Beyond success: unveiling the hidden potential of radiotherapy and immunotherapy in solid tumors
Abstract
Immunotherapy, particularly with immune checkpoint inhibitors, has significantly transformed cancer treatment. Despite its success, many patients struggle to respond adequately or sustain long-lasting clinical improvement. A growing consensus has emerged that radiotherapy (RT) enhances the response rate and overall efficacy of immunotherapy. Although combining RT and immunotherapy has been extensively investigated in preclinical models and has shown promising results, establishing itself as a dynamic and thriving area of research, clinical evidence for this combination strategy over the past five years has shown both positive and disappointing results, suggesting the need for a more nuanced understanding. This review provides a balanced and updated analysis of the combination of immunotherapy and RT. We summarized the preclinical mechanisms through which RT boosts antitumor immune responses and mainly focused on the outcomes of recently updated clinical trials, including those that may not have met expectations. We investigated the optimization of the therapeutic potential of this combined strategy, including key challenges, such as fractionation and scheduling, lymph node irradiation, and toxicity. Finally, we offered insights into the prospects and challenges associated with the clinical translation of this combination therapy, providing a realistic perspective on the current state of research and potential future directions.
List of abbreviations
-
- ICI
-
- immune checkpoint inhibitor
-
- RT
-
- radiotherapy
-
- IMRT
-
- intensity-modulated radiotherapy
-
- SBRT
-
- stereotactic body radiotherapy
-
- ISABR
-
- immunotherapy and stereotactic ablative radiotherapy
-
- IGRT
-
- image-guided radiotherapy
-
- CTLA-4
-
- cytotoxic T lymphocyte-associated protein 4
-
- HNSCC
-
- head and neck squamous cell carcinoma
-
- NSCLC
-
- non-small cell lung cancer
-
- RIT
-
- radioimmunotherapy
-
- OS
-
- overall survival
-
- TME
-
- tumor microenvironment
-
- APC
-
- antigen-presenting cell
-
- TAA
-
- tumor-associated antigen
-
- DAMP
-
- damage-associated molecular pattern
-
- HMGB-1
-
- high-mobility group box 1
-
- ATP
-
- adenosine triphosphate
-
- DLN
-
- draining lymph node
-
- MHC
-
- major histocompatibility complex
-
- dsDNA
-
- double-stranded DNA
-
- cGAS
-
- cyclic GMP-AMP synthase
-
- cGAMP
-
- cyclic GMP-AMP
-
- TBK1
-
- TANK-binding kinase 1
-
- IRF3
-
- interferon regulatory factor 3
-
- IFN
-
- interferon
-
- TNF
-
- tumor necrosis factor
-
- TREX1
-
- three prime repair exonuclease 1
-
- MDSC
-
- myeloid-derived suppressor cell
-
- TAM
-
- tumor associated macrophage
-
- LPS
-
- lipopolysaccharide
-
- iNOS
-
- inducible nitric oxide synthase
-
- CSF1
-
- macrophage colony-stimulating factor
-
- SABR
-
- stereotactic ablative radiotherapy
-
- CR
-
- complete response
-
- BED
-
- biologically effective dose
-
- MPR
-
- Major pathological response
-
- HFRT
-
- hypofractionated radiotherapy
-
- ORR
-
- overall response rate
-
- PDAC
-
- pancreatic ductal adenocarcinoma
-
- NSCLC
-
- non-small cell lung cancer
-
- PD-1
-
- programmed cell death protein 1
-
- PD-L1
-
- programmed death-ligand 1
-
- DC
-
- dendritic cell
-
- MHC
-
- major histocompatibility complex
-
- NK
-
- natural killer
-
- Treg
-
- regulatory T cell
-
- LA
-
- locally advanced
-
- CRT
-
- chemo- radiotherapy
-
- cCRT
-
- concurrent chemo- radiotherapy
-
- sCRT
-
- sequential chemo- radiotherapy
-
- CFRT
-
- conventionally fractionated radiotherapy
-
- STING
-
- stimulator of interferon genes
-
- CXCL
-
- C-X-C motif chemokine ligand
-
- TIL
-
- tumor infiltrating lymphocytes
-
- Foxp3
-
- forkhead box protein P3
-
- TGF-β
-
- transforming growth factor-β
-
- CCL
-
- C-C motif chemokine
-
- CCR
-
- C-C chemokine receptor type
-
- YTHDF2
-
- YT521B homology domain family 2
-
- AE
-
- adverse effects
-
- TPS
-
- tumor proportion score
-
- SCLC
-
- small cell lung cancer
-
- LDHRT
-
- low-dose hypofractionated radiotherapy
-
- MSS
-
- microsatellite stable
1 BACKGROUND
Immunotherapy, specifically immune checkpoint inhibitors (ICIs), offers a new paradigm for treating several solid tumors, including melanoma [1, 2], non-small cell lung cancer (NSCLC) [3, 4], and head and neck squamous cell carcinoma (HNSCC) [5]. However, most patients with cancer do not respond optimally to immunotherapy alone [6, 7]. Accordingly, combining immunotherapy with other established cancer treatments, including radiotherapy (RT), has garnered considerable attention [8-12].
RT, a widely used and efficacious cancer treatment modality, can enhance both localized and systemic antitumor immune responses [13, 14]. RT has evolved considerably over time, driven by advancements in diagnostic imaging and delivery techniques. A pivotal leap occurred in the development of electron linacs in the 1960s and the 1970s. The evolution of RT techniques, including intensity-modulated RT, stereotactic body RT (SBRT), image-guided RT, and proton therapy, has ushered in a new era of precision radiation for solid tumors, with lower toxicities and higher conformality of the radiation fields targeting the tumor [14-18].
The concept of radioimmunotherapy was first proposed in 2005, triggering many preclinical studies that explored the potential synergy between RT and immunotherapy [19]. However, evidence from relevant clinical trials is limited. In 2016, Bernstein et al. [20] introduced the definitive concept of immunotherapy and stereotactic ablative RT (ISABR). In 2018, they further advocated comprehensive irradiation of multiple lesions in the ISABR field [21] (Figure 1).
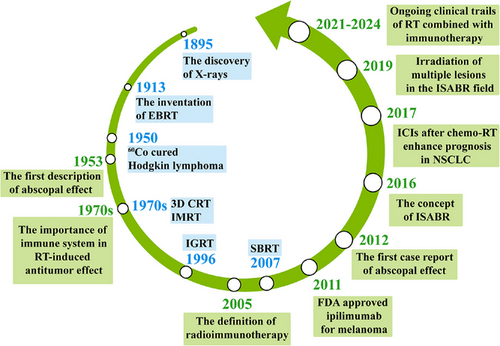
First, a secondary analysis of the KEYNOTE-001 trial (NCT01295827) provided intriguing insights into this combination strategy at the clinical trial level. The primary objective of the phase I KEYNOTE-001 trial was to assess the safety and antitumor activity of pembrolizumab (an anti-programmed cell death protein 1 [anti-PD-1] antibody) in patients with advanced NSCLC [22]. Shaverdian et al. [23] assessed patients with advanced NSCLC who had received RT before pembrolizumab treatment. They found that the overall survival (OS) and progression-free survival (PFS) were significantly longer in patients who had previously received RT than in those who had not, with an acceptable safety profile. This benefit was observed despite the significant interval of 9.5 months between RT and pembrolizumab treatment. Another major milestone occurred when Antonia et al. [24] found that in the PACIFIC trial, patients who started durvalumab (anti-programmed death-ligand 1 [anti-PD-L1] antibody) within 2 weeks after completing chemo-RT (CRT) survived longer than those who started durvalumab at 4 weeks. The PACIFIC trial, a randomized phase III trial (NCT02125461), enrolled patients with stage III unresectable NSCLC who received at least 2 cycles of platinum-based CRT. These patients were then assigned to receive durvalumab or placebo. This trial demonstrated improved OS and PFS in patients with NSCLC receiving durvalumab post-CRT [24, 25]. The latest analyses demonstrated robust and sustained OS and durable PFS benefits [26]. PACIFIC-R (NCT03798535) is a large, real-world, retrospective study of patients who received the PACIFIC regimen. Better real-world PFS outcomes were also observed among patients who received durvalumab closer to the end of RT, which is consistent with the findings from the PACIFIC trial [27]. These findings provide compelling evidence supporting the potential of RT to elicit a systemic antitumor immune response, inducing an abscopal effect. However, certain factors, including PD-1/PD-L1, impede RT-induced abscopal effects, highlighting the role of ICIs in enhancing the efficacy of RT.
The purpose of this review is to provide a comprehensive and balanced examination of the combination of RT and immunotherapy in cancer treatment. We aim to present an overview of the numerous clinical trials, such as the SPRINT, DOLPHIN, PEMBRO-RT, and MDACC trials, which have released their findings in the past five years. Our goal is to shed light on the successes and setbacks of these trials, highlighting the need for a nuanced understanding of this combination therapy. In addition to summarizing the preclinical mechanisms that enhance antitumor immune responses through RT, our objective extends to exploring the potential optimization of this combined strategy, including challenges such as fractionation and scheduling, lymph node irradiation, and toxicity management. Ultimately, this review seeks to provide insights into the potential and hurdles of translating this combination therapy into clinical practice, offering a realistic view of the current state of research and possible future directions.
2 MECHANISMS AND PRECLINICAL EVIDENCE
Several reviews have discussed the preclinical mechanisms of synergy between RT and immunotherapy [28-30]. In this section, we present an updated summary of recent preclinical studies on the impact of RT on the immune system via in situ vaccination and immune reprogramming.
2.1 In situ vaccination
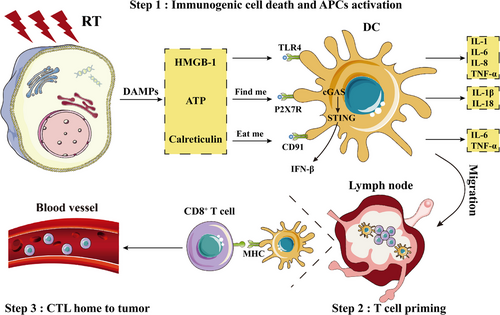
The immune mechanisms triggered by RT encompass three essential processes: immunogenic cell death and arousal of antigen-presenting cells, T cell priming in lymph nodes, and effector T cells homing to tumors [31, 32] (Figure 2). RT-damaged tumor cells release various tumor-associated antigens and damage-associated molecular patterns (DAMPs), including high-mobility group box 1 [33, 34] and adenosine triphosphate [35, 36]. Increased DAMPs activate dendritic cells (DCs) and trigger the MyD88 pathway, inducing a cascade of cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-6, and IL-8 [37, 38]. Furthermore, RT enhances the expression of calreticulin on the cell surface, acting as an “eat me” signal [39-41], and upregulates the expression of major histocompatibility complex (MHC) class I on tumor cells [42, 43]. RT enhances antigen cross-presentation within draining lymph nodes (DLNs) [43, 44]. During this process, activated DCs migrate to DLNs, where they present antigens to T cells [45]. After education, T cells, mainly CD8+ T cells, leave the DLNs and circulate throughout the body, patrol for tumor antigens, and target both irradiated and non-irradiated tumor deposits, thereby promoting the regression of distant tumors, an intriguing phenomenon known as the abscopal response [46-49].
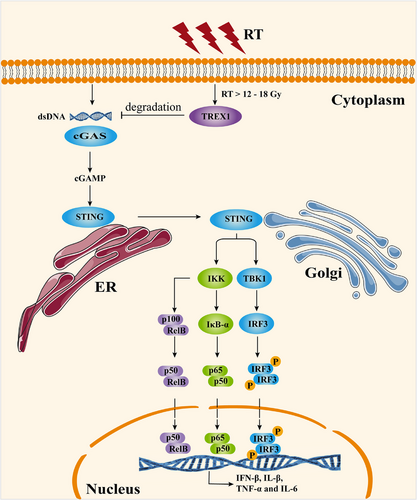
Cellular responses after RT are intricate and multifaceted, involving various signaling pathways that affect immune system function. There is a recurring consensus that non-tumor cell stimulator of interferon genes (STING) is a crucial factor [50, 51] (Figure 3). Radiation-generated cytoplasmic double-stranded DNA fragments trigger cyclic GMP-AMP (cGAMP) synthase activation, leading to the synthesis of the secondary messenger cGAMP [52]. This, in turn, recruits TANK-binding kinase 1 and IκB kinase [53, 54], initiating the transcription of inflammatory cytokines, especially interferon-β (IFN-β) [50, 55]. Notably, Vanpouille-Box et al. [56] showed that RT ranging from 12 Gy to 18 Gy activates three prime repair exonuclease 1 (TREX1) within tumor cells, thereby orchestrating the degradation of radiation-induced cytoplasmic double-stranded DNA.
2.2 Immune reprogramming
RT also induces immune reprogramming by dynamically altering the immune milieu in response to treatment [57]. This causes the release of chemokines, including C-X-C motif chemokine ligand 9 (CXCL9) [58], CXCL10 [58, 59], and CXCL16 [60, 61], leading to the infiltration and accumulation of immune cells and reprogramming of the tumor microenvironment (TME) [62-64]. DCs [46], CD8+ T cells [65], natural killer (NK) cells [66], as well as regulatory T cells (Tregs) [67, 68] and myeloid-derived suppressor cells (MDSCs) [69], are involved in this process (Figure 4).
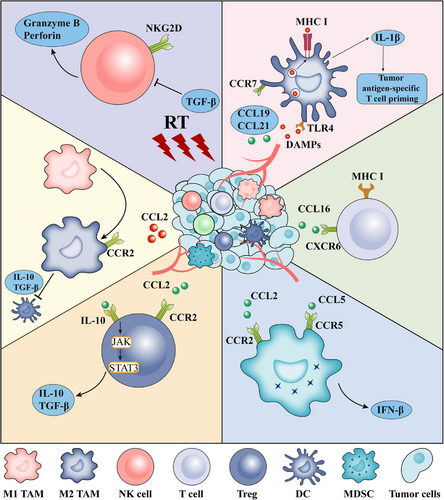
Immune cells exhibit differential sensitivities to radiation. For instance, immature DCs can tolerate radiation doses of 10 Gy to 30 Gy, while retaining their viability and functionality. However, mature DCs, which are crucial for immune activation, may be more radiation-sensitive [70]. Tregs are more resistant to RT than other lymphocytes [71, 72]. Recently, irradiated DCs were found to decrease the secretion of IL-12 and IL-23 cytokines, a reduction, in turn, mitigated by irradiated fibroblasts [73].
Significant CD4+ T helper 1 and CD8+ T cytotoxic 1 polarization was observed in tumor DLNs after RT [74]. To delve deeper into the underlying potentiation of RT and ICIs, Rudqvist et al. [75] identified the separate contributions of each therapy (RT and anti-cytotoxic T-lymphocyte-associated antigen 4 [anti-CTLA-4] antibody) to the T cell population. They found that the anti-CTLA-4 antibody expanded CD4+ T helper 1 cells and RT expanded exhausted CD8+ T cells. However, in the combination group, Tregs were reduced while CD8 effector memory, early activation, and precursor exhausted T cells were expanded compared to those in the control and monotherapy groups.
NK cells are cytotoxic innate lymphoid cells essential for the innate immunosurveillance of tumors [76, 77]. NK cells were activated by irradiated tumor cells and could regulate the response to RT and CTLA-4 blockade [66, 78]. Combining RT with the adoptive transfer of NK cells has been shown to prolong survival compared with that of RT alone [79].
Tumor-associated macrophages (TAMs) are the most abundant tumor-infiltrating lymphocytes in the TME [80-83]. TAMs are phenotypically and functionally diverse and can be broadly divided into two types: pro-inflammatory M1 and anti-inflammatory M2 macrophages [84-87]. RT can shift macrophage differentiation to the M1 phenotype, indirectly increasing tumor infiltrating lymphocytes (TIL) frequency [88, 89]. Moreover, depletion of TAMs can reverse immunosuppression and promote RT efficacy [90].
Tregs, characterized by high forkhead box protein P3 (Foxp3) and CD25 expression, inhibit antitumor immunity, thereby promoting tumor development [91-93]. Different doses of RT can increase the expression of CTLA‑4 on Tregs as well as the level of transforming growth factor-β (TGF-β) secreted by Tregs [94]. Moreover, in a murine model of HNSCC, RT upregulated C-C motif chemokine 2 (CCL2) chemokine production in tumor cells, resulting in the C-C chemokine receptor type 2 (CCR2)-dependent accumulation of CCR2+ Tregs. This reduces the efficacy of RT [95]. Treg depletion combined with RT can significantly enhance immune-promoting effects, as well as reduce tumor burden and improve OS [44, 96, 97].
MDSCs are a cluster of cells with immunosuppressive effects that are classified into 2 distinct subsets: polymorphonuclear and monocytic MDSCs [98-100]. RT induces MDSC expansion and recruitment in murine models and humans [69]. Interestingly, a transient significant increase in the percentage of MDSCs was observed 3 days after RT, which then decreased at day 14 after RT [101]. The upregulation of CCL2, CCL7, and CCL12 after high-dose radiation (20 Gy) led to the accumulation of CCR2+ MDSC in the TME [102]. The CCR2 blockade abrogates RT-induced MDSCs [102, 103]. A recent study found that RT-induced YT521B homology domain family 2 (YTHDF2) expression and YTHDF2 deficiency reversed the accumulation of MDSC following local RT, improving the effects of combined RT and/or anti-PD-L1 treatment [104].
In summary, while RT-recruited immunosuppressive cells can potentially hinder immune-stimulatory responses, it has been observed that RT can ultimately increase the effector-to-suppressor cell ratio in the TME [33]. This, combined with the upregulation of PD-L1 expression induced by RT, suggests that combining RT with ICIs could be a promising strategy for treating solid tumors [105-108] (Table 1).
| Preclinical study | Tumor model | RT | Immunotherapy | Results |
|---|---|---|---|---|
| Chen et al., 2021 [182] | Lewis lung carcinoma | 4 fractions of 10 Gy | anti-PD-L1 | Suppressed the tumor growth, improved survival, and prolonged immune memory |
| Hong et al., 2020 [183] | MFC gastric cancer | 3 fractions of 5 Gy | anti-PD-1 | Improved antitumor activities in RT-insensitive tumor models |
| Philippou et al., 2020 [184] | TRAMP-C1 and MyC-CaP prostate cancer | 3 fractions of 5 Gy | anti-PD-L1 | Tumor growth delay and immune cell changes |
| Grapin et al., 2019 [185] | Subcutaneous CT26 colorectal carcinoma | 3 fractions of 8 Gy, 2 fractions of 18 Gy and 1 fraction of 16.4 Gy | anti-PD-L1, anti-TIGIT | 3 fractions of 8 Gy were the most effective protocol |
| Oweida et al., 2018 [186] | MOC2 and LY2 squamous cell carcinoma | 1 fraction of 20 Gy, 3 fractions of 8 Gy | anti-PD-L1, anti-TIM-3 | Targeting Tregs enhances therapeutic response to RT and dual immune checkpoint blockade |
| Rodriguez-Ruiz et al., 2017 [187] |
Subcutaneous MC38 colon adenocarcinoma |
3 fractions of 8 Gy | anti-PD1, anti-CD137 or both | Brachytherapy with immunotherapy can potentiate the abscopal effect |
| Young et al., 2016 [188] | Subcutaneous CT26 colorectal carcinoma | 1 fraction of 20 Gy | anti-CTLA-4 and anti-TNFRSF4 | Ideal timing of RT is dependent on the mechanism of action of the respective immunotherapy utilized |
| Hao et al., 2016 [189] | Subcutaneous LLC | 1 fraction of 6 Gy | anti-CD40 | RT combined with anti-CD40 boosts the abscopal effect |
| Habets et al., 2016 [190] | Subcutaneous 67NR mammary carcinoma | 3 fractions of 8 Gy | Flt3L | HFRT with Flt3L induces abscopal effect |
| Twyman-Saint et al., 2015 [191] | Subcutaneous TSA and B16-F10 | 3 fractions of 8 Gy or 1 fraction of 20 Gy | anti-CTLA-4, anti-PD-1, and anti-PD-L1 | Anti-CTLA-4 decreased Tregs, anti-PD-L1 reinvigorates exhausted T cells, and RT increases the TCR repertoire |
| Deng et al., 2014 [105] | Subcutaneous TUBO mammary carcinoma and MC38 | 1 fraction of 12 Gy | anti-PD-L1 |
Increased T-cell infiltration boosts abscopal effect |
| Lee et al., 2009 [45] | Subcutaneous 4T1 and B16-CCR7 | 2 fractions of 12 Gy | TNFSF14 | Increased abscopal effect was CD8+ T cell-mediated |
| Dewan et al., 2009 [192] | Subcutaneous 4T1 and MC38 | 5 fractions of 6 Gy, 3 fractions of 8 Gy, or 1 fraction of 20 Gy | anti-CTLA-4 | Fractionated but no single fraction RT with immunotherapy induced abscopal effect |
| Demaria et al., 2005 [193] | Subcutaneous 4T1 mammary carcinoma | 2 fractions of 12 Gy | anti-CTLA-4 | A promising new strategy against poorly immunogenic metastatic cancers |
| Demaria et al., 2004 [194] | Subcutaneous 67NR | 1 fraction of 2-6 Gy | Flt3L | Synergism through T cell-mediated abscopal effect |
| Chakravarty et al., 1999 [195] | Subcutaneous LLC | 60 Gy | Flt3L | Improved survival and reduced pulmonary metastases |
- Abbreviations: CTLA-4, cytotoxic T-lymphocyte associated protein 4; Flt3L, Flt3 ligand; HFRT, hypofractionated radiotherapy; LLC, Lewis lung carcinoma; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; RT, radiation therapy; TCR, T cell receptor; TIGIT, T cell immunoreceptor with immunoglobulin and ITIM domain; TIM-3, T cell immunoglobulin and mucin domain-3; TNFRSF4, TNF receptor superfamily member 4; Treg, regulatory T cell.
3 EVIDENCE FROM CLINICAL TRIALS
Previous case reports and limited clinical trials have demonstrated promising results when combining RT with immunotherapy [109-112]. Despite the recent disclosure of the results of several clinical trials, the future of this field remains uncertain. SBRT, also known as SABR, is the most commonly used treatment in clinical trials [113]. In this section, we discussed the results of clinical trials and findings revealed over the past five years, focusing primarily on NSCLC, where the most promising results have been observed. We also summarized the relevant clinical trials combining RT with ICIs, with a predominant emphasis on NSCLC (Table 2) and other solid tumors (Table 3).
| Condition | NCT number | Status | Phase | Immunotherapy | RT (dose and fractionation) | Timing and sequencing |
|---|---|---|---|---|---|---|
| Early-stage NSCLC | NCT03383302 | Unknown | Phase I/II | Nivolumab | SBRT (N/A) | RT prior to immunotherapy |
| Early-stage NSCLC | NCT04271384 | Recruiting | Phase II | Nivolumab | SBRT (N/A) | Concurrent |
| Stage I NSCLC | NCT03446547 | Unknown | Phase II | Durvalumab | SBRT (N/A) | RT prior to immunotherapy |
| Stage I NSCLC | NCT04944173 | Not yet recruiting | Phase II | Durvalumab | SBRT (N/A) | Concurrent |
| Stage I-II NSCLC | NCT03383302 | Unknown | Phase I/II | Nivolumab | SBRT (N/A) | RT prior to immunotherapy |
| Stage I-IIA NSCLC | NCT03110978 | Active, not recruiting | Phase II | Nivolumab | SBRT (N/A) | Concurrent |
| Stage III NSCLC | NCT05157542 | Recruiting | Phase I | Durvalumab | Low dose RT (N/A) | Concurrent |
| Stage II-III NSCLC | NCT04310020 | Recruiting | Phase II | Atezolizumab | HFRT (N/A) | RT prior to immunotherapy |
| Metastatic NSCLC | NCT03168464 | Completed | Phase I/II | Ipilimumab/nivolumab | SBRT (30 Gy in 6 fractions) | Concurrent |
| Stage IV NSCLC | NCT01860430 | Completed | Phase I | Pembrolizumab | Palliative EBRT (N/A) | Concurrent |
| Stage IV NSCLC | NCT02303990 | Completed | Phase I | Pembrolizumab | SBRT (N/A) | Concurrent |
| Locally advanced or metastatic NSCLC | NCT05111197 | Recruiting | Phase III | anti-PD-1 or anti-PD-L1 | SBRT (24 Gy in 3 fractions) | Concurrent |
| Advanced NSCLC | NCT02839265 | Completed | Phase II | FLT3 ligand (CDX-301) | SBRT (N/A) | Concurrent |
| Advanced NSCLC | NCT04491084 | Recruiting | Phase I/II | FLT3 ligand (CDX-301) and anti-CD40 antibody (CDX-1140) | SBRT (N/A) | Concurrent |
| Metastatic NSCLC | NCT03825510 | Completed | Phase II | Nivolumab/Pembrolizumab | SBRT (N/A) | RT prior to immunotherapy |
| Stage III NSCLC | NCT04577638 | Active, not recruiting | Phase II | Nivolumab | IMRT (N/A) | Concurrent |
| Stage IV NSCLC | NCT03867175 | Active, not recruiting | Phase III | Pembrolizumab | SBRT (N/A) | Concurrent |
| Stage IV NSCLC | NCT02608385 | Active, not recruiting | Phase I | Pembrolizumab | SBRT (N/A) | Concurrent |
| Stage IV NSCLC | NCT02221739 | Completed | Phase I/II | Ipilimumab | IMRT (30 Gy in 5 fractions) | Concurrent |
| Metastatic NSCLC | NCT03224871 | Completed | Phase I | IL-2 | HFRT (N/A) | Concurrent |
| NSCLC | NCT03217071 | Completed | Phase II | Pembrolizumab | SBRT (a single 12 Gy dose) | Immunotherapy prior to RT |
| Metastatic NSCLC | NCT02463994 | Completed | Early phase I | MPDL3280A (Anti-PD-L1) | IMRT (N/A) | RT prior to immunotherapy |
| Stage IV NSCLC | NCT03812549 | Completed | Phase I | Sintilimab (Anti-PD-1) | SBRT (N/A) | Immunotherapy prior to RT |
| Stage IIIb or Stage IV NSCLC | NCT00879866 | Completed | Phase I | EMD 521873 (immunocytokine) | RT (20 Gy in 5 fractions) | RT prior to immunotherapy |
| NSCLC | NCT02221739 | Completed | Phase I/II | Ipilimumab | IMRT (N/A) | Concurrent |
| Metastatic NSCLC | NCT03158883 | Completed | Early phase I | Avelumab | SBRT (N/A) | N/A |
| Stage IIIa/b NSCLC | NCT02434081 | Completed | Phase II | Nivolumab | RT (66 Gy in 33 fractions) | Concurrent |
|
Advanced NSCLC (PEMBRO-RT) |
NCT02492568 | Completed | Phase II | Pembrolizumab | SBRT (24 Gy in 3 fractions) | RT prior to Immunotherapy |
| Stage IV NSCLC | NCT04929041 | Recruiting | Phase II/III | ICIs | SBRT (N/A) | Concurrent |
| Stage IV NSCLC | NCT03223155 | Active, not recruiting | Phase I | Ipilimumab/nivolumab | SBRT (N/A) | Concurrent and sequential |
| Metastatic colorectal cancer or NSCLC | NCT02888743 | Active, not recruiting | Phase II | Tremelimumab and durvalumab | High/low dose RT (N/A) | Immunotherapy prior to RT |
| Metastatic or locally advanced NSCLC | NCT05000710 | Recruiting | Phase II | Tremelimumab and durvalumab | Low dose RT (N/A) | Concurrent |
| NSCLC with brain metastases | NCT04889066 | Not yet recruiting | Phase II | Durvalumab | SBRT (N/A) | Concurrent |
| NSCLC | NCT02444741 | Active, not recruiting | Phase I/II | Pembrolizumab | SBRT (N/A) or conventional RT (N/A) | Concurrent |
| NSCLC (KEYNOTE-799) | NCT03631784 | Active, not recruiting | Phase II | Pembrolizumab | RT (60 Gy in 30 daily fractions) | Concurrent |
| Stage II/III NSCLC | NCT04013542 | Recruiting | Phase I | Ipilimumab and nivolumab | RT (N/A) | Concurrent |
| Stage IV NSCLC | NCT03705403 | Recruiting | Phase II | L19-IL2 | SBRT (N/A) | Concurrent |
| Metastatic NSCLC | NCT05034055 | Not yet recruiting | Phase II | Atezolizumab/tiragolumab | SBRT (N/A) | RT prior to immunotherapy |
- Abbreviations: SBRT, stereotactic body radiation therapy; RT, radiation therapy; EBRT, external beam radiation therapy; NSCLC, non-small cell lung cancer; IMRT, intensity modulated radiation therapy; L19-IL2, human recombinant scFv fragment directed against fibronectin containing extra domain, designated L19, combined with the pro-inflammatory interleukin-2; CIRT, carbon ion radiotherapy; HFRT, hypofractionated radiotherapy; HDCRT, high-dose conformal radiotherapy; ICI, immune checkpoint inhibitor; N/A, not applicable.
| Condition | NCT number | Status | Phase | Immunotherapy | RT (dose and fractionation) | Timing and sequencing |
|---|---|---|---|---|---|---|
| Metastatic castration-resistant prostate cancer | NCT01807065 | Completed | Phase II | Sipuleucel-T | SBRT (N/A) | RT prior to immunotherapy |
| Metastatic castration-resistant prostate cancer | NCT01818986 | Completed | Phase II | Sipuleucel-T | SBRT (N/A) | Concurrent |
| Glioblastoma | NCT02313272 | Completed | Phase I/II | Pembrolizumab | EBRT (N/A) | Concurrent |
| Glioblastoma | NCT02968940 | Completed | Phase II | Avelumab | HFRT (30 Gy in 5 fractions) | N/A |
| Advanced melanoma | NCT01497808 | Completed | Phase I | Ipilimumab | Palliative SBRT (N/A) | RT prior to immunotherapy |
| Advanced melanoma | NCT01449279 | Completed | Phase I | Ipilimumab | Palliative EBRT (N/A) | Immunotherapy prior to RT |
| Advanced melanoma | NCT01689974 | Terminated | Phase II | Ipilimumab | SBRT (30Gy in 5 fractions) | Immunotherapy starts day 4 of RT |
| Advanced melanoma | NCT01557114 | Terminated | Phase I | Ipilimumab | EBRT (dose escalation, 9, 15, 18, 24 Gy in 3 fractions) | RT starts week 4 of immunotherapy |
| Advanced melanoma | NCT02406183 | Completed | Phase I | Ipilimumab | SBRT (N/A) | Immunotherapy prior to RT |
|
Melanoma with brain metastases |
NCT01703507 | Completed | Phase I | Ipilimumab | SBRT (N/A) or whole brain EBRT (N/A) | Concurrent |
|
Melanoma with brain metastases |
NCT02115139 | Completed | Phase II | Ipilimumab | Whole brain EBRT (30 Gy in 10 fractions) | Concurrent |
| Melanoma with brain metastases | NCT02097732 | Terminated | Phase II | Ipilimumab | SBRT (N/A) | Immunotherapy prior to RT |
| Stage III-IVb HNSCC | NCT01935921 | Completed | Phase I | Ipilimumab | IMRT (N/A) | Concurrent |
| HNSCC | NCT04220775 | Completed | Phase I/II | Bintrafusp alfa | SBRT (N/A) | Immunotherapy prior to RT |
| HNSCC | NCT02684253 | Completed | Phase II | Nivolumab | SBRT (27 Gy in 3 fractions) | Concurrent |
| Metastatic clear cell renal cell carcinoma | NCT01896271 | Completed | Phase II | IL-2 (proleukin) | SBRT (N/A) | Concurrent |
| Renal cell carcinoma | NCT03065179 | Completed | Phase II | Nivolumab/ipilimumab | SBRT (N/A) | Concurrent |
| Metastatic urothelial cancer | NCT02826564 | Completed | Phase I | Pembrolizumab | SBRT (N/A) | Concurrent and sequential |
| Soft tissue sarcoma | NCT01347034 | Completed | Phase II | Autologous DC intra-tumoral vaccination | EBRT (N/A) | Concurrent |
|
Stage IV soft tissue sarcoma |
NCT02180698 | Completed | Phase I | TLR4 | Palliative EBRT (N/A) | Concurrent |
| Metastatic breast cancer | NCT01862900 | Completed | Phase I | MEDI6469 (anti-OX40) | SBRT (3 dose escalation cohorts of 15 Gy, 20 Gy or 25 Gy to liver or lung metastases) | Concurrent |
| Metastatic breast cancer | NCT01421017 | Completed | Phase I/II | TLR7 imiquimod | SBRT (6 Gy in 5 fractions) | Concurrent |
| Advanced solid tumors with liver and lung metastases | NCT02239900 | Completed | Phase I | Ipilimumab |
SBRT (50 Gy in 4 fractions to 1-4 lesion[s]) |
Immunotherapy prior to RT |
| Recurrent or metastatic solid tumors | NCT02318771 | Unknown | Phase I | Pembrolizumab | SBRT (N/A) | Concurrent |
| Metastatic colorectal cancer | NCT02437071 | Active, not recruiting | Phase II | Pembrolizumab | IMRT (N/A) | Concurrent |
|
Colorectal cancer with liver Metastases |
NCT03101475 | Completed | Phase II | Durvalumab | SBRT (10 Gy in 3 fractions) | RT starts day 8 of immunotherapy |
| Locally advanced rectal cancer | NCT04663763 | Not yet recruiting | Phase II | Sintilimab(anti-PD-1)/capecitabine/Oxaliplatin | Shor-course RT (25 Gy in 5 fractions) | RT prior to immunotherapy |
| Penile cancer | NCT03686332 | Active, not recruiting | Phase II | Atezolizumab | RT (33 fractions of 1.5 Gy or 1.8 Gy) | Concurrent |
| Metastatic anaplastic thyroid cancer | NCT03122496 | Completed | Phase I | Durvalumab/tremelimumab | SBRT (27 Gy in 3 fractions) | Immunotherapy prior to RT |
| Solid tumors | NCT02086721 | Completed | Phase I | L19-IL2 (immunocytokine) | SBRT (N/A) | Concurrent |
| Solid tumors | NCT05097781 | Recruiting | Phase II | Anti-PD-1 antibody | RT (N/A) | N/A |
| Solid tumors | NCT05229614 | Recruiting | Phase II | Pembrolizumab | CIRT (N/A) | Immunotherapy prior to RT |
| Solid tumors | NCT02239900 | Completed | Phase I | Ipilimumab | SBRT (N/A) | Concurrent and sequential |
| Solid tumors | NCT03220854 | Completed | Phase II | Anti-PD-1/PD-L1 antibody | SBRT (N/A) | N/A |
| Solid tumors | NCT02987166 | Completed | Phase I | Pembrolizumab | HDCRT (N/A) | Concurrent and sequential |
| Solid tumors | NCT02474186 | Completed | Phase I/II | GM-CSF | RT (35 Gy in 10 fractions) | Concurrent |
| Solid tumors | NCT03313804 | Recruiting | Phase II | ICIs | SBRT (N/A) | Immunotherapy prior to RT |
- Abbreviations: CIRT, carbon ion radiotherapy; DC, dendritic cell; EBRT, external beam radiation therapy; GM-CSF, granulocyte-macrophage colony-stimulating factor; HDCRT, high-dose conformal radiotherapy; HFRT, hypofractionated radiotherapy; HNSCC, head and neck squamous cell carcinoma; ICI, immune checkpoint inhibitor; IMRT, intensity modulated radiation therapy; L19-IL2, human recombinant scFv fragment directed against fibronectin containing extra domain, designated L19, combined with the pro-inflammatory interleukin-2; mCRPC, metastatic castration-resistant prostate cancer; N/A, not applicable; RT, radiation therapy; SBRT, stereotactic body radiation therapy.
3.1 Early-stage NSCLC
The RTOG 0236 trial showed that SBRT achieved a high rate of tumor control in patients with medically inoperable early-stage NSCLC [114]. These patients are typically treated with definitive SBRT as the standard of care [115]. However, a longer follow-up period revealed additional cancer recurrences [116, 117]. Preclinical evidence suggests that ICIs combined with SBRT may facilitate preoperative immunotherapy without compromising antitumor efficacy, making it a safer option for neoadjuvant therapy [118].
An open-label phase II trial (NCT02904954) compared durvalumab alone with durvalumab plus SBRT (3 × 8 Gy) for early-stage NSCLC [119]. Major pathological response (MPR) rates were significantly higher in the durvalumab plus SBRT group (16/30 patients) than in the durvalumab alone group (2/30 patients). Notably, half of the patients in the dual therapy group with MPR showed complete pathological response [119].
A recent open-label phase II trial (NCT03110978) evaluated the efficacy and safety of SBRT alone and in combination with nivolumab for early-stage NSCLC. Compared with that of the SBRT group, the combination therapy group showed significantly improved four-year event-free survival with tolerable toxicity [120]. ISABR (NCT03148327), a multicenter prospective clinical trial, recently reported the safety and efficacy of the combination of SBRT and durvalumab in 18 patients [121]. These results suggest that pulmonary toxicity risk is the greatest concern for combination therapy [121].
3.2 Oligometastases
The concept of an oligometastatic state was first postulated in 1995 [122]. This hypothesis posits that metastases may be limited to specific organs in limited numbers [123], implying potential curability with localized interventions, including RT or surgery [124]. Earlier evidence supports the safety and efficacy of SBRT for oligometastases [125]. In limited metastatic NSCLC, SBRT before maintenance chemotherapy significantly improved PFS compared with maintenance chemotherapy alone [126]. The SABR-COMET phase II trial showed that SBRT improved OS [127].
Harnessing the innate and adaptive immunity is vital for restricting metastatic development [128-130]. Pitroda et al. [131] identified that the upregulation of immune-related genes in colorectal liver metastases was associated with better clinical outcomes. Accordingly, the integration of immunotherapy with SBRT has been proposed for oligometastases [132] (Table 4). Luke et al. [133] conducted a phase I trial and found that pembrolizumab and SBRT combination therapy had an acceptable safety profile. A subsequent clinical study (NCT02316002) showed improved PFS with reduced quality of life after SBRT for oligometastatic NSCLC [134].
| Condition | NCT number | Status | Phase | Immunotherapy | RT (dose and fractionation) | Timing and sequencing |
|---|---|---|---|---|---|---|
| Melanoma | NCT01565837 | Unknown | Phase II | Ipilimumab | SBRT (N/A) | Immunotherapy prior to RT |
| Melanoma | NCT01416831 | Active, not recruiting | Phase II | IL-2 | SBRT (N/A) | Concurrent |
| Melanoma | NCT02107755 | Unknown | Phase II | Ipilimumab | SBRT (N/A) | SBRT starts 5 weeks after first dose of ipilimumab |
| Solid tumors | NCT05259319 | Not recruiting | Phase I | Atezolizumab and tiragolumab | SBRT (24 Gy in 3 fractions) | Sequential |
| NSCLC | NCT03275597 | Terminated | Phase I | Durvalumab and tremelimumab | SBRT (N/A) | RT prior to immunotherapy |
| NSCLC | NCT03965468 | Active, not recruiting | Phase II | Durvalumab | SBRT (N/A) | Concurrent |
| NSCLC | NCT04549428 | Recruiting | Phase II | Atezolizumab | RT (a single fraction of 8 Gy) | Concurrent |
| NSCLC | NCT04238169 | Recruiting | Phase II | Toripalimab | SBRT (N/A) | Concurrent |
- Abbreviations: IL-2, interleukin-2; N/A, not applicable; NSCLC, non-small cell lung cancer; RT, radiation therapy; SBRT, stereotactic body radiation therapy.
The results from a randomized phase II trial have established the use of SBRT to all lesions, becoming the standard of care for patients with oligometastatic NSCLC [135]. A multicenter prospective observational study aimed to determine whether concomitant anti-PD-1 and SABR could enhance tumor response in metastatic NSCLC and melanoma. In this study, all patients received concurrent pembrolizumab or nivolumab and SABR to 1 to 5 lesions, with the anti-PD-1 treatment continuing until further progression, unacceptable toxicity, or a medical/patient decision to discontinue. The objective response rate (ORR) was 42%, and the median PFS was 14.2 months [136]. This approach achieved high response rates and extended the clinical benefits of immunotherapy by delaying further progression and developing a new systemic therapy.
3.3 Locally advanced (LA)-NSCLC
Approximately one-third of patients with NSCLC are initially diagnosed with LA disease [137]. Based on the finding of the PACIFIC trial [24, 25], concurrent CRT (cCRT) followed by consolidation durvalumab (the PACIFIC regime) became the standard of care for patients with LA-NSCLC. With updated suboptimal 5-year OS and PFS rates [26], novel treatment strategies are under investigation to improve clinical outcomes. One such approach was the GEMSTONE-301 phase III trial (NCT03728556) [138] and the PACIFIC-6 phase II trial (NCT03693300) [139]. Both trials demonstrated that ICI after sequential CRT (sCRT) is an effective consolidation therapy for LA-NSCLC, suggesting that sCRT followed by ICI could be an alternative for patients unsuitable for the PACIFIC regimen [140].
Trials exploring the use of ICIs concurrently with cCRT or sCRT have also been conducted. KEYNOTE-799 is a nonrandomized phase II trial of pembrolizumab concurrent with cCRT as the initial therapy for the treatment of LA-NSCLC, with an ORR of 70.5% [141]. The PACIFIC-2 trial (NCT03519971) randomized patients to receive durvalumab or a placebo concurrently with CRT. On November 14, 2023, the news that durvalumab administered concurrently with CRT failed to achieve statistical significance for PFS compared with CRT alone was announced [142]. The failure of the PACIFIC-2 trial could be attributed to several factors, including the toxicity caused by immature treatment regime. Thus, the optimal regime of RT still requires further exploration and research.
Furthermore, the induction of ICIs before CRT in patients with LA-NSCLC is currently being explored in clinical trials. The prospective AFT-16 study (NCT03102242) evaluated the safety and efficacy of atezolizumab before CRT. The primary endpoint of the disease control rate at 12 weeks was 77.4% [143]. Recently, the analysis of secondary endpoints was updated. The median PFS was 23.7 months. The median OS is not yet estimable [144]. Owing to the encouraging PFS and OS rates without unexpected safety signals, further studies are warranted. In a retrospective study, patients with LA-NSCLC received standard of care or induction ICIs, followed by standard of care. Although the OS and PFS rates between the 2 groups were similar, the induction ICIs group had a significantly lower distant metastasis rate [145].
For patients unable to complete the PACIFIC regimen due to chemotherapy-induced adverse effects (AEs), combining RT with immunotherapy can reduce toxicity while maintaining survival [146]. The SPRINT study (NCT03523702) is a prospective phase II trial that tested sequential pembrolizumab and RT. Patients with LA-NSCLC (n = 25) having PD-L1 tumor proportion score (TPS) ≥ 50% were enrolled. The primary endpoint was PFS. The actuarial 1-year PFS rate was 74% and the actuarial 1-year OS rate was 95% [147]. The promising initial results suggest that sequential pembrolizumab and RT may be an alternative treatment approach for patients in this setting. Further clinical trials should be designed to optimize the SPRINT regimen and compare it with the standard of care. The DOLPHIN study was a nonrandomized, single-arm, phase II trial. Patients with PD-L1 positive, LA-NSCLC received RT (60 Gy) concurrently with durvalumab, followed by maintenance durvalumab therapy. The 12-month PFS rate was 72.1%, far exceeding the 28% set under the original hypothesis. The median PFS was 25.6 months and the ORR was 90.9% [148]. This study is expected to support further development of phase III clinical trials. The START-NEW-ERA study (NCT05291780) was a single-arm phase II trial that explored the efficacy of SBRT combined with immunotherapy in patients with LA-NSCLC. The median OS was 55 months [148, 149]. Early outcomes suggest that SBRT followed by ICIs may be a suitable treatment regimen for these patients. The TRADE-hypo study (NCT04351256), a prospective randomized phase II trial, addressed the safety and efficacy of durvalumab combined with either conventional (30 × 2.00 Gy) or hypofractionated (20 × 2.75 Gy) RT. The primary end point was ORR [150]. Preliminary results were showcased at the 2024 ESMO meeting. Interim futility analysis was conducted in the conventional RT arm and was positive with 11/18 patients achieving tumor response (5 stable disease, 2 progressive disease). This suggests that for patients who are not suitable for chemotherapy, a novel combination of duvalizumab and conventional chest RT could potentially be beneficial. Additional safety, efficacy, and biomarker data are expected to be provided in May 2025.
3.4 Metastatic NSCLC
The PEMBRO-RT (NCT02492568) and MDACC (NCT02444741) trials provided solid evidence for combining RT and ICIs for metastatic NSCLC. Both trials enrolled patients with metastatic NSCLC who were randomly assigned to receive either pembrolizumab alone or SBRT. In the phase II PEMBRO-RT trial, pembrolizumab was administered within 7 days of the completion of SBRT (3 × 8 Gy). The primary endpoint of 12-weeks ORR was improved from 20% in the pembrolizumab alone arm to 50% in the pembrolizumab after RT arm [151]. Intriguingly, subgroup analyses showed the largest benefit from the addition of RT in patients with PD-L1-negative tumors, which significantly improved PFS and OS. In the phase I/II MDACC trial, SBRT was 50 Gy/4 fractions or 45 Gy/15 fractions. No significant differences in ORR or PFS were observed; however, exploratory analyses suggested that, for patients with low PD-L1 expression, a longer median PFS was observed in the ICIs plus RT group [152]. None of the trials met the preset criteria for meaningful clinical benefit owing to the small sample size. A pooled analysis showed significantly longer median PFS (9.0 vs. 4.4 months) and median OS (19.2 vs. 8.7 months) in the pembrolizumab plus RT group, with no new safety concerns [153]. Unfortunately, this pooled study could not draw conclusions regarding the optimal dose and timing of RT to induce a distant response, which should be further confirmed in specialized, large, randomized trials.
While RT combined with ICIs shows promise for NSCLC, the enhanced antitumor effect appears to be limited to other tumor types [154]. Indeed, evidence to date has shown that combination therapy has limited benefits for most patients with HNSCC. A phase II trial (NCT02684253) enrolled patients with metastatic HNSCC who were randomly assigned to receive nivolumab alone or nivolumab in combination with SBRT. Regrettably, no improvement in clinical outcomes or evidence of the abscopal effect was found [155]. In a phase II study involving 18 patients with relapsed small cell lung cancer (SCLC) (NCT02701400), durvalumab and tremelimumab were administered with or without SBRT (3 × 9 Gy). SBRT was administered as an immune sensitizer prior to ICIs treatment. However, neither OS nor PFS showed a significant difference in the two arms [156]. In a recent trial (NCT03104439) involving patients with hepatocellular carcinoma and portal vein tumor thrombus, camrelizumab and apatinib were administered with or without SBRT (36-40 Gy/6-8 Gy). Longer median OS (12.7 vs. 8.6 months) and median PFS (4.6 vs. 2.5 months) were observed in the ICIs plus SBRT group [157]. This combination regimen showed clinical benefits with an acceptable safety profile, and may be a promising first-line therapy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Regarding immune desert tumors, including microsatellite-stable colorectal cancer and pancreatic ductal adenocarcinoma, despite their limited response to immunotherapy alone, a phase II trial (NCT03104439) showed that RT can enhance the immunotherapy response even in these cases [158].
Collectively, RT combined with ICIs has demonstrated encouraging outcomes in patients with NSCLC and certain solid tumors. However, many clinical studies investigating the efficacy of combining RT with ICIs have not included ICI or RT monotherapy. This omission obstructed the ability to discern a synergistic therapeutic benefit from combination therapy compared with the effects of either monotherapy independently.
4 CHALLENGES AND CONCERNS
4.1 Fractionation and scheduling
For decades, conventionally fractionated radiotherapy (CFRT) has typically been delivered at doses of 1.8 to 2.0 Gy per fraction, 5 days per week, for 5-8 weeks. In contrast, hypofractionated radiotherapy (HFRT) delivers large doses in one-fifth fractions and is increasingly used in clinical practice. The most prominent example of an HFRT is SBRT. Biological differences between SBRT and CFRT exist [159]. Low-dose irradiation with a single dose (0.5-1.0 Gy) has been suggested to modulate the TME and activate immune responses [88, 160, 161]. Low-dose HFRT refers to a higher dose per fraction but a lower total dose of radiation for cancer treatment, both used in preclinical models [88] and clinical trials [162, 163].
Preclinical studies have emphasized the fractionation and scheduling of RT with ICIs to establish a long-lasting antitumor immune response [164, 165]. The optimal regimen of RT combined with ICIs to maximize the antitumor immune response remains controversial [166]. Recent clinical trials have evaluated the safety and effectiveness of HFRT and low-dose hypofractionated radiotherapy (LDHRT) in combination with ICIs. However, in patients with microsatellite stable (MSS) colorectal cancer, the abscopal effect was not observed in either radiation regime, and the median PFS and OS were limited [167]. A randomized study (NCT02888743) in 2022 evaluated ICIs combined with LDFRT or HFRT in patients with metastatic NSCLC [168]. The study did not identify any significant benefit in ORR for either the LDFRT or HFRT regimens, and no significant differences in PFS or OS were observed between the treatment arms.
A latest phase I/II clinical trial (NCT02239900) compared the administration of concurrent or sequential SBRT with ipilimumab in patients with metastatic cancer [169, 170]. All patients received ipilimumab and were randomly assigned to 5 treatment groups based on tumor size and location. The group that received sequential ipilimumab with SBRT to the lungs showed the highest rate of clinical benefit. A recent systematic literature review sought to reach a consensus among experts on the combination of SBRT and ICIs [171]. The consensus reached was that anti-PD-L1 or anti-PD-1 treatment should continue during SBRT delivery without omission of treatment cycles and that nivolumab plus ipilimumab should not be administered on the same day as SBRT. However, in an observational cohort study of patients with early-stage NSCLC, all patients were treated with SBRT (27 Gy/1 fraction or 50 Gy/5 fractions) targeting different lung lesions [172]. This large retrospective analysis found no statistically significant differences in the 5-year OS or 5-year PFS rates, raising the question of whether the fraction and location matter. Indeed, irradiation of liver metastases in patients with NSCLC has been shown to result in stronger activation of antitumor immunity than irradiation of pulmonary metastases [170]. Several ongoing clinical trials have persistently scrutinized diverse regimens of RT combined with ICIs [173, 174]. Emerging evidence will furnish precise directives for an optimal regime.
4.2 DLNs irradiation and dose
Notably, the total dose of the DLNs irradiation is a topic worthy of discussion. DLNs serve as the site where DCs prime antigen-specific CD8+ T cells, and irradiation of lymphoid organs can result in lymphopenia [45, 175]. A study published in 2018 highlighted that irradiation of the DLN impeded adaptive immune responses and attenuated the efficacy of SBRT and ICIs [67]. A concept termed lymphocyte-sparing RT has been proposed that advocates the sparing of lymphocytes whenever possible [176]. Therefore, precautionary irradiation of the DLNs is recommended [177]. A nonrandomized phase II trial (NCT01463423) enrolled patients into 3 groups based on the tumor stage. The results suggest that individual doses and fractionation of SBRT, including doses lower than those routinely administered, are associated with local tumor control [178]. Therefore, individualized dosing should be considered in future studies.
4.3 Toxicity
Pneumonitis and radiation pneumonitis are the most common AEs associated with the administration of CRT [141]. The overall incidence of pneumonitis in the PACIFIC trial was 33.9% [25, 27]. In real-world studies, the incidences of all-grade and grade ≥3 pneumonitis were 35% and 6%, respectively [179]. The incidence of pneumonitis varies with race and age [146, 179]. The MDACC trial found that most AEs were self-limiting, and no patient in the ICI combined with SBRT group experienced grade 4 or 5 toxic effects [152]. However, another multicenter analysis demonstrated that SBRT with concurrent ICI increased the risk of grade 3 pneumonitis compared with that of SBRT alone [180]. Closer monitoring should be considered in patients who are administered ICIs and RT. Differentiating between RT-induced pneumonitis and immune-related pneumonitis is challenging [27]. Radiomics holds great promise in aiding correct diagnosis [181]. To date, evidence indicates that the observed risk of severe toxicity for SBRT plus anti-PD1/PD-L1 monotherapy is low [171], and it remains to be seen whether the combination simply increases or amplifies toxicity. In the absence of objective data showing that simultaneous administration leads to a significant increase in toxicity, RT combined with ICI is a reasonable strategy in clinical practice.
5 CONCLUSIONS
The immune system's capacity to reject a tumor depends on the presence of neoantigens within cancer cells. RT increases the number of neoantigens via in situ vaccination. Additionally, RT reprograms the TME to foster a durable and systemic immune response. Ongoing studies have explored precision RT based on gene expression profiles to complement the precision of cancer medicine using immunotherapy.
In conclusion, the integration of RT with immunotherapy represents a paradigm shift in cancer treatment. This field awaits the results of the ongoing clinical trials. Over the past five years, promising clinical trial outcomes have been predominantly observed in NSCLC. Conversely, trials investigating other solid malignancies, including HNSCC and colorectal cancer, are limited in number, and the outcomes have been less encouraging. The inherent heterogeneity of tumors may dictate disparate responses to the combination of RT and immunotherapy. A severe limitation of ongoing clinical trials is that they are not biomarker-driven. Biomarker implementation and the identification of distinct patient subsets are priorities. However, challenges, including determining the optimal dosing, fraction, and schedule with the lowest toxicity of the combination therapy, remain. Further research is imperative to refine combination therapy and identify predictive biomarkers for the individualized treatment of solid tumors.
AUTHOR CONTRIBUTIONS
Yuze Wu drafted the manuscript and prepared the figures. Ming Yi, Mengke Niu, and Binghan Zhou helped in collecting the related literatures and participated in discussion. Kongming Wu and Qi Mei designed the review and revised the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (82073370 and 82272794).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




