ and CD4+ neo-antigen-specific T cells. Another research team obtained relevant mutant neo-antigens from a mouse lung cancer cell line, LL2, and cultured them with DCs in vitro, followed by injection into mice [156], which resulted in continuous activation of CD8+ T cells and a large amount of IFN-γ production. In addition, gliomas with low mutations exhibited specific T-cell responses against the tumor using neo-antigens [157]. Based on these reports, neo-antigen DNA or RNA could be delivered as a vaccine to induce a positive immune response [103, 158].
In addition to tumor antigens, a group of DC vaccines loaded with immunomodulatory molecules enhanced the immune regulation [159]. DCs electroplated with TriMix mRNA encoded three immune-modulating molecules: TLR-4, CD40L, and CD70 (TriMixDC-MEL) [133]. According to other reports, melanoma-associated antigen was fused with human leukocyte antigen class II targeting DC-lysosomal membrane proteins to constitute the whole TriMixDC-MEL vaccine [159, 160]. TriMixDCs combined with the anti-CTLA-4 monoclonal antibody ipilimumab demonstrated vital T cell-specific activation ability in multiple experiments [159]. Compared with ipilimumab monotherapy, this combination therapy was beneficial for obtaining long-term clinical responses in melanoma patients [133]. Given the fewer side effects and durable clinical responses, TriMixDC in combination with ipilimumab may be more beneficial [133, 161]. Another engineered DC, SmartDC-tyrosinase-related protein 2 (TRP2), was constructed using a lentivirus expressing IL-4, TRP2 (melanoma antigen), and GM-CSF [162]. SmartDC-TRP2 was effectively transferred from the injection site to the local draining lymph nodes in a mouse model, where it persisted for a few weeks to induce anti-melanoma responses and T cell expansion [162]. These studies suggest that DCs equipped with immunomodulatory molecules exhibited enhanced efficacy, which provided a novel strategy for DC vaccines.
The advent of genetic engineering technology has opened a new era of personalized therapy, such as neoantigen DC vaccines; however, the following problems still need to be resolved: 1) the manufacturing cycle is lengthy and expensive; 2) the extraction of neo-antigens is complex, and the purification process is inefficient; 3) The efficacy of mono-therapy remains limited in the TME [158, 163].
4.3 New DC vaccine delivery system
In addition to antigen-loaded DC vaccines, effective delivery systems have been the focus of research (Figure 2C) [14, 130]. Mannose (MN)-labeled poly lactic-co-glycolic acid (PLGA) nanoparticles (MN-PLGA-NPs) were synthesized to encapsulate the TAA [164]. These nanoparticles target the MN receptor to initiate antigen presentation by DCs through the pattern recognition receptor [164]. Similarly, another team reported that TAA encapsulated in PLGA nanoparticles enhanced the antigen presentation ability of DCs [165]. Tateshita et al. [166] reported that an mRNA vaccine comprising lipoplex and vitamin E scaffolds exhibited greater cytokine release and enhanced CTL activity than that of an mRNA vaccine without lipoplex delivery. A multi-functional compound with lipids was also synthesized to improve stability by avoiding the catalytic hydrolysis of mRNA, which resulted in insufficient stimulation of DC cells [167, 168]. This compound activates TLR7 (or TLR8)/RLR (RIG-I and MDA5) and condenses with mRNA into lipid nanoparticles to promote cellular endocytosis and reduce mRNA degradation [167]. In addition, this study also demonstrated that nanoparticles loaded with OVA mRNA rapidly induced potent T cell responses, increased IFN-γ levels, and inhibited the growth of secondary inoculated tumor cells by producing durable immune effects. The delivery system is a critical component of DC vaccine therapy and can considerably impact the effectiveness of the treatment.
4.4 Combination therapy
Combining DC vaccines with ICBs improved the response rate in patients and prolonged their survival (Figure 2D) [122, 169].
Although PD-1/PDL-1 antibodies demonstrated restoration of immune cell killing ability, more than 50% of patients experienced poor efficacy [170]. PD-L1 was highly expressed in both peripheral and infiltrated DCs in lung cancer patients. Blocking PD-L1 in DCs enhanced T cell activation and proliferation, thereby prolonging patient survival [171]. Therefore, immunoregulation by DCs is critical in therapies targeting PD-L1, which was also proved by the fact that anti-PD-L1 antibodies did not control tumor growth and the CD8+ T cell population in the mice did not increase in the cDC1 function-deficient mice [172]. Dammeijer et al. [173] established two animal models of PD-L1 blockade targeting either tumor-draining lymph nodes or the entire body. They demonstrated that blocking PD-L1 in cDC2 cells induced effective tumor immunity. Furthermore, DCs induced tumor-specific T cell responses to ICBs via the stimulation of interferon genes activation [174]. Given that ICB therapy depends on DCs, the efficacy of combination therapy involving DC vaccines and ICBs was superior to that of single treatment modality [13, 133].
In addition to targeting immunosuppressive molecules (PD-1/PD-L1), therapies that leverage immuno-costimulatory molecules, such as CD40, enhance DC targeting strategies. CD40, which is expressed on B lymphocytes, DCs, and monocytes, is a crucial regulator of cellular and humoral immunity [175]. Its ligand, CD40L, is primarily expressed in activated T lymphocytes and platelets [176]. Activation of the CD40 signal on DCs triggered several immunological responses: upregulation of the expression of co-stimulated receptors and MHC molecules, enhancement of antigen presentation, production of pro-inflammatory cytokines (such as IL-12), and promotion of T cell activation [177]. Therapies targeting CD40 and FMS-related tyrosine kinase 3 ligand increased cDC infiltration and restored immune surveillance in pancreatic cancer [178]. Various agonists targeting CD40 have also been developed. Their safety profiles and enhanced immune cell function have been demonstrated in clinical trials [176]. In combination therapy using CD40 mAb and gemcitabine for the treatment of pancreatic ductal adenocarcinoma, 11 of 21 patients showed a favorable clinical response [179]. Salomon et al. [180] designed a new bispecific antibody (BsAb) that preferentially targeted cDC1 cells and activated CD40 signaling to further improve safety and efficacy. Compared to CD40 mAbs, BsAb mediated more robust T cell activation and anti-tumor activity [180]. In addition, a CD40 agonist-conjugated TAA and TLR5 binding domain directly generated a triple functional molecule to target DCs [181]. The activation signal of CD40 significantly improved the activity of DCs in the TME and provided a more durable anti-tumor response in combination therapy, which may be beneficial for patients who are not responsive to conventional immunotherapy [175, 176]. Another BsAb targeting DCs and T cells on PD-L1 and CD3 respectively, achieved durable anti-tumor activity through T-cell rejuvenation [182], which highlights the restoration of T cell function by DCs.
Both traditional combination therapies and novel approaches have demonstrated promising results. Fan et al. [183] designed novel antibody-engineered tDC-Exo-expressing anti-CD3 and anti-EGFR antibodies to mimic CAR-T therapy. This approach promoted the binding of T cells to cancer cells and achieved better efficiency when combined with an anti-PD-L1 antibody. T-cell-engaging BsAbs (T-BsAbs) such as teclistamab, blinatumomab, and mosunetuzumab, demonstrated improved anti-tumor activity than those of their parent mAbs. However, continuous exposure to T-BsAbs may induce T-cells exhaustion [184]. Fortunately, DCs counteracted this exhaustion by maintaining and guiding the differentiation of the precursors of the exhausted T cells [185]. This insight provides a new strategic approach for cancer therapy: combining T-BsAbs such as anti-CD3/tissue factor BsAb, anti-CD3/Lewis Y BsAb, and anti-CD3/EGFRvIII BsAb from our laboratories with therapies targeting DCs [186-188]. Another BsAb targeting PD-L1 and lymphocyte activation gene 3 demonstrated potent anti-tumor activity by promoting the activation of DCs and conjugation of T and tumor cells [189]. This was investigated in a first-in-human trial (NCT05101109). To summarize, therapies involving DCs achieve synergistic effects resulting in “1 + 1> 2” activity when combined with CAR-T cells [190, 191], ICBs [192], NK cells [193], cytokines [194] or other immunotherapies.
5 CONCLUSIONS
DCs are the most potent antigen-presenting cells that has the ability to induce immune memory responses and activate naïve T cells in cancer therapy. Recent progress in understanding the role of DCs in immune responses against cancer highlights the potential of DCs in improving clinical outcomes [195]. Cancer immunotherapy is more effective when synchronized with DCs’ functions [196]. Therefore, regardless of the immunotherapy applied, DCs may be crucial in inducing durable immune responses.
DCs are often tolerogenic or dysfunctional in the TME. Therefore, remodeling the immunogenic TME may be an effective method to overcome DC dysfunction. Moreover, further exploration of the mechanisms underlying DC dysfunction in the TME may facilitate the restoration of the biological function of DCs. In conclusion, many strategies targeting DCs have been explored to improve their curative effect against cancer [197-200], although DCs often appear dysfunctional in the TME. Modifying the TME and understanding the causes of DC dysfunction may contribute to improved outcomes in the near future.
AUTHOR CONTRIBUTIONS
Jie Chen and Yuhang Duan contributed equally to this review article. Writing/ Original draft (DC dysfunction and its molecular mechanisms): Yuhang Duan; Writing, editing and final proofreading: Jie Chen; Review: Junye Che; Study conceptualization and revision: Jianwei Zhu.
ACKNOWLEDGEMENTS
We thank the National Natural Science Foundation of China (grant numbers 81773621 and 82073751 to JZ) and the National Science and Technology Major Project of the Ministry of Science and Technology, China (grant number 2019ZX09732001-019 to JZ) for supporting this study.
CONFLICT OF INTEREST STATEMENT
The authors have no potential conflicts of interest to declare.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




