The regulatory roles and clinical significance of glycolysis in tumor
Abstract
Metabolic reprogramming has been demonstrated to have a significant impact on the biological behaviors of tumor cells, among which glycolysis is an important form. Recent research has revealed that the heightened glycolysis levels, the abnormal expression of glycolytic enzymes, and the accumulation of glycolytic products could regulate the growth, proliferation, invasion, and metastasis of tumor cells and provide a favorable microenvironment for tumor development and progression. Based on the distinctive glycolytic characteristics of tumor cells, novel imaging tests have been developed to evaluate tumor proliferation and metastasis. In addition, glycolytic enzymes have been found to serve as promising biomarkers in tumor, which could provide assistance in the early diagnosis and prognostic assessment of tumor patients. Numerous glycolytic enzymes have been identified as potential therapeutic targets for tumor treatment, and various small molecule inhibitors targeting glycolytic enzymes have been developed to inhibit tumor development and some of them are already applied in the clinic. In this review, we systematically summarized recent advances of the regulatory roles of glycolysis in tumor progression and highlighted the potential clinical significance of glycolytic enzymes and products as novel biomarkers and therapeutic targets in tumor treatment.
List of Abbreviations
-
- 1,3-BPG
-
- 1,3-bisphosphoglycerate
-
- 1H MRS
-
- proton magnetic resonance
-
- 2-[18F] FDG
-
- 2-[18F] fluoro-2-deoxy-d-glucose
-
- 2-DG
-
- 2-Deoxy-D-glucose
-
- 2-PGA
-
- 2-phosphoglycerate
-
- 3-BrPA
-
- 3-Bromopyruvate
-
- 3-PGA
-
- 3-phosphoglycerate
-
- 3PO
-
- 3-(3-Pyridinyl)-1-(4-pyridinyl)-2-propen-1-one
-
- AF
-
- Amentofavone
-
- Akt
-
- protein kinase B
-
- ALDO
-
- aldolase
-
- ALDOA
-
- Aldolase A
-
- Atg7
-
- autophagy-related gene
-
- ATO
-
- Arsenic Trioxide
-
- CCND1
-
- cyclin D1
-
- CDK
-
- cyclin-dependent kinase
-
- CML
-
- chronic myeloid leukemia
-
- c-Myc
-
- Myc proto oncogene
-
- CRC
-
- colorectal cancer
-
- CTL
-
- cytotoxic T lymphocyte
-
- DC
-
- dendritic cell
-
- DFS
-
- disease-free survival
-
- DHAP
-
- dihydroxyacetone phosphate
-
- ECM
-
- extracellular matrix
-
- EGFR
-
- epidermal growth factor receptor
-
- EMT
-
- epithelial-mesenchymal transition
-
- ENO
-
- enolase
-
- ETS1
-
- ETS proto-oncogene 1
-
- F-1,6-BP
-
- fructose-1,6-bisphosphate
-
- F-2,6-BP
-
- fructose-2,6-bisphosphate
-
- F-6-P
-
- fructose-6-phosphate
-
- FGF
-
- fibroblast growth factor
-
- FOXM1
-
- forkhead box protein M1
-
- G-3-P
-
- glyceraldehyde-3-phosphate
-
- G-6-P
-
- glucose-6-phosphate
-
- GAPDH
-
- glyceraldehyde-3-phosphate dehydrogenase
-
- GBM
-
- glioblastoma
-
- GLUT
-
- glucose transporter protein
-
- HCC
-
- hepatocellular carcinoma
-
- HIF-1α
-
- hypoxia inducible factor-1α
-
- HK
-
- hexokinase
-
- HK1
-
- hexokinase 1
-
- IL-6
-
- interleukin 6
-
- IL-8
-
- interleukin 8
-
- LC3
-
- microtubule-associated protein 1 light chain 3
-
- LDH
-
- lactate dehydrogenase
-
- LDHA
-
- lactate dehydrogenase A
-
- LUAD
-
- lung adenocarcinoma
-
- MRS
-
- magnetic resonance spectrum
-
- mTOR
-
- mammalian target of rapamycin
-
- NF-κB
-
- nuclear transcription factor-kappaB
-
- NK
-
- natural killer
-
- NPM1
-
- nucleophosmin
-
- OS
-
- overall survival
-
- OSCC
-
- oral squamous cell carcinoma
-
- PAI-1
-
- plasminogen activator inhibitor-1
-
- PB2
-
- proanthocyanidin B2
-
- PDAC
-
- pancreatic ductal adenocarcinoma
-
- PD-L1
-
- programmed death-ligand 1
-
- PEP
-
- phosphoenolpyruvate
-
- PET
-
- positron emission tomography
-
- PET/CT
-
- positron emission tomography/computed tomography
-
- PFK-1
-
- phosphofructokinase-1
-
- PFK15
-
- 1-(4-pyridinyl)-3-(2-quinolinyl)-2-propen-1-one
-
- PFK-2
-
- phosphofructokinase-2
-
- PFKFB3
-
- 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3
-
- PGAM
-
- phosphoglycerate mutase
-
- PGAM1
-
- phosphoglycerate mutase 1
-
- PGI
-
- phosphohexose isomerase
-
- PGK
-
- phosphoglycerate kinase
-
- PhAH
-
- phosphonoacetohydroxamicacid
-
- Phx-3
-
- 2-aminophenoxazine-3-one
-
- PI3K
-
- phosphatidylinositol 3 kinase
-
- PIK3C3
-
- phosphatidylinositol 3-kinase catalytic subunit type 3
-
- PK
-
- pyruvate kinase
-
- PKM2
-
- pyruvate kinase M2
-
- RA
-
- retinoic acid
-
- STAT3
-
- signal transducer and activator of transcription 3
-
- TA
-
- tannic acid
-
- TADC
-
- tumor-associated dendritic cell
-
- TAM
-
- tumor-associated macrophage
-
- TAN
-
- tumor-associated neutrophil
-
- TME
-
- tumor microenvironment
-
- TNF-α
-
- tumor necrosis factor-alpha
-
- Treg
-
- regulatory T cell
-
- VDAC
-
- voltage-dependent anion channels
-
- VEGF
-
- vascular endothelial growth factor
-
- VK
-
- vitamin K
1 BACKGROUND
Metabolic reprogramming is a series of metabolic changes in response to the stress of various stimuli, involving the pathways of amino acid metabolism, lipid metabolism, and glucose metabolism, which has been closely correlated with the development of multiple diseases. Glucose is the most important substance for maintaining the viability of all kinds of cells and tissues, and its catabolic pathways mainly include anaerobic oxidation (glycolysis), aerobic oxidation (oxidative phosphorylation), and pentose phosphate pathways. Among them, glycolysis is a necessary process of glucose catabolism and refers to the transformation of intracellular lactate from glucose under anaerobic circumstances with the catalysis of various enzymes. Accumulating evidence has revealed that glycolysis is closely connected with a number of disorders containing atherosclerosis [1], neurodegenerative diseases [2], rheumatoid arthritis [3], and so on. By the same token, the indispensable roles of glycolysis in the development of tumors have attracted widespread attention.
Glycolysis catabolizes carbohydrates into lactate under anaerobic conditions, which occurs in the cytoplasm. Glucose is transferred to the cytoplasm by glucose transporter proteins (GLUT), a family containing 14 members [4]. Subsequently, glucose is catalyzed sequentially by hexokinase (HK), phosphohexose isomerase (PGI), phosphofructokinase-1 (PFK-1), aldolase (ALDO), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phosphoglycerate kinase (PGK), phosphoglycerate mutase (PGAM), enolase (ENO), and pyruvate kinase (PK) to produce pyruvate, in which HK, PFK-1 and PK are rate-limiting enzymes. Finally, under low oxygen conditions, lactate dehydrogenase (LDH) catalyzes the formation of lactate from pyruvate. Glycolytic enzymes and products have been shown to be closely implicated in tumor progression, including proliferation, invasion, metastasis, autophagy and immune evasion (Figure 1).
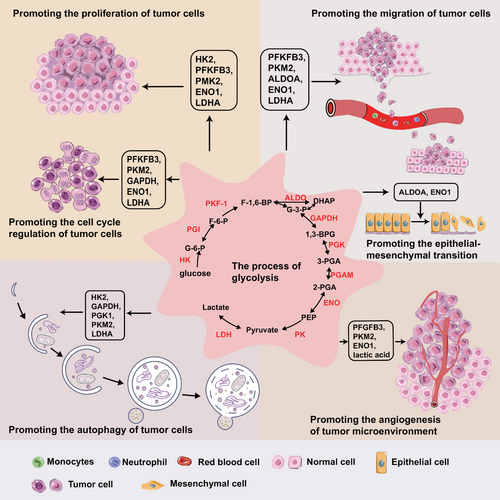
Tumor cells boost their metabolism through “metabolic reprogramming” to maintain rapid growth, which is most commonly altered by increased glycolysis [5]. Large amounts of evidence have proven that glycolysis serves an extremely vital role in tumor growth and development [6, 7]. In contrast to normal tissue cells, tumor cells exhibited unique glycolytic properties in that they generate the substance needed for rapid proliferation, manifested by elevated glucose absorption and lactate generation even under aerobic circumstances [8, 9]. The intermediate metabolites produced during glycolysis can be used as substrates for the biosynthesis of necessary substances like nucleotides, lipids, and non-essential amino acids [9]. Enhanced glycolysis can promote the angiogenesis in tumor microenvironment (TME), which provides sufficient nutrients for rapid tumor growth. In addition, lactate produced in glycolysis makes the TME acidic, which suppresses the activity of immune cells and promotes immune escape, thus promoting tumor invasion and metastasis [10, 11]. Therefore, understanding the mechanism of how glycolysis facilitates tumor growth and exploring the feasibility of glycolysis as biomarkers and therapeutic targets can provide new ideas to inhibit the occurrence and progression of tumors. This review intended to present an overview of regulatory roles and molecular mechanisms of glycolysis on tumor biological behaviors, and focused on the latest advances in glycolysis in the diagnosis and treatment of tumors.
2 THE REGULATORY ROLE OF GLYCOLYSIS IN TUMOR DEVELOPMENT
2.1 Promoting the proliferation and cell cycle of tumor cells
Numerous reports have documented that glycolysis can promote the proliferation and cell cycle of tumor cells including glycolytic enzymes and glycolytic products [12, 13]. Moreover, tumor cells could up-regulate the levels of glycolytic enzymes in an attempt to increase glycolysis efficiency and maintain their rapid growth, forming a positive feedback loop [14]. Elevated glycolysis levels have been detected in many tumor types, for instance, pancreatic cancer [7] and breast cancer [15].
Cell cycle, an ordered, irreversible process, is choreographed by cyclin-dependent kinases (CDKs) together with their cyclin chaperones [16]. One of the important mechanisms how glycolysis promotes tumor development is the acceleration of cell cycle process. For example, the glycolytic enzyme pyruvate kinase M2 (PKM2) was found to accelerate the cell cycle process of gliomas by interacting with Cdk1-cyclin B, thereby promoting tumor proliferation [17]. Several types of glycolytic enzymes, like PKM2 and ALDO, were found to periodically translocate to nucleus, which links metabolism to cell cycle processes [18, 19].
A study on hepatocellular carcinoma (HCC) showed that inhibition of hypoxia inducible factor-1α (HIF-1α) could reduce the growth rate of liver tumors through indirectly inhibiting HK2 expression, the most efficient isozyme of the glycolytic rate-limiting enzyme HK [20]. Besides, reduced HK2 expression could disrupt HK2/voltage-dependent anion channels (VDAC) complex in the mitochondrial membrane of tumor cells, which resulted in mitochondrial membrane potential loss and tumor cell death [20]. Furthermore, in vitro experiments revealed that 2-deoxy-D-glucose (2DG), an inhibitor of HK2, caused urothelial bladder carcinoma cells to be blocked in the S phase [21]. The above results suggested that HK2 might accelerate the cell cycle and promote the proliferation of tumor cells.
The most active isozyme of phosphofructokinase-2 (PFK-2), 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3), catalyzes fructose-6-phosphate to fructose-2,6-bisphosphate (F-2,6-BP), which is a strong stimulant of PFK-1, the key enzyme in the secondary rate-limiting phase of glycolysis [22]. PFK-2, activated in late G1, could indirectly promote glycolysis during late G1 phase and achieve the G1/S phase transition in tumor cells [23]. It was found in renal cell carcinoma (RCC) that the knockdown of PFKFB3 inhibited cell cycle G1/S transition [24]. F-2,6-BP stimulated the phosphorylation of the Kip/Cip protein P27, an efficient CDK suppressor and G1/S transition inhibitor, causing the proteasome degradation of P27 and facilitating cellular G1/S phase progression [22]. In vivo and in vitro, the use of specific PFKFB3 inhibitors suppressed HCC cell growth and promoted cell apoptosis [25], implying that PFKFB3 is a viable target for HCC treatment. The above studies suggested that slowing down G1/S transition and hindering proliferation by inhibiting PFKFB3 is a crucial strategy to hinder tumor advancement.
It was found that the nuclear translocation of ALDO, an important glycolytic enzyme family, was associated with cell proliferation and DNA synthesis [26], and the enhanced ALDO activity might accelerate the S phase progression in tumor cell cycle [26]. Aldolase A (ALDOA), a member of the ALDO family, plays an important role in cellular glycolysis and the maintenance of glucose homeostasis [27]. A recent study showed that knockdown of ALDOA blocked cervical cancer cells at G2/M stage and inhibited the proliferation of tumor cells [28]. These results implied that it was possible to block tumor progression by targeting ALDOA for the purpose of tumor treatment.
In various tumor cells, glycolytic enzyme GAPDH was overexpressed during the G2 phase, and further studies has shown that GAPDH induced the advancement of cyclin B-Cdk1 peak and accelerated the G2 phase of tumor cells [29]. When small molecule inhibitors of GAPDH were applied to treat lung adenocarcinoma (LUAD), breast cancer, and colon cancer cells, the proliferation of tumor cells was found to be inhibited, suggesting that GAPDH could be used as a therapeutic target for tumor treatment [30].
Enolase-1 (ENO1), the most potent enzyme among the four isozymes of ENO [31], is critical to tumor cell growth. It was found that ENO1 promoted pancreatic cancer proliferation through the phosphatidylinositol 3 kinase/protein kinase B (PI3K/Akt) signaling pathway, while silencing ENO1 significantly reduced tumor growth in breast cancer [12, 32]. ENO1 expression could be upregulated by the activation of hypoxia-associated mammalian target of rapamycin (mTOR)/HIF-1α signaling pathway, which could promote the growth of thyroid cancer cells [33]. Furthermore, the knockdown of ENO1 resulted in gastric cancer cell cycle arrest in G1, a critical period for the synthesis of RNA and proteins to support necessary materials for DNA synthesis in S phase [34]. Similarly, silencing ENO1 resulted in G2/M phase arrest and markedly decreased the expression of cell cyclin-related proteins, such as cell division cycle 25 (CDC25), cyclin B1 and CDC2, in breast cancer [32]. The above studies indicated that ENO1 played a remarkably important role in accelerating cell cycle and promoting proliferation of tumor cells, suggesting that it was possible to prevent tumor progression by targeting ENO1.
PKM2, one of the isoenzymes of PK, maintained the growth of mouse bladder cancer cells through triggering the signal transducer and activator of transcription 3 (STAT3)-HIF-1α/vascular endothelial growth factor (VEGF) signaling axis [35]. Furthermore, PKM2 was found to be translocated to nucleus in G1 phase [36], which could upregulate Myc proto oncogene (c-Myc) expression and promote trans-activation of β-catenin, causing upregulation of cyclin D1 (CCND1) [37]. Meanwhile, c-Myc feedback promoted the upregulation of GLUT1 and multiple glycolytic enzymes (including lactate dehydrogenase A [LDHA] and PKM2) [38]. Finally, aerobic glycolysis, c-Myc expression and cell cycle progression were simultaneously activated by PKM2 translocation [37]. A study of osteosarcoma showed that PKM2 knockdown induced G1 arrest and cell apoptosis in vitro [39]. These results indicated that glycolytic enzymes, proto-oncogenes, and cell cycle proteins could interact together to promote the G1 phase of cell cycle, which ultimately drove tumor development. Moreover, the glycolytic enzyme PKM2 bound and enhanced the transcriptional activity of β-catenin [22], and PKM2-β-catenin complex promoted cyclin D1 expression and accelerated the G1/S transition [40]. In summary, targeting PKM2 might delay tumor progression by blocking cell cycles and directly inhibiting tumor proliferation.
LDH facilitates the interconversion of lactate and pyruvate, and its five isozymes (LDH1-5) consist of LDHA and LDHB subunits in varying ratios, where LDHA primarily catalyzes pyruvate transformation to lactate, while the reverse reaction is catalyzed by LDHB [41]. Downregulation of LDHA induced growth arrest in mouse HCC cells, suggesting that LDHA has a significant contribution to the promotion of tumor cell growth [42]. In a study of prostate cancer, sodium oxamate (SO) was found to suppress the proliferation of tumor cells by inhibiting the activity of LDHA [43]. Further, co-treatment of prostate cancer cells with docetaxel (DOC) in combination with SO showed that the tumor cells were blocked at the G2/M phase by mechanisms that could be attributed to the cumulative cytotoxic effects and inhibition of LDHA expression, which provided inspiration for the treatment of prostate cancer [43]. In addition, it was found that the level of LDHA expression was positively correlated with the G2/M checkpoint, and that knockdown of LDHA caused tumor cells to arrest in the G2/M phase by activating the nuclear transcription factor-kappaB (NF-κB) signaling pathway in LUAD [13]. Moreover, as the produced lactate in glycolysis process could be secreted into extracellular space, the intracellular environment of most tumor cells is alkaline. It was shown that intracellular pH > 7.2 enhanced the efficiency of cell cycle into G2/M phase. However, intracellular pH ≤ 7.2 restricted the CDK 1-cyclin B activity, a key mitotic regulatory complex [44], therefore inhibiting cell cycle entry into G2/M phase. The above results demonstrated that LDHA and intracellular pH played an enormously important role in accelerating the cell cycle and promoting tumor proliferation, which provides inspiration for the clinical treatment of tumors. The above studies suggested that glycolytic enzymes could promote tumor cell proliferation and accelerate cell cycle in tumor cells, ultimately causing the development of tumors. Moreover, targeting glycolytic enzymes may stall tumor cells at different stages, thus achieving the goal of treating tumors. Therefore, future studies are necessary to explore the glycolytic enzymes associated with tumor proliferation and cell cycle, and to investigate the mechanisms by which they promote tumor progression, ultimately facilitate the exploration of efficient targeted drugs for tumor treatment.
2.2 Regulating the TME
Excess metabolites were produced by enhanced glycolysis and secreted out of tumor cells, which altered the TME to provide a more advantageous environment. Interestingly, the pH value of TME was found to be generally lower than that of normal tissues, which promoted angiogenesis and facilitated the development of tumor cells [45]. In addition, glycolytic metabolites inhibit the tumor-killing effects of immunocytes in TME, which promotes immune escape of tumor cells (Figure 2).
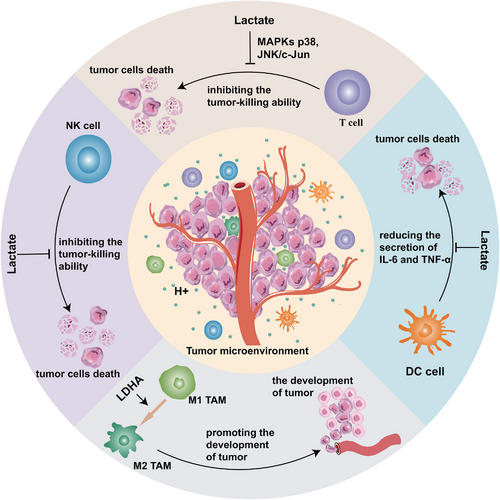
2.2.1 Promoting the angiogenesis in TME
Angiogenesis mainly encompasses degrading the vascular basement membrane, proliferating and migrating vascular endothelial cells, and forming new vessels [46], which involves multiple regulatory molecules, for instance, fibroblast growth factor (FGF), VEGF, and interleukin-8 (IL-8) [47]. Numerous studies found that the upgraded glycolysis of tumor cells activated the expression of angiogenesis-associated proteins, which in turn transported more nutrients for tumor cells to promote cell growth and tumor metastasis [48-50].
Both enzymes and products involved in glycolytic process can promote tumor angiogenesis. In a study of mouse transplantation models of breast and colon cancers, intracellular lactate was observed to stimulate angiogenesis of tumors through the NF-κB/IL-8 pathway [51]. In addition, by inducing endothelial cell proliferation and adhesion, the dimer of glycolytic enzyme PKM2 initiated and promoted tumor angiogenesis [52]. Furthermore, under hypoxic conditions, PKM2 translocated to the nucleus and induced VEGF secretion by upregulating HIF-1α expression through the NF-κB/p56 pathway, thereby facilitating tumor angiogenesis [53]. In tumor cells, the activity of VEGF was correspondingly enhanced due to the upregulation of glycolytic enzyme PFKFB3, and VEGF expression was decreased by the inhibition of PFKFB3 [54], suggesting that glycolytic enzyme PFKFB3 promotes tumor angiogenesis by modulating VEGF activity.
Lactate and H+ produced during glycolysis could acidify TME and promote the secretion of angiogenic factors like VEGF and IL-8 [55], which increase angiogenesis in HCC. Pyruvate, one of the products in glycolytic process was found to promote HIF-1α expression, which accelerated angiogenesis in the TME of HCC by upregulating the expression of plasminogen activator inhibitor-1 (PAI-1) and VEGF [56]. Elevated plasma levels of PKM2 in patients with pancreatic and colon cancers were found to promote tumor angiogenesis in a mouse xenograft model [52]. Further study on colon cancer showed that PKM2 promoted angiogenesis through PI3K/Akt and Wnt/β-catenin signaling pathways [57]. Knockdown of PKM2 reduced the expression of angiogenesis-related proteins (including N-cadherin, Vimentin, and VEGFA), thereby inhibiting angiogenesis in thyroid tumor [58]. Additionally, PKM2 knockout caused impaired angiogenesis and inhibited the growth of pancreatic cancer cells [53]. Besides, breast cancer with the silenced ENO1 gene exhibited a reduction in neovascularization and tumor size [59], indicating that ENO1 has an essential function in promoting tumor angiogenesis and proliferation. The above findings suggested that glycolytic enzymes and their products could promote tumor angiogenesis and tumor progression, which provided new ideas for targeting angiogenesis in tumor treatment.
2.2.2 Promoting the immune escape of tumor cells
The extracellular environment that is excessively acidic and hypoxic has been shown to dampen immune cell activity and aid in the immune evasion of tumor cells [60]. Most antitumor immune cells could be inhibited directly by lactate according to several studies. For example, lactate could inhibit the growth and cytotoxicity of cytotoxic T lymphocytes (CTLs) [61], diminish the secretion of cytokines from CTLs [61], and ultimately repress the antitumor capacity of T cells [61]. Recent research demonstrated that lactate inhibited the antitumor function of CTLs by inhibiting JNK/c-Jun and MAPK-p38 signaling pathway [62].
Additionally, there has been extensive research into the modulation of macrophages by lactate. Tumor-associated macrophages (TAMs) consist of two subtypes: M1 macrophages have been mainly involved in the immune response against tumors, while M2 macrophages repress the inflammatory reaction and facilitate the evasion of immune surveillance. Macrophages in an acidic environment are more inclined to polarize into M2 macrophages to exert immunosuppressive effects [63]. Furthermore, LDHA expression was also found to correlate positively with CD163, a marker of M2-like macrophages, in extramammary Paget's disease [64] and head and neck cancer [65]. The above evidence suggested that glycolysis regulated the polarization of macrophages within the TME, opening up new avenues for tackling tumor cell immune evasion.
A study on in vitro model of tumor-associated dendritic cells (TADCs) showed that the presence of lactate reduced the production of multiple antitumor cytokines in DCs, such as IL-6 and tumor necrosis factor-alpha (TNF-α) [66], suggesting that lactate restrains the antitumor effects of DCs and encourages immune escape of tumor cells. Elevated expression of LDH was found to induce lactate accumulation in melanoma, which suppressed the immune function of natural killer (NK) cells and T cells, and promoted immune escape [67]. Elevated lactate levels in TME inhibited glycolysis in monocytes, which decreased the secretion of TNF-α [68], thus suppressing antitumor immunity. Lactate and the glycolytic enzyme ENO1 were essential for the enhancement and maintenance of the immunosuppressive capacity of regulatory T cells (Tregs) [69, 70], demonstrating that glycolysis can promote immune escape by regulating Treg cells in multiple dimensions. The aforementioned results suggest that glycolysis products and enzymes can interfere the antitumor effect of immune cells, providing a fresh angle for the therapy of tumors by targeting glycolysis.
Since neutrophils have few mitochondria, they mainly rely on aerobic glycolysis for their biological functions [71]. Tumor-associated neutrophils (TANs) are classified into two phenotypes, anti-tumorigenic (N1) and pro-tumorigenic (N2) [72]. Elevated levels of glycolysis in tumor cells led to increased levels of lactate in TME, which promoted N2 polarization of neutrophils, exerting their pro-tumorigenic effects and leading to immune escape of tumor cells [73]. Metabolomic analysis of neutrophil lysates from pancreatic ductal adenocarcinoma (PDCA) showed that several glycolytic metabolites, such as glucose-6-phosphate, glucose-1-phosphate, fructose-6-phosphate, and lactate, were significantly up-regulated in TANs, indicating enhanced neutrophil glycolysis in the PDCA TME [74]. Further, when LDHA-overexpressed neutrophil-like differentiated HL-60 (dHL-60) cells were co-cultured with PDCA cells, enhanced proliferation of tumor was found, revealing that glycolysis exerts its pro-tumor effects by regulating neutrophils [74]. Moreover, lactate drove the expression of programmed death-ligand 1 (PD-L1) in infiltrating neutrophils through the monocarboxylate transporter 1 (MCT1)/NF-κB/Cyclooxygenase 2 (Cox2) pathway, which reduced the sensitivity of HCC cells to immunotherapy and led to the progression of tumor [75]. The above results implied that the tumor-promoting effects of neutrophils could be suppressed by inhibiting the glycolytic function, which provides a new direction for clinical treatment of tumors.
2.2.3 Promoting the invasion and metastasis of tumor cells
The metastasis of tumor, one of the characteristics of tumor aggressiveness [76], affects the patient's outcome and contributes to poor prognosis, which is a challenge that we need to address urgently at present. Recent reports unveiled that glycolytic enzymes and products serve a significant function in promoting tumor metastasis, which has attracted attention [77, 78]. The discovery indicated that lactate concentration was markedly increased in areas with tumor cell metastasis compared to those without tumor cell metastasis [79, 80], suggesting that lactate might promote the migration of tumor cells. Several pieces of research have demonstrated that ENO1 expression was positively correlated with tumor cell invasion and metastasis [77, 81]. ENO1 could function as a receptor for plasminogen and accelerate the activation of the fibrinolytic system, resulting in fibrinolysis and the decomposition of extracellular matrix (ECM), which provides conditions for tumor cell invasion and metastasis [82, 83]. Through downregulating the expression of ENO1, retinoic acid (RA) reduced the migration of follicular thyroid cancer cells [84], suggesting that ENO1 promoted the migration of tumor cells.
Transcription factors including c-Myc, forkhead box protein M1 (FOXM1), and ETS proto-oncogene 1 (ETS1) promoted the glycolysis process by activating the promoters of glycolytic genes (for example LDHA, PFKFB3, and hexokinase 1 [HK1]), thus increasing the capability of pancreatic cancer to invade and metastasize [85, 86]. Moreover, pancreatic cancer could promote glycolysis through post-translational modification and the alteration of glycolytic enzyme activity, which further strengthened the viability of tumor cells [87]. In addition, studies focused on HCC showed that more aggressive cell lines (such as HCC-LM3) exhibited stronger glycolytic activity than less aggressive cell lines (such as HepG2) [88, 89], indicating that the degree of glycolytic activity might influence the level of tumor invasiveness.
Multiple investigations have demonstrated that glycolysis could promote epithelial-mesenchymal transition (EMT) [90-92]. EMT is a biological process that involves the transformation of epithelial cells to cells with a mesenchymal phenotype, with distinct features including reduced levels of cell adhesion proteins (for instance E-cadherin) and alteration of the cytokeratin cytoskeleton to a cytoskeleton based on vimentin [93]. The occurrence of EMT makes tumor cells more motile and invasive, and therefore, more prone to metastasis [94, 95]. ALDOA, one of the isoenzymes of glycolytic enzyme ALDO, has been found to be positively correlated with EMT [96]. The researchers found that E-cadherin expression was down-regulated and metastasis was more likely to occur in pancreatic cancer tissues with high levels of ALDOA relative to those with low levels [96]. Moreover, the silencing of ALDOA was associated with increased E-cadherin expression levels and decreased vimentin levels [96], indicating that ALDOA might accelerate tumor progression through promoting EMT and metastasis. Additionally, ALDOA might contribute to bladder cancer invasion and metastasis through the E-cadherin-epidermal growth factor receptor (EGFR) signaling pathway [78]. Furthermore, ENO1 promoted EMT through interacting with macrophages and hepatocyte growth factor receptor (HGFR, also called c-Met), thereby facilitating the metastasis of oral squamous cell carcinoma (OSCC) [97] and lung cancer [98], respectively. Moreover, by inducing the upregulation and nuclear relocation of STAT3, PKM2 could induce the metastasis of colon cancer cells [99].
The GLUT family contains 14 members, which are key gating proteins responsible for the entry and exit of glucose into and out of the cell, and play an important role in maintaining homeostasis of intracellular and extracellular glucose [100]. Impaired membrane transport of GLUT is one of the most important reasons for the disruption of glucose levels in the body and the development of hyperglycemia and diabetes [101]. GLUT1 has a high affinity with glucose, and its expression is regulated by HIF-1α [102, 103]. Studies have shown that GLUT1 was highly expressed in a variety of tumor cells, such as lymphoma, kidney, lung, and prostate cancers, which enhanced the uptake of glucose by cancer cells [104-106]. In addition, GLUT2 and GLUT3 have been found to be highly expressed in certain tumors, including HCC, breast cancer, gastric cancer and so on [103]. A recent study has demonstrated that colorectal cancer (CRC) patients with high GLUT3 expression had worse prognosis, and further mechanistic research revealed that GLUT3 promoted EMT in tumor cells through the transforming growth factor-β (TGF-β)/c-Jun N-terminal kinase (JNK)/activating transcription factor-2 (ATF2) signaling pathway, thereby promoting tumor invasion and metastasis [107]. In summary, glycolysis can affect EMT and promote tumor cell invasion and migration, suggesting that it is possible to delay tumor progression by antagonizing glycolytic enzymes, which provides a new idea for the therapy of malignant tumors in the clinic.
2.3 Promoting the autophagy of tumor cells
Autophagy is the process by which lysosomes degrade the structures of cytoplasmic proteins and organelles [108]. The failure of autophagy could cause cell abnormalities or even death, thus autophagy has an effect on regulating cellular homeostasis [109]. Autophagy acts a pivotal part in tumor development by promoting the proliferation of early-stage tumors and increasing the metastasis of advanced tumors [110, 111]. In mouse models of lung cancer, deletion autophagy-related gene 7 (Atg7), an essential autophagy gene, could inhibit tumor growth and promote tumor cell death, revealing a crucial function of autophagy in malignant tumor development [112]. Interestingly, the promotion of tumor progression by glycolysis is associated with its regulation on autophagy, which has attracted close attention [113].
A variety of glycolytic enzymes could promote cancer development and progression by inducing autophagy in tumor cells [114, 115]. HK2, the key enzyme of glycolysis, made ovarian cancer more resistant to cisplatin by promoting autophagy [116], and silencing HK2 suppressed autophagy and induced apoptosis in myeloma cells [117], indicating that HK2 could accelerate tumor progression by enhancing autophagy. Similarly, elevated levels of GAPDH expression promoted the upregulation of autophagy-related genes (including Beclin 12, ATG3, phosphatidylinositol 3-kinase catalytic subunit type 3 [PIK3C3], and microtubule-associated protein 1 light chain 3 [LC3]) in esophageal cancer [115], and accelerated the autophagy of colon cancer cells [118]. It was found that PKM2 enhanced the phosphorylation of autophagy protein Beclin-1 and the triggering of autophagy in leukemic cells with nucleophosmin 1 (NPM1) gene mutations [119], suggesting that PKM2 has a function in promoting tumor autophagy. The study has shown that the oxaliplatin resistance in CRC might be associated with enhanced autophagy due to upregulation of PFKFB3 [120], implying that we could inhibit autophagy by restraining PFKFB3 to increase tumor drug sensitivity. Likewise, the correlation between LDHA and autophagy has been found in breast cancer cells, indicating that LDHA promoted the survival of tumor cells [121]. Additionally, lactate creates an acidic environment, which could also promote the induction of autophagy in multiple tumor cells [122]. The above reports exhibited that elevated glycolysis promotes autophagy in tumor cells and provide them a protective mechanism, hinting that we can destroy the pro-tumorigenic effect of autophagy by targeting glycolysis.
3 THE ROLE OF GLYCOLYSIS IN TUMOR DIAGNOSIS AND TREATMENT
Due to the specificity of abnormal glycolytic function in tumor cells, the value of glycolysis in diagnosis and treatment of tumors has been increasingly noticed. Besides, the overexpression of glycolytic enzymes and the accumulation of glycolytic products are instructive in assessing tumorigenesis or progression. Furthermore, novel drugs targeting glycolysis have been developed to provide expanded therapeutic options for tumor patients (Figure 3).
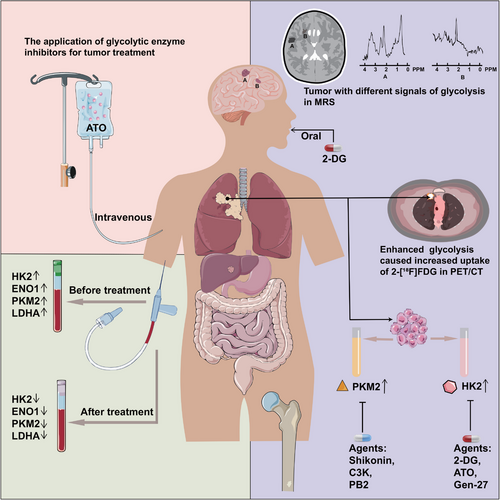
3.1 Application of glycolysis in diagnostic radiology
2-DG is a glucose analogue synthesized by replacing the 2-hydroxyl group of glucose with hydrogen, which enters cells via glucose transporters. Because of the enhanced glucose uptake by tumor cells and the relative non-toxicity of 2-DG, the role of 2-DG in tumor imaging diagnosis has been explored. Positron emission tomography (PET) is a method of diagnosing and analyzing diseases by injecting essential metabolic substances labeled with radionuclides, which reflects the glucose uptake and metabolism by observing the accumulation of specific substances in various body tissues, and has been applied to the diagnosis and prognosis prediction of many solid tumors, like neuroblastoma [123], cervical cancer [124], and lung cancer [125]. Currently, the metabolic substance widely used in PET analysis is 2-[18F] fluoro-2-DG (2-[18F] FDG) [126], which has a relatively brief half-life of 110 minutes. However, normal brain tissue had a stronger uptake of 2-[18F] FDG and exhibited a higher background signal, making a poor diagnostic ability of 2-[18F] FDG PET/computed tomography (CT) for brain tumors [127]. Encouragingly, a study in a mouse model of orthotopic glioma found that 2-DG could freely cross the blood-brain barrier, indicating its potential utility in the diagnosis of remaining intracranial tumors [127].
Magnetic resonance spectrum (MRS) is the only current technique that allows non-invasive observation of metabolic and biochemical changes in living tissues, and proton magnetic resonance (1H MRS) can assist in the diagnosis of tumors by measuring the amount of lactate in tumor cells [128]. 13C-labeled pyruvate and lactate could be detected by MRS in a mouse model of pancreatic precancerous lesion to follow disease progression [129]. The size of the lactate peak in MRS depended on the malignancy of tumor, therefore MRS is important in identifying benign and malignant tumors and speculating on patients’ prognosis [130]. In addition, based on abnormal lactate metabolism, MRS could predict tumor response to treatment and whether the tumor would relapse during the treatment period [131]. Furthermore, in a study applying [1-13C] pyruvate, the safety and feasibility of MRS were confirmed in patients with prostate cancer [132]. MRS was able to distinguish between dedifferentiated liposarcomas and atypical lipomatous tumors, two benign lipomas, based on the differences in their metabolism of glucose, lactate and other substances [133]. The simplicity and accuracy of MRS have allowed its widespread application in the diagnosis and evaluation of brain tumors [134].
The advent of PET/CT and MRS based on the enhanced glycolysis in tumor cells has provided stronger evidence for the accurate diagnosis and disease management of tumor patients [125, 135]. The applications of glycolysis in PET/CT and MRS have prompted us to develop more diagnostic methods based on tumor glycolysis features to provide more favorable means for tumor diagnosis and evaluation.
3.2 Glycolysis-related tumor biomarkers
A growing number of studies have indicated that glycolytic enzymes are abnormally expressed in tumor cells compared to normal tissues, which may be linked to a less favorable prognosis in tumor sufferers (Table 1). Gaining insights into the characteristics of glycolytic enzyme expression in tumor cells can promote accurate diagnosis and prognosis assessment in cancer patients, which provides informative implications for clinical decisions to prolong the survival of cancer patients.
| Enzyme | Tumor | Expression pattern | Detectable samples | Biomarker application | Effects | Reference |
|---|---|---|---|---|---|---|
| HK2 | Glioma | Upregulated | Tumor tissue (n = 1,764) | Diagnosis and prognosis | Promoting metastasis | [228] |
| PDAC | Tumor tissues (n = 51) and adjacent normal tissues (n = 51); serum samples from patients with tumors (n = 78) and healthy controls (n = 30) | Promoting proliferation | [142] | |||
| Bladder carcinoma | Tumor tissues (n = 76) | Prognosis | Promoting growth and angiogenesis | [21] | ||
| CC | Tumor tissues (n = 30) | Promoting growth | [229] | |||
| SCLC | Tumor tissues (n = 90) | Promoting growth | [230] | |||
| CRC | Tumor tissues (n = 104) | Promoting growth and invasion | [231] | |||
| PFKFB3 | RCC | Upregulated | Tumor tissues (n = 90) and adjacent normal tissues (n = 90) | Diagnosis and prognosis | Promoting proliferation and cell cycle | [24] |
| LAC | Tumor tissues (n = 263) and tissues from healthy controls (n = 20) | Accelerating cell cycle and promoting metastasis | [146] | |||
| GC | Tumor tissues (n = 134) and adjacent normal tissues (n = 134) | Promoting proliferation and migration | [232] | |||
| ALDOA | GC | Upregulated | Tumor tissues (n = 114) | Diagnosis and prognosis | Promoting immune escape | [233] |
| LUAC | N/A | Promoting immune escape | [234] | |||
| GC | Tumor tissues (n = 252) | Promoting growth, EMT and migration | [235] | |||
| PGK1 | LUAC | Upregulated | Tumor tissues (n = 91) and adjacent normal tissues (n = 91) | Diagnosis | Promoting growth and immune escape | [236] |
| EC | Tumor tissues (n = 54), tumor adjacent (n = 9), and normal tissues (n = 9) | Promoting invasion | [237] | |||
| LUAC | Tumor tissues (n = 91) and adjacent normal tissues (n = 91) | Prognosis | Promoting growth and immune escape | [236] | ||
| GC | Tumor tissues (n = 10) | Promoting invasion | [238] | |||
| PGAM1 | NSCLC | Upregulated | Tumor tissues (n = 277) | Prognosis | Promoting proliferation | [239] |
| OSCC | Tumor tissues (n = 122) | Promoting migration | [240] | |||
| ENO1 | Bladder cancer | Upregulated | Tumor tissues (n = 165), tumor adjacent (n = 58), and normal tissues (n = 10) | Diagnosis and prognosis | Promoting growth, invasion and immune escape | [241] |
| LC | Tumor tissues (n = 72) and benign lung disease tissues (n = 60); serum samples from patients with tumors (n = 72), patients with benign lung disease (n = 69) and healthy control (n = 60) | Promoting invasion | [147] | |||
| TC | Tumor tissues (n = 45) and adjacent normal tissues (n = 45) | Promoting invasion | [33] | |||
| CM | Tumor tissues (n = 112) | Prognosis | Promoting proliferation and migration | [242] | ||
| Breast cancer | Tumor tissues (n = 24) | Promoting proliferation | [243] | |||
| ccRCC | Downregulated | Tumor tissues (n = 360) | N/A | [244] | ||
| PKM2 | Bladder cancer | Upregulated | Tumor tissues (n = 215) and adjacent normal tissues (n = 90) | Diagnosis | Promoting growth | [137] |
| Bladder cancer | Urine from patients (n = 50) and healthy control (n = 10) | Promoting growth | [138] | |||
| CRC | Feces (n = 7) and benign intestine disease (n = 141); | N/A | [136] | |||
| HNSCC | Tumor tissues (n = 88) | Prognosis | Promoting proliferation and migration | [245] | ||
| Bladder cancer | Tumor tissues (n = 215) and adjacent normal tissues (n = 90) | Promoting growth | [137] | |||
| Bladder cancer | Urine from patients (n = 50) and healthy control (n = 10) | Promoting growth | [138] | |||
| NSCLC | Tumor tissues (n = 305) | N/A | [139] | |||
| LDHA | Pancreatic cancer | Upregulated | Tumor tissues (n = 136) and control tissues (n = 130) | Diagnosis and prognosis | Promoting metastasis | [246] |
| ccRCC | Tumor tissues (n = 385) and control tissues (n = 85) | N/A | [247] | |||
| BC | Tumor tissues (n = 112) | Prognosis | N/A | [248] | ||
| LC | Tumor tissues (n = 462) | Promoting angiogenesis and invasion | [145] |
- Abbreviations: BC, breast cancer; CC, cervical cancer; ccRCC, clear cell renal carcinoma; CM, cutaneous melanoma; CRC, colorectal cancer; EC, endometrial cancer; GC, gastric cancer; HCC, hepatocellular carcinoma; HNSCC, head and neck squamous cell carcinoma; LC, lung cancer; LUAC, lung adenocarcinoma; N/A, not applicable; NSCLC, non-small cell lung cancer; OSCC, oral squamous cell carcinoma; PDAC, pancreatic ductal adenocarcinoma; RCC, renal cell carcinoma; SCLC, small cell lung cancer; TC, thyroid carcinoma.
Recent research revealed that PKM2 expression was upregulated in CRC as well as correlated with more advanced tumor stage [136]. In addition, Huang et al. [137] reported that PKM2 expression was strongly linked to unfavorable disease-free survival (DFS), suggesting its possible utility as a prognostic biomarker for patients with urothelial carcinoma. A study has analyzed the disparities in urine composition between patients and healthy subjects, revealing heightened PKM2 levels in the urine of individuals with bladder cancer [138]. It was found in a study of lung cancer receiving platinum-based chemotherapy that the increased expression of PKM2 was correlated with worse overall survival (OS) and PKM2 might serve as a valuable biomarker of chemotherapy sensitivity [139]. The expression levels of GLUT and several glycolytic enzymes have been identified to be closely linked to the prognosis of tumor patients. It was found that high levels of GLUT1 expression were associated with worse prognosis in a variety of tumors, including breast cancer, esophageal cancer [140], and locally advanced rectal cancer treated with neoadjuvant radiotherapy [141]. Furthermore, HK2 exhibited overexpression in both tumor tissues and serum from pancreatic ductal adenocarcinoma (PDAC) patients, which was also related to tumor progression [142]. High HK2 expression was correlated with poor prognosis, larger tumor volume, more lymph node involvement, and more advanced clinical stage in CRC and HCC [143]. The above studies showed that elevated expression of GLUT1 and glycolytic enzyme HK2 was associated with adverse outcomes, indicating that GLUT1 and HK2 could be utilized as prognostic markers in tumor patients.
Additionally, the expression levels of LDHA have been closely linked to the prognosis of various solid tumors, including breast cancer [144], lung cancer [145] and so on. Therefore, serum LDH levels have been currently used to assist in the diagnosis and prognostic assessment of tumors. Moreover, PFKFB3 and ENO1 were highly expressed in lung cancer tissues as opposed to normal lung tissues, and PFKFB3 expression was demonstrated to have a negative correlation with survival time, indicating that PFKFB3 and ENO1 have an important role in assessing tumor prognosis [146, 147]. However, PGK1 expression was reduced in patients with gallbladder cancer compared to non-cancerous gallbladder tissue, so a reduction in PGK1 should be more noticeable in patients with gallbladder disease [148]. Elevated levels of lactate, the final product of glycolysis, have been linked to worse OS in patients with metastatic lung cancer [149] and head and neck cell carcinoma [150], implying that lactate may be a prognostic factor in tumor patients. These results suggested that glycolytic enzymes and products could be used as biomarkers for a variety of tumors, which provided favorable evidence for tumor diagnosis and prognostic assessment.
3.3 Targeting glycolysis in tumor treatment
In recent years, as the understanding of glycolysis's pivotal role in the proliferation, metastasis and immune escape of tumor cells, numerous novel drugs targeting glycolytic enzymes have been developed to treat tumors and prolong patients' survival time (Table 2). As the resistance to chemotherapy drugs has been a great obstacle in the process of fighting tumors, many inhibitors targeting glycolytic enzymes have been found to reduce drug resistance and enhance the sensitivity to chemotherapy drugs, inducing better anti-tumor effects.
| Enzyme | Agent | Tumor | Phase | Regulatory role | Reference or NCT number |
|---|---|---|---|---|---|
| HK2 | 2-DG | HCC | Phase II | Against sorafenib resistance | [172] |
| PCa and BC | Phase II | Enhancing the ability of thioredoxin to kill cancer cells | [249] | ||
| HNC | Phase II | Enhancing cytotoxicity of cisplatin | [250] | ||
| OS and LC | Phase II | Enhancing the anti-cancer effect of adriamycin and Paclitaxel | [251] | ||
| PCa | Phase I/II | Evaluating the safety of 2-DG | NCT00633087 | ||
| LC, BC, PC, HNC, and GC | Phase I | Evaluating the maximum tolerated dose of 2-DG | NCT00096707 | ||
| 3-BrPA | CRC | Phase I | Against oxaliplatin resistance | [252] | |
| Leukemic | Phase I | Enhancing the sensitization of leukemic cells to Daunorubicin | [177] | ||
| LN | BC | Phase III | Evaluating the therapeutic value of chemotherapy combined with LN | [178] | |
| OVC | Phase II | Enhancing the activity of anti-cancer drugs | [179, 253] | ||
| GL-V9 | BC | Preclinical | Inducing mitochondrial cytotoxicity and apoptosis of cancer cells | [183] | |
| Gen-27 | HCC | Preclinical | Enhancing the antitumor effect of sorafenib | [20] | |
| ATO | APL | Preclinical | Inducing apoptosis of cancer cells | [186] | |
| OVC and EC | Phase I | Evaluating the efficacy, safety and tolerability of ATO | NCT04489706 | ||
| PCa | Phase II | Evaluating the efficacy and toxicity of ATO | NCT00004149 | ||
| Shikonin | Esophageal cancer | Preclinical | Suppressing of glycolytic activity and cell growth | [184] | |
| PFKFB3 | 3PO | RCC | Preclinical | Inhibiting glycolytic activity of cancer cells | [24] |
| PFK15 | CML | Preclinical | Enhancing the sensitivity of CML to tyrosine kinase inhibitor prolonging survival | [211] | |
| CRC | Preclinical | Enhancing cytotoxicity of oxaliplatin | [120] | ||
| PFK158 | OVC and CC | Preclinical | Inducing apoptosis of cancer cells | [210] | |
| ALDOA | TDZD-8 | BC | Preclinical | Suppressing of glycolytic activity and cell growth | [214] |
| Raltegravir | LC | Preclinical | Inhibiting cell metastasis | [215] | |
| PGAM1 | MJE3 | BC | Preclinical | Covalent labelling PGAM1 | [218] |
| ENO1 | PhAH | Glioma | Preclinical | Promoting apoptosis | [220] |
| PKM2 | Shikonin | Bladder cancer | Preclinical | Inhibiting the expression of PKM2 | [191] |
| Metformin | OS | Preclinical | Impairing the resistance of cancer cells to cisplatin | [192] | |
| RCC | Preclinical | Suppressing cell growth | [193] | ||
| C3k | LC, CC, and colon cancer | Preclinical | Suppressing cell growth | [196] | |
| MC-4 | RCC | Preclinical | Inducing apoptosis of cancer cells | [198] | |
| PB2 | HCC | Preclinical | Triggering apoptosis | [199] | |
| LDHA | oxamate | ALL | Preclinical | Inducing apoptosis of cancer cells | [201] |
| PCa | Preclinical | Promoting susceptibility of cancer cells to Docetaxel | [43] | ||
| FX11 | Lymphoma and PC | Preclinical | Inhibiting tumorigenesis and progression | [203] | |
| GF | BC | Preclinical | Promoting apoptosis | [204] | |
| Gossypol | BC | Phase I/II | Causing cell cycle arrest | [205] | |
| Adrenal cancer | Phase I/II | Suppressing cell growth | [206] |
- Abbreviations: 2-DG, 2-deoxy-d-glucose; 3-BrPA, 3-bromopyruvate; ALL, acute lymphoblastic Leukemia; APL, acute promyelocytic leukemia; ATO, arsenic trioxide; BC, breast cancer; CC, cervical cancer; CRC, colorectal cancer; CML, chronic myelogenous leukemia; EC, endometrial cancer; GF, galloflavin; LN, lonidamine; HCC, hepatocellular carcinoma; HNC, head and neck cancer; RCC, renal cell carcinoma; LC, lung cancer; OS, Osteosarcoma; OVC, Ovarian cancer; PB2, proanthocyanidin B2; PC, pancreatic cancer; PCa, prostate cancer; PhAH, phosphonoacetohydroxamicacid.
3.3.1 Inhibitors of GLUT1
High expression of GLUT1 provides tumor cells with more glucose to participate in glycolysis, thereby promoting tumor progression [151]. Currently, a series of GLUT1 inhibitors have been identified, such as cytochalasin B, CG-5, BAY-876, STF-31, and WZB117. Cytochalasin B, a cell-permeable fungal toxin, reduced glucose uptake and inhibited proliferation of breast cancer by inhibiting GLUT1 [152]. In addition, STF-31 was found to inhibit the growth of renal cancer cells by directly binding to GLUT1 and reducing glucose uptake [153]. WZB117 inhibited glucose transport by binding to the glucose-binding site of GLUT1, thereby inhibiting glycolytic function in tumor cells and inducing cell cycle arrest [154-156]. Furthermore, WZB117 could be used in combination with cisplatin, paclitaxel and other anti-tumor drugs for the treatment of lung cancer and breast cancer [154]. BAY-876 could inhibit the activity of GLUT1 by targeting it for the treatment of tumors, which has been demonstrated in a variety of tumors such as breast cancer [157] and ovarian cancer [158]. In recent years, a variety of GLUT1 inhibitors have been discovered, such as rapaglutin A [159, 160], EF24 [161], quinazolines [162], and phenylalanine amides [163]. Additionally, some natural products including trehalose [164], melatonin [165], bezielle [166], resveratrol [167], and caffeine [168] have been found to inhibit GLUT1. It was found that natural product compounds generally had a better safety and less toxicity than synthetic compounds in inhibiting tumor progression [169]. The above studies have shown that targeting GLUT might be an effective strategy for the treatment of tumors.
3.3.2 Inhibitors of HK2
As HK2 has been discovered to be upregulated in tumor cells and is prominent in promoting tumor development [6, 170], targeting HK2 can safely and effectively inhibit tumor cell growth [171]. Now, many small molecule inhibitors have been extensively identified for the purpose of inhibiting HK. Preclinical studies showed that the glucose analogue 2-DG inhibited glycolysis in tumor cells by competitively inhibiting HK2 [172]. The anti-tumor effects of 2-DG can be enhanced when combined with other drugs such as metformin [173], sorafenib [174], and 2-aminophenoxazine-3-one (Phx-3) [175] in the treatment of HCC. As 3-bromopyruvate (3-BrPA), a brominated derivative of pyruvate, directly inhibits the activity of HK2 and displays a strong glycolytic inhibitory effect [176], Rai et al. [177] discovered that the sensitivity of leukemic cells to daunorubicin was heightened by 3-BrPA through the inhibition of HK2 efficiency. Lonidamine (LN) is an indazole derivative targeting glycolytic enzyme HK2, which has undergone clinical trials for the treatment of a variety of tumors, like breast [178] and ovarian cancers [179]. In studies involving the treatment of ovarian cancers, prostate, and breast, the combination of LN with doxorubicin was found to exhibit enhanced efficacy [180, 181]. Moreover, metformin, the commonly prescribed drug for managing type 2 diabetes was found to inhibit the activity of HK1 and HK2, and reduce glycolytic function, ultimately attenuating cell proliferation in lung cancer [182]. In recent years, many flavonoid derivatives including amentofavone (AF) [183], GL-V9 [183], Gen-27 [20] and natural compounds such as shikonin [184] and astragalin [185] have been discovered to inhibit HK2 and exert anti-tumor effects in multiple solid tumors. In addition, numerous natural compounds also exhibited HK2 inhibitory effect, such as arsenic trioxide (ATO), the main active ingredient of arsenic, which had the capability to reduce the proliferation of gastric cancer cells through down-regulating HK2 expression [186]. Furthermore, the combination with vitamin C might enhance sensitivity of osteosarcoma cells to ATO by strengthening the inhibition of glycolysis [187]. Additionally, an in vivo mouse experiment showed that metformin attenuated the toxic effects of ATO on the liver [188]. However, the inhibitory effect of ATO in combination with metformin on HK2 has not been investigated, which requires further exploration. By inhibiting the expression of HK2, resveratrol induced apoptosis in HCC cells [189]. In summary, various HK2 inhibitors have been demonstrated to serve as effective antitumor drugs in preclinical studies, and accelerated clinical studies are expected to promote the application of HK2 inhibitors in the early treatment of tumor patients.
3.3.3 Inhibitors of PKM2
Several novel inhibitors targeting PKM2 have been discovered, including shikonin, metformin, and vitamin K (VK). Among them, the most specific and effective PKM2 inhibitor is shikonin, and its analogue alkannin has potential therapeutic value by targeting PKM2 [190]. A previous study found that shikonin could down-regulate PKM2 expression through the AKT/mTOR/SREBP-1c signaling pathway, thus inhibiting bladder cancer cell growth [191]. Besides, metformin can enhance osteosarcoma stem cell responsiveness to cisplatin by down-regulating PKM2 expression, thereby exerting an anti-tumor effect [192]. In addition, metformin reduced kidney cancer cell intrusion and metastasis by activating AMPK and down-regulating PKM2 [193]. Moreover, VK is a lipid-soluble substance, with VK3 and VK5 exhibiting more potent inhibitory effect on PKM2 compared to PKM1 [194]. Chen et al. [195] demonstrated that VK3 and VK5 could reduce glycolysis by targeting PKM2, which inhibited the proliferation of cervical cancer cells. Furthermore, C3k [196], tannic acid (TA) [197], and some natural compounds, such as MC-4 [198], proanthocyanidin B2 (PB2) [199], and wogonin [200] were also demonstrated to display the inhibitory effect on PKM2. Altogether, a variety of PKM2 inhibitors can restrict the reproduction, invasion and spread of tumor cells, thereby delaying the progression of tumor and prolonging patient survival.
3.3.4 Inhibitors of LDHA
As LDHA is one of the important tumor biomarkers and lactate (produced from pyruvate catalyzed by LDH) significantly contributes to promoting tumor proliferation and migration, the development of drugs targeting LDHA is a promising anti-tumor strategy. Oxamate impeded the proliferation and metastasis of tumor cells by competitively inhibiting LDHA activity in leukemia [201] and prostate cancer [43]. Besides, oxamate increased the drug responsiveness of glioblastoma (GBM) cells to temozolomide [202]. Moreover, it was found that FX11, a small molecule inhibitor of LDHA, inhibited lactate production by targeting LDHA, thereby restraining the progression of pancreatic cancer and lymphoma [203]. Galloflavin inhibited breast cancer cell proliferation by blocking glycolysis and reduced ATP production through targeting LDHA [204]. In addition, gossypol, a natural compound targeting LDHA, has exhibited anti-tumor effects in diverse types of tumors, for example breast cancer [205], glioma [206] and adrenal cancer [206], but the non-specific toxicity of gossypol limits its clinical application [207].
3.3.5 Inhibitors of PFKFB3
Many inhibitors targeting PFKFB3 have been discovered, such as 3-(3-Pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) [24, 208], 1-(4-pyridinyl)-3-(2-quinolinyl)-2-propen-1-one (PFK15) [209], and PFK158 [210]. 3PO was found to rapidly decrease glucose absorption in leukemic cells, thereby reducing the production of lactate and ATP, which caused leukemia proliferation inhibition [208]. PFK15, a derivative of 3PO, has higher PFKFB3 inhibitory activity, which was found to improve the efficacy of imatinib in chronic granulocytic leukemia [211], and it could be used in combination with oxaliplatin for better treatment in CRC [120]. In addition, benzoindoles [212], BrAcNHEtOP [213], and YZ9 [213], and other compounds have been identified to hinder PFK function, and delay the progression of tumor cells.
3.3.6 Inhibitors of glycolytic non-rate-limiting enzymes
Glycolytic non-rate-limiting enzymes also contribute significantly to tumor progression, so it is necessary to explore their inhibitors for tumor treatment. TDZD-8, specifically targeting ALDOA, inhibited the glycolytic function in cancer cells and acted as an anti-breast cancer agent [214]. Raltegravir prolonged the patient's survival by inhibiting the metastasis of lung cancer cells through targeting ALDOA [215]. PGK1 inhibitors and their molecular mechanisms in tumors have not been elucidated [216], and the function of its potential suppressors, like CBR-470-1, terazosin, and bisphosphonates, in tumor therapy needs to be further explored [217]. MJE3 inhibited breast cancer cell growth by targeting phosphoglycerate mutase 1 (PGAM1), which is one of the isoenzymes of the glycolytic enzyme PGAM [218]. Furthermore, phosphonoacetohydroxamicacid (PhAH) was found to decrease ENO's enzymatic function, thus inhibiting the growth of pancreatic cancer and GBM [219, 220].
Despite the current widespread development of anti-tumor drugs, the incidence of tumor drug resistance has increased, leading to poor therapeutic efficacy in tumor patients. Therefore, it is an urgent problem to diminish the resistance to anti-tumor drugs, and many studies have shown that metabolic reprogramming may be related to the chemoresistance of tumors [221]. Several studies illustrated that enhanced glycolysis was associated with insensitivity to sorafenib in HCC cells [88, 199]. The upregulation of HK2 in breast cancer cells could cause tumor cell resistance to paclitaxel [222] and tamoxifen [223]. Moreover, PKM2 overexpression decreased the sensitivity of acute promyelocytic leukemia cells to RA, whereas inhibiting PKM2 attenuated RA resistance in APL cells [224], indicating that PKM2 could serve as a target to ameliorate drug resistance in leukemia. In tumors with cisplatin resistance, such as cervical, colorectal, and advanced bladder cancers, shikonin reduced the resistance to platinum by inhibiting PKM2 activity [190]. In addition, the overexpression of PFKFB3 has been linked to resistance to sorafenib in HCC [88] patients and to the tyrosine kinase inhibitor imatinib in chronic myeloid leukemia (CML) patients [211]. The above studies demonstrated that overexpressing glycolytic enzymes can lead to tumor cell resistance to antitumor drugs, ultimately resulting in poor therapeutic outcomes and even reduced survival time in tumor patients. Furthermore, some of the glycolysis inhibitors do not specifically target a particular glycolytic enzyme, which is not conducive to further drug discovery and clinical drug selection. Therefore, it is urgent to explore more effective, safe and specific inhibitors of glycolytic enzymes to improve the therapeutic efficiency of tumors.
4 CONCLUSIONS AND FUTURE PROSPECTS
Numerous studies have shown that tumor cells produce a large amount of substance needed for growth and proliferation by increasing the glycolytic process to maintain their rapid progression. High expression of glycolytic enzymes and excess products during glycolysis may also promote the advancement and progression of tumors. In this review, we discussed the important roles of glycolytic enzymes and products in regulating proliferation, metastasis, invasion, autophagy of tumors, altering the TME, and so on. Powerful diagnostic imaging methods such as PET/CT and MRS have been developed through taking advantage of the glycolytic properties of tumor cells. Additionally, the abnormal expression of several glycolytic enzymes in tumor cells enables their utilization as diagnostic and prognostic biomarkers, which provides scientific evidence for the clinical diagnosis and management of individuals with tumors. During the recent period, numerous studies have shown that targeting glycolytic enzymes can inhibit tumor progression, thereby improving prognosis and prolonging survival time of tumor patients. Moreover, glycolytic enzyme inhibitors significantly contribute to combating tumor drug resistance. Therefore, glycolytic enzymes have a critical function in tumor diagnosis and prognostic assessment, and the application of glycolytic enzyme inhibitors is the promising approach for tumor treatment.
Despite the development of various inhibitors targeting glycolytic enzymes have been designed for tumor therapy, the application of glycolytic enzyme inhibitors is likely to affect glucose metabolism in normal tissues because glycolytic enzymes are nonspecifically expressed in various tissues. For example, the ubiquitously expressed HK1 is highly structurally similar to HK2, an overexpressed protein in tumor cells [225, 226], and HK2 inhibitors could degrade HK1 activity, thereby affecting glycolysis in normal tissues [227]. Nevertheless, the mechanism of interaction between glycolytic enzymes and their inhibitors is not fully elucidated, which requires continued exploration. Furthermore, although some glycolytic enzymes and products have been demonstrated to serve as potential biomarkers, most of them still lack the specificity and sensitivity to be applied in clinical practice, which needs to be verified by further clinical trials. Nevertheless, glycolytic enzymes and their inhibitors hold potential for future applications in the diagnosis and treatment of tumors.
DECLARATIONS
AUTHOR CONTRIBUTIONS
Qiqi Qiao and Shunfeng Hu wrote this manuscript and created figures and tables. Xin Wang and Shunfeng Hu revised the manuscript. Xin Wang provided guidance throughout the preparation of the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
This study was funded by National Natural Science Foundation (No.82270200, No. 82070203, and No.81770210), Key Research and Development Program of Shandong Province (No.2018CXGC1213), Taishan Scholars Program of Shandong Province (No.tspd20230610 and No.tsqnz20231251), Translational Research Grant of NCRCH (No.2021WWB02 and No.2020ZKMB01), Shandong Provincial Engineering Research Center of Lymphoma, Academic Promotion Programme of Shandong First Medical University (No. 2019QL018), China Postdoctoral Science Foundation (No. 2023M741506), and Shandong Provincial Natural Science Foundation (No. ZR2023QH193).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




