Interleukin-10 gives exhausted chimeric antigen receptor (CAR) T cells a second breath
List of Abbreviations
-
- ACT
-
- adoptive cell therapy
-
- CAR
-
- chimeric antigen receptor
-
- ICB
-
- immune checkpoint blockade
-
- IL
-
- interleukin
-
- MPC1
-
- mitochondrial pyruvate carrier 1
-
- STAT
-
- signal transducer and activator of transcription
-
- TCF7
-
- T-cell factor 7
-
- TEFF-EX
-
- effector exhausted T cell
-
- TEX
-
- terminally exhausted T cell
-
- TFs
-
- transcription factors
-
- TILs
-
- tumor-infiltrating lymphocytes
-
- TINT-EX
-
- intermediate-exhausted T cell
-
- TPEX
-
- precursor exhausted T cell
-
- TSCM
-
- stem-like memory T cell
Adoptive cell therapy (ACT) represents a major pillar of modern immuno-oncology. Naturally or synthetically endowed with the ability to recognize tumor-associated antigens, tumor-infiltrating lymphocytes (TILs) or T cells engineered to transgenically express T cell receptors or chimeric antigen receptors (CARs) are expanded and infused to tumor patients to lyse tumor cells. Yet, despite tremendous response rates against liquid tumors, many patients undergo relapses, and treatment outcomes in solid tumors have been disappointing so far [1]. The harsh tumor microenvironment, including nutrient deprivation, acidification, hypoxia, and immunosuppressive signals [2, 3], in conjunction with persistent antigen stimulation triggers a program of exhaustion in T cells (Figure 1A). Exhausted T cells exhibit reduced cytotoxicity and minimal proliferation potential, physiologically balancing tissue health with control of chronic viral infection or of tumor growth [4, 5]. Strategies to disengage or rewire the “erroneously” deployed exhaustion program to exploit the full potential of tumor-fighting T cells are highly sought after (Figure 1B). The characteristic ex vivo handling steps to manufacture the cell product offer near-endless opportunities for their selective pharmacologic or genetic manipulation [1, 2].
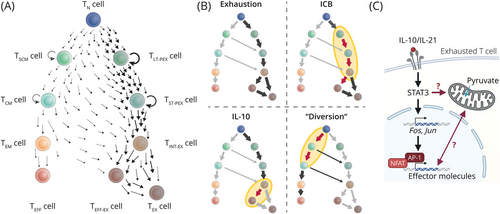
Recently, Zhao et al. [6] reported in Nature Biotechnology that CAR T cells transgenically overexpressing interleukin-10 (IL-10) excelled over second-generation CAR T cells in various syn- and xenogeneic models of liquid and solid tumors. While IL-10 is best known for its anti-inflammatory and even exhaustion-promoting effects [1, 4], cytokines exert pleiotropic effects, and the administration of PEGylated IL-10 [7] or of an IL-10-Fc fusion protein had previously been shown to support expansion and cytotoxicity of exhausted TILs, potentiating ACT, immune checkpoint blockade (ICB) [8], and tumor immune surveillance [7]. Now, the authors took this principle to the next level.
Exhaustion is driven by mitochondrial dysfunction and, at least in part, by subsequent increases in reactive oxygen species. Thus, protecting mitochondrial integrity appears to be fundamental to successful ACT of solid tumors [3]. IL-10 increased mitochondrial fitness of therapeutic T cells as IL-10-expressing CAR T cells had mitochondria with dense, functional morphology and high membrane potential, which culminated in enhanced oxidative phosphorylation, tumor infiltration, and effector functions. Inhibition or genetic deletion of mitochondrial pyruvate carrier 1 (MPC1) largely abrogated the benefit of IL-10 overexpression in in vitro experiments [6] (Figure 1C), suggesting that greater mitochondrial fitness was a prerequisite to preserve effector functions. Remarkably, even the anti-tumor efficacy of CAR T cells using the 4-1BB co-stimulatory domain, which favors oxidative phosphorylation for enhanced metabolic fitness [2], was strengthened by IL-10 overexpression [6].
To understand how mitochondrial fitness bolstered therapeutic efficacy, Zhao et al. [6] investigated intratumoral CAR T cells by single-cell RNA-sequencing. T cell exhaustion is thought to be a continuous process following the same hierarchy as memory T cell differentiation: the least differentiated memory T cells [stem-like memory T (TSCM) cells] show the highest degree of plasticity, proliferation potential, and self-renewal, which they gradually loose with continued proliferation and acquisition of effector functions [9] (Figure 1A). Molecularly, stemness features are reflected in the expression of transcription factors (TFs) T-cell factor 7 (TCF7) and MYB in these cells. Likewise, TCF1 and MYB identify the exhausted T cell subset with the greatest proliferative capacity, designated as (long-term) precursor exhausted T (TPEX) cells [5, 9]. TPEX cells serve as the reservoir for the more differentiated intermediate-exhausted T (TINT-EX) and effector or terminally exhausted T (TEFF-EX/TEX) cells and are thus required for successful ICB (Figure 1A) [4, 5, 9].
Notably, CAR T cells either with or without IL-10 transgene transcriptionally mapped with TEFF-EX/TEX cells in the analysis of Zhao et al. [6], while genes upregulated in intratumoral IL-10-expressing CAR T cells included effector molecules such as granzymes, perforin 1, and interferon-γ as well as the activator protein 1 (AP-1) TFs Jun and Fos (Figure 1C). Unfortunately, the underlying map does not further differentiate TEFF-EX/TEX states. Yet, based on the transcriptional signature and congruent with the effect of IL-21/signal transducer and activator of transcription 3 (STAT3) signaling [5, 9], IL-10 presumably favored TEFF-EX over TEX cell differentiation at the TINT-EX junction [10] (Figure 1B). Indeed, in their previous study, the authors had clearly demonstrated that, unlike ICB [4, 5, 9], IL-10-Fc did not require the proliferation of TPEX cells but reinvigorated TEFF-EX/TEX cells (Figure 1B), which re-gained some effector functions and proliferative capacity [8]. The combination treatment with half-life extended IL-10 and ICB currently undergoes clinical testing with mixed results and a notable rate of adverse events [7]. Given the limited tolerability of high systemic IL-10 doses required for therapeutic efficacy, CAR T cells “armored” with transgenic IL-10 offer the appealing benefit to increase local IL-10 concentrations sufficiently in the absence of systemic toxicity, making the strategy a prime candidate for clinical testing.
Some interesting questions emerge. Typically, AP-1 TFs downstream of STAT3 signaling are known to balance induction of the TF nuclear factor of activated T cells (NFAT) in control of the initiation and progression of exhaustion (Figure 1C), among others, by promoting TPEX maintenance [5, 10]. How IL-10 CAR T cells pass through this developmental checkpoint to form TEFF-EX cells and which genetic programs prevent their attrition may reveal exciting starting points for further improvements of ACT. Interestingly, inhibition of MPC1 or of lactate dehydrogenase A during the CAR T cell manufacturing procedure promoted the cells’ functionality [2], and synergies of these approaches with synthetic IL-10 expression are conceivable. Exploring designer variants of IL-10 [7], including orthogonal IL-10/IL-10R systems, might help to restrain the impact of IL-10 to therapeutic T cells which may further boost their efficacy and safety. Lastly, initial data hinting at improved memory formation of IL-10-expressing CAR T cells might be confounded by differences in antigen load at the analysis timepoint [5, 6] and require further analysis in a more controlled setting.
The development of advanced engineering approaches diverting chronically stimulated T cells from exhaustion programing (Figure 1B) or driving natural or synthetic T cell states that retain both cytotoxicity and persistence, as exemplified in this study, holds great promise of next-generation cell therapies [1]. Reference maps need to be refined and extended to accurately reflect the entire T cell differentiation space including non-physiological differentiation states that can occur, e.g., when using orthologous cytokine systems or synthetic genetic circuits. This will provide higher resolution of developmental trajectories as well as of the transcriptional and epigenetic programs engaged by therapeutic T cells, and aid to identify potential synergies between different optimization strategies.
In summary, the current study highlights IL-10-expressing CAR T cells as an exciting opportunity for clinical translation that may offer a potent solution for solid tumor therapy and a decisive step forward for long-lasting control of liquid tumors.
AUTHOR CONTRIBUTIONS
Christoph Heuser-Loy drafted and revised the manuscript and the figures.
ACKNOWLEDGMENTS
The author thanks the members of the Division for Functional Immune Cell Modulation at the Leibniz Institute for Immunotherapy, Regensburg, Germany, for critical discussion. This work has been supported by funding from the European Innovation Council and Small and Medium-sized Enterprises Executive Agency (Grant Agreement No. 101070740).
CONFLICT OF INTEREST STATEMENT
The author declares no conflict of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




