Cellular metabolism: A key player in cancer ferroptosis
Xianjie Jiang and Qiu Peng contribute equally to the work.
Abstract
Cellular metabolism is the fundamental process by which cells maintain growth and self-renewal. It produces energy, furnishes raw materials, and intermediates for biomolecule synthesis, and modulates enzyme activity to sustain normal cellular functions. Cellular metabolism is the foundation of cellular life processes and plays a regulatory role in various biological functions, including programmed cell death. Ferroptosis is a recently discovered form of iron-dependent programmed cell death. The inhibition of ferroptosis plays a crucial role in tumorigenesis and tumor progression. However, the role of cellular metabolism, particularly glucose and amino acid metabolism, in cancer ferroptosis is not well understood. Here, we reviewed glucose, lipid, amino acid, iron and selenium metabolism involvement in cancer cell ferroptosis to elucidate the impact of different metabolic pathways on this process. Additionally, we provided a detailed overview of agents used to induce cancer ferroptosis. We explained that the metabolism of tumor cells plays a crucial role in maintaining intracellular redox homeostasis and that disrupting the normal metabolic processes in these cells renders them more susceptible to iron-induced cell death, resulting in enhanced tumor cell killing. The combination of ferroptosis inducers and cellular metabolism inhibitors may be a novel approach to future cancer therapy and an important strategy to advance the development of treatments.
List of abbreviations
-
- TCA
-
- tricarboxylic acid
-
- HK
-
- hexokinase
-
- GSH
-
- glutathione
-
- PUFA
-
- polyunsaturated fatty acid
-
- SFA
-
- saturated fatty acid
-
- MUFA
-
- monounsaturated fatty acid
-
- G6P
-
- glucose 6-phosphate
-
- ROS
-
- reactive oxygen species
-
- G6PD
-
- glucose-6-phosphate dehydrogenase
-
- BECN1
-
- beclin 1
-
- USF2
-
- upstream stimulatory factor 2
-
- PDK4
-
- pyruvate dehydrogenase kinase 4
-
- IDH2
-
- isocitrate dehydrogenase 2
-
- OXPHOS
-
- oxidative phosphorylation
-
- AA
-
- arachidonic acid
-
- AdA
-
- adrenoyl
-
- PE
-
- phosphatidylethanolamine
-
- ACSL4
-
- acyl-coA synthetase long-chain family 4
-
- OA
-
- oleic acid
-
- POA
-
- palitoleic acid
-
- LPLATs
-
- lysophospholipid acyltransferases
-
- SCD
-
- steroyl CoA desaturase
-
- PEBP1
-
- phosphatidylethanolamine binding protein 1
-
- TD52
-
- tumor protein D52
-
- PLIN2
-
- perilipin2
-
- H2S
-
- hydrogen sulfide
-
- GCL
-
- glutamate-cysteine ligase
-
- GDH
-
- glutamate dehydrogenase
-
- GOT2
-
- glutamic-oxaloacetic transaminase 2
-
- TFR1
-
- membrane protein TF receptor 1
-
- STEAP3
-
- six-transmembrane epithelial antigens of the prostate 3
-
- DMT1
-
- divalent metal transporter 1
-
- PCBP1
-
- poly-(rC)-binding protein 1
-
- LIP
-
- labile iron pool
-
- FPN
-
- ferroportin
-
- HSe
-
- selenide
-
- γ-GCS
-
- γ-glutamylcysteine synthase
-
- HCAR1
-
- hydroxycarboxylic acid receptor1
-
- GPX4
-
- glutathione peroxidase 4
-
- PDH
-
- pyruvate dehydrogenase
-
- BSO
-
- l-buthionine sulfoximine
-
- γ-GCS
-
- γ-glutamylcysteine synthase
-
- acyl-CoA
-
- acyl coenzyme A
-
- NADH
-
- nicotinamide adenine dinucleotide
-
- NADPH
-
- nicotinamide adenine dinucleotide phosphate
-
- KDM5C
-
- lysine demethylase 5C
-
- MCT1
-
- monocarboxylate transporter 1
-
- SREBP1
-
- sterol regulatory element-binding protein 1
-
- SCD1
-
- stearoyl-coenzyme A desaturase-1
-
- RSL3
-
- ras-selective lethal small molecule 3
-
- PFKP
-
- phosphofructokinase
-
- PKM2
-
- pyruvate kinase M2
-
- AMPK
-
- AMP-activated protein kinase
-
- JAK2
-
- janus kinase 2
-
- STAT3
-
- signal transducer and activator of transcription 3
-
- ELOVL5
-
- elongation of very long-chain fatty acid protein 5
-
- FADS1
-
- fatty acid desaturase 1
-
- iPLA2β
-
- Ca2+ independent phospholipase A2β
-
- LPCAT3
-
- lysophosphatidylcholine acyltransferase 3
-
- ALOX
-
- arachidonic acid lipoxygenase
-
- POR
-
- cytochrome p450 oxidoreductase
-
- RAB7A
-
- RAS oncogene family member 7A
-
- PNPLA2
-
- patatin like phospholipase domain containing 2
-
- EAAT3
-
- excitatory amino acid transporter 3
-
- ASCT1
-
- alanine/serine/cysteine/threonine transporter 1
-
- GSSG
-
- glutathione disulfide
-
- SLC1A5
-
- solute carrier family 1 member 5
-
- α-KG
-
- α-ketoglutarate
-
- GLS
-
- glutaminase
-
- GOT1
-
- glutamic-oxaloacetic transaminase 1
-
- TFRC
-
- transferrin receptor
-
- PSTK
-
- phosphoseryl-tRNA kinase
-
- TrxR
-
- thioredoxin reductase
-
- DIAPH3
-
- diaphanous related dormin 3
-
- iFSP1
-
- FSP1 inhibitor
-
- icFSP1
-
- FSP1-specific inhibitor
1 BACKGROUND
Cancer is currently the leading cause of death that shortens life expectancy worldwide [1]. According to the World Health Organization estimates, in 2019, cancer ranked second among factors causing death before age 70, posing a serious threat to human life and health security [1, 2]. Cancer is a result of variation in the regulation of certain cells in the body that are free from the limits imposed by cell death programs, allowing unlimited cancer cell proliferation and invasive metastasis. Programmed cell death is an important guarantee for normal cell renewal in the body, and programmed cell death impairment triggers unrestricted proliferation of normal cells and promotes the transition into tumor cells, ultimately leading to the occurrence of cancer. Programmed cell death, including apoptosis, necroptosis, pyroptosis, and autophagic death, is a regulated process in cells under the control of a series of signaling pathways. Additionally, a previously unnamed type of cell death called ferroptosis was discovered in 2012 [3].
Ferroptosis is a programmed cell death resulting from membrane damage caused by lipid peroxidation in an ion-dependent manner, and it is different from apoptosis, necrosis, pyroptosis and autophagic death [4-6]. When cells undergo ferroptosis, their morphology changes, tending toward increased roundness, resembling cells undergoing necroptosis. However, in contrast to necroptosis, ferroptosis does not cause cytoplasmic or organelle swelling or membrane rupture, and the nucleus remains relatively intact [3, 7]. In addition, ferroptotic cells do not exhibit chromatin condensation or apoptotic body formation through plasma membrane blebbing, both of which are hallmarks of apoptosis [8]. In addition, the formation of multiple closed vesicles with double membranes observed in autophagic cells and the loss of lipid membrane integrity seen in cells undergoing pyroptosis are not evident in ferroptotic cells [9]. Despite these differences, mitochondrial wrinkling and increased density compared to mitochondria in normal cells are the distinguishing morphological characteristics of ferroptotic cells [9-12]. Differences between ferroptosis and other types of programmed cell death are summarized in Table 1. Studies have shown that the fundamental mechanism underlying ferroptosis is based on the dysregulation of intracellular redox homeostasis, which triggers lipid peroxidation and ultimately disrupts membrane integrity, leading to cell death [4, 13, 14]. Recent research indicates that ferroptosis is induced primarily by the dysregulation of the solute carrier family 7 member 11 (SLC7A11)-glutathione (GSH)-glutathione peroxidase 4 (GPX4), nicotinamide adenine dinucleotide (phosphate) (NAD(P)H)-ferroptosis suppressor protein 1 (FSP1)-coenzyme Q10 (CoQ10), GTP cyclohydrolase-1 (GCH1)-tetrahydrobiopterin (BH4), dihydroorotate dehydrogenase (DHODH)-ubiquinol (CoQH2), and acyl-CoA synthetase long-chain family member 4 (ACSL4)-polyunsaturated fatty acids (PUFAs) signaling pathways and disruptions to iron metabolism [15, 16]. Ferroptosis is closely linked to the development of diseases, and studies have shown that ferroptosis plays an important role in neurological diseases, ischemia-reperfusion injury, kidney injury, hematological diseases, and tumorigenesis. Increasing evidence has shown that ferroptosis exerts an important inhibitory effect on the progression of tumors [12]. In most tumor cells, ferroptosis signaling is inhibited [6]. This finding suggests that suppression of ferroptosis signaling may play a crucial role in tumorigenesis and that targeted induction of ferroptosis may lead to a significant breakthrough in cancer therapy. Therefore, understanding the regulation of iron-mediated cell death signaling in tumor cells is crucial for the accurate diagnosis and effective treatment of tumors.
| Cell death | Morphological characteristic | Biochemical characteristic | Symbol | Risk factor | Immune feature | Key gene | Reference |
|---|---|---|---|---|---|---|---|
| Ferroptosis | Cellular mitochondria become smaller, mitochondria membrane density increases, and cristae decrease | Iron overload, lipid peroxidation | Lipid peroxidation | Disruption of redox balance | Pro-inflammatory | GPX4, SLC7A11, FSP1, ACSL4, TP53, Nrf2, DHODH, TFRC, ferritin | [4, 6] |
| Apoptosis | Cell size consolidation and chromatin condensation aggregation | Caspase activate, DNA degradation and fragmentation | Apoptotic bodies formation | Autoclasia, immune cell interactions | Mostly anti-inflammatory | Caspase, TP53, Fas, Bcl-2, Bax | [8] |
| Necroptosis | Cell rupture and release of contents | Decreased ATP levels, RIP1, RIP3 and MLKL activation | MLKL phosphorylation | Stimulation by pathogens | Mostly pro-inflammatory | LEF1, RIP1, RIP3 | [3, 7] |
| Pyroptosis | Cells swollen with bubble-like protrusions, intact nuclei, and DNA fragmentation | Inflammasome formation, caspase and gasdermin activation, a large number of pro-inflammatory cytokines release | Inflammasome formation | Stimulation by pathogens | Pro-inflammatory | Caspase1, GSDMD, IL-1β | [9, 10] |
| Autophagy | The organelles were swollen, the cytoplasm was amorphous, the cytoplasm was extensively vacuolated, and the nucleus was broken | Conversion of LC3-I to LC3-II, autophagic substrate degradation | Autophagosome formation | Nutritional deficiencies, oxidative stress and protein aggregation, etc. | Mostly anti-inflammatory | ATG5, ATG7, DRAM3, TFEB | [11, 12] |
- Abbreviations: ACSL4, acyl-CoA synthetase long-chain family member 4; ATG5, autophagy related 5; ATG7, autophagy related 7; DRAM3, DNA damage regulated autophagy modulator 3; FSP1, ferroptosis suppressor protein 1; GPX4, glutathione peroxidase 4; GSDMD, gasdermin D; IL-1β, interleukin 1 beta; LC-3, microtubule associated protein light chain 3; LEF1, lymphoid enhancer binding factor 1; MLKL, mixed lineage kinase domain-like protein; Nrf2, NFE2 like bZIP transcription factor 2; RIP1, receptor-interacting serine/threonine-protein kinase 1; RIP3, receptor-interacting serine/threonine-protein kinase 3; SLC7A11, solute carrier family 7 member 11; TP53, tumor protein p53; TFEB, transcription factor EB; TFRC, transferrin receptor.
Cellular metabolism is the basis of normal physiological cellular functions. It contains the metabolism of biological macromolecules and other factors, such as trace elements (including iron and selenium). Biological macromolecule metabolism involves glucose, fatty acid, and amino acid metabolism, which generates ATP and provides the building blocks necessary for nucleic acid, lipid, and protein synthesis, thereby supporting cellular life processes. Trace element metabolism, such as iron and selenium metabolism, plays a crucial role in regulating the activity of various enzymes within cells and the essential cofactors needed for normal cellular growth [17]. Simultaneously, the modulation of cellular signaling is influenced by alterations in cellular metabolism. In recent years, numerous studies have demonstrated the pivotal regulatory role of cell metabolism in tumor cell ferroptosis [18, 19].
In this review, we expound upon the roles of glucose metabolism, fatty acid metabolism, amino acid metabolism, iron metabolism and selenium metabolism in tumor ferroptosis. Additionally, we described the specific regulatory mechanisms of different cellular pathways during tumor cell ferroptosis to gain a deeper understanding of the connection between cellular metabolism and tumor cell ferroptosis.
2 THE CELLULAR METABOLISM-RELATED MECHANISMS UNDERLYING CANCER FERROPTOSIS
2.1 Glucose metabolism
Glucose is the main source of cellular energy and an important substrate for the synthesis of biomolecules in cells [20]. After entering cells through transporters, extracellular glucose is consumed by cells through glycolysis and the tricarboxylic acid (TCA) cycle to produce energy and intermediate metabolites for cellular biosynthesis. Glycolysis is the first step of intracellular glucose metabolism. Glucose entering the cell is catabolized in the cytoplasm by hexokinase (HK) to yield glucose 6-phosphate (G6P), which is subsequently transformed into fructose 6-phosphate under the action of G6P isomerase, and fructose 6-phosphate participates in the next step of the glucose catabolism process, which eventually produces nicotinamide adenine dinucleotide (NADH) and pyruvate. Some of this generated pyruvate is converted to lactate under the action of lactate dehydrogenase to complete glycolysis. The other portion of the generated pyruvate enters mitochondria and is consumed in the TCA cycle, which produces energy as well as intermediate metabolic products [21]. Mitochondria are the main sources of energy production in cells. Energy production is an oxygen-consuming process, and the TCA cycle drives the transfer of electrons across the mitochondrial inner membrane, where large amounts of reactive oxygen species (ROS), including H2O2, OH-, ·OH, etc.) are also produced. ROS plays multifaceted roles in cells. On the one hand, ROS can be signaling molecules that stimulate cell proliferation; on the other hand, ROS at high concentrations can damage cells and cause cell death. Thus, glycolysis and the TCA cycle maintain a dynamic balance in cells [22-24]. Notably, in the 1920s, scientists discovered that in most tumor cells, energy is primarily produced through glycolysis, even in the presence of sufficient oxygen, a phenomenon known as the Warburg effect. The glycolysis reaction is rapid and can produce a large amount of energy in a short period. Moreover, a pathway that bypasses glycolysis, known as the pentose phosphate pathway (PPP pathway), produces large amounts of ribose 5-phosphate (a precursor of DNA synthesis), 4 phosphate and the reducing equivalent nicotinamide adenine dinucleotide phosphate (NADPH) to induce the rapid proliferation of tumor cells [25]. Furthermore, NADPH is involved in the synthesis of the reduced form of glutathione (GSH), an important reducing agent, which can effectively attenuate oxidative damage in tumor cells [26]. As mentioned above, the primary cause of ferroptosis is disrupted redox balance. Abnormalities in glucose metabolism in tumor cells can alter redox homeostasis, leading to the sensitivity or resistance of tumor cells to ferroptosis [27]. Therefore, the normal regulation of intracellular glucose metabolism plays an important role in tumor ferroptosis (Figure 1).
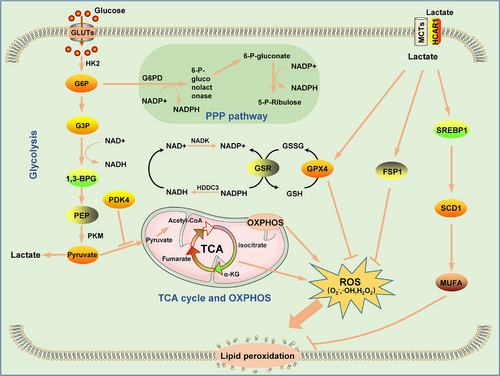
Glucose-6-phosphate dehydrogenase (G6PD) is a rate-limiting enzyme in the PPP. Abnormal expression of G6PD in tumor cells can lead to the disruption of intracellular NADPH and GSH metabolism, causing intracellular redox imbalance and contributing to tumor ferroptosis [28, 29]. Extensive glycogen accumulation and mutations in histone-modifying genes are important to the development of clear cell renal carcinoma. For example, a high frequency of histone methylase lysine demethylase 5C (KDM5C) mutations has been found in patients with clear cell renal cell carcinoma, and restoration of KDM5C expression in clear renal cell carcinoma cells led to a reduction in glycogen production, inhibition of G6PD expression and PPP activation, decreased NADPH and GSH production, and ferroptosis inhibition [28]. In addition, accumulating evidence has shown that G6PD can inhibit ferroptosis by regulating the expression of cytochrome P450 oxidoreductase. In hepatocellular carcinoma (HCC), G6PD expression is elevated and negatively correlated with the prognosis of HCC patients [29]. Further study showed that G6PD inhibited the expression of P450, thereby inhibiting the ferroptosis of HCC cells and enhancing the proliferation, invasion and metastasis of HCC cells [29]. Lactate is a metabolite of glycolysis, and mucosal melanoma cells can take up extracellular lactate through mononuclear transport carriers, thereby increasing PPP pathway signaling and upregulating ferroptosis-related GPX4 and FSP1 expression. In addition, lactate induces an increase in the intracellular levels of NADH, NADPH and GSH, leading to the resistance of tumor cells to ferroptosis [30]. In addition, lactate can activate the hydroxycarboxylic acid receptor 1 (HCAR1)/monocarboxylate transporter 1 (MCT1)-sterol regulatory element-binding protein 1 (SREBP1)-stearoyl-coenzyme A desaturase-1 (SCD1) signaling pathway to induce monounsaturated fatty acid (MUFA) generation, thereby inhibiting the ferroptosis of tumor cells [31]. Ras-selective lethal small molecule 3 (RSL3) is a ferroptosis inducer that targets GPX4 and promotes ferroptosis. Furthermore, in addition to targeting GPX4, RSL3 downregulated the expression of glycolysis-related proteins such as hexokinase 2 (HK2), phosphofructokinase (PFKP), and pyruvate kinase M2 (PKM2) and reduced the glycolysis rate in glioma cells, while the addition of sodium pyruvate greatly reduced the killing effect of RSL3 on glioma cells, reflecting the role of glycolysis in anti-ferroptosis effects [32]. AMP-activated protein kinase (AMPK) is a sensory receptor of intracellular ATP and is activated during energy stress in tumor cells, while activated AMPK can promote GPX4-dependent ferroptosis via the action of the janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3)/tumor protein p53 (p53) signaling axis in kidney cancer cells [33]. AMPK can also promote ferroptosis by promoting the phosphorylation of beclin1 (BECN1), which leads to the inactivation of the SLC7A11 protein [34]. These findings confirm that highly efficient glycolysis can inhibit ferroptosis in tumor cells because AMPK is inactivated when glycolysis is accelerated. In contrast, the activation of AMPK activates ACACA, which inhibits the synthesis of PUFAs, thereby inhibiting ferroptosis [35]. This finding suggests that AMPK plays a dual role in regulating ferroptosis in cancer cells, which mainly depends on substrate AMPK activation. Pyruvate is an important product of glycolysis and initiates the mitochondrial TCA cycle. In pancreatic cancer, upstream stimulatory factor 2 (USF2) is an upstream regulator of PKM2; specifically, USF2 binds to the promoter of PKM2 and upregulates the expression of PKM2 to increase the intracellular GSH concentration and the expression of GPX4, thereby inhibiting pancreatic cancer cell ferroptosis [36].
Mitochondria are sites of the TCA cycle, oxidative phosphorylation (OXPHOS) and ROS production. Therefore, mitochondrial metabolism exerts an important impact on ferroptosis [37]. Notably, cysteine deficiency leads to mitochondrial membrane hyperpolarization and the accumulation of lipid peroxides, leading to ferroptosis [38, 39]. Inhibition of the TCA cycle or OXPHOS can alleviate this phenomenon [38]. Pyruvate is an important raw material for the TCA cycle. Pyruvate enters the TCA cycle through the action of pyruvate dehydrogenase to form acetyl coenzyme A (CoA). In a study on pancreatic ductal adenocarcinoma, researchers found that pyruvate dehydrogenase kinase 4 (PDK4) drove resistance to ferroptosis. Specifically, PDK4 inhibited the entry of pyruvate into the TCA cycle by preventing pyruvate oxidation, thereby reducing the fatty acid synthesis rate and ultimately inhibiting ferroptosis [40]. However, the role of mitochondria in ferroptosis in tumor cells is unclear because HT-1080 human fibrosarcoma cells undergo ferroptosis even though they lack the mitochondrial electron transport chain [41]. Hence, ferroptosis signaling may differ in different tumors. Isocitrate dehydrogenase 2 (IDH2) is a key enzyme in the TCA cycle, playing an important role in NADH production and an important factor involved in mitochondrial GSH turnover. Studies have shown that the downregulation of IDH2 expression enhances the sensitivity of tumor cells to ferroptosis, which may be associated with the reduction in NADH production caused by IDH2 downregulation [42]. Fumarate hydratase is also an important metabolic enzyme in the TCA cycle, and inhibition of this enzyme activity can inhibit ferroptosis [38]. With research advancements, increasing evidence has shown that mitochondrial metabolism plays an important role in the ferroptosis of tumor cells [43, 44]. Glycolysis and mitochondrial OXPHOS are dynamic processes, and inhibition of aerobic glycolysis in tumor cells can cause metabolism in these cells to shift toward OXPHOS. In fact, inducing this metabolic shift is considered an effective strategy for tumor treatment, and the increase in OXPHOS in tumor cells may further increase the sensitivity of tumor cells to ferroptosis.
In general, NADPH NADH, GSH, and ROS produced by glucose metabolism affect the intracellular redox balance, and when the number of oxidized intracellular products increases, lipid peroxidation is induced, leading to cell ferroptosis. Thus, resistance to intracellular oxidation is an effective strategy to defend against ferroptosis.
2.2 Fatty acid metabolism
Thousands of lipid molecules are produced in the human body. In addition to being the main substance of biofilms, lipid molecules play important roles in energy storage and signal transduction [45]. Lipid metabolism is involved in several processes of cellular life activities, and abnormal lipid metabolism exerts an impact on the normal life activities of many cells, including ferroptosis. During lipid metabolism, peroxidation of PUFAs is a central trigger of ferroptosis [46]. We describe the role of lipid metabolism in ferroptosis from the perspective of the synthesis, regulation, storage and transport of PUFAs (Figure 2).
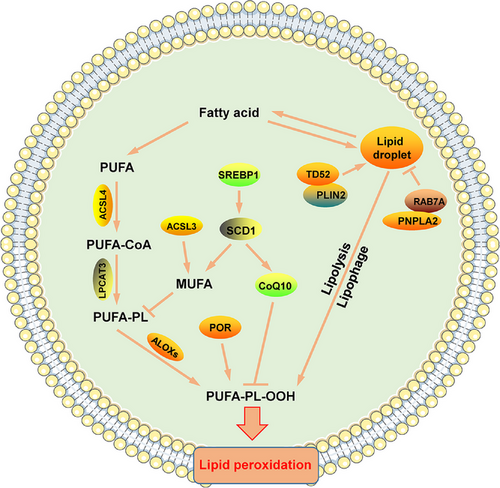
The fatty acyl groups of membrane phospholipids are very diverse in terms of chain length and saturation (double vs. single bonds), which determines the biophysical properties of the cell membrane, including its fluidity, curvature and subdomain structures [47]. These membrane characteristics, in turn, affect membrane-related cellular functions and determine the sensitivity of cells to ferroptosis [48]. The phospholipid molecule is made up of a number of different fatty acids, including saturated fatty acids (SFAs), MUFAs, and PUFAs. The phospholipid molecular layer, which contains many unsaturated fatty acids, is susceptible to ROS damage because the C = C bond in unsaturated organic molecules is particularly prone to chain reaction oxidation. PUFAs constitute a class of fatty acid molecules with two or more C = C bonds in the carbon chains [49]. The double bond adjacent to the methylene group weakens the hydrogen bond connecting a dienyl methyl group to the PUFA chain, thereby increasing the sensitivity of the carbon chain to hydrogen ion acquisition, eventually leading to their oxidation. In addition, when PUFAs are oxidized, they are converted into reactive free radical groups, which propagate a lipid peroxidation chain reaction [50]. Thus, the content and location of PUFAs in the phospholipid bilayer determine the sensitivity of cells to ferroptosis [51].
Phosphatidylethanolamine (PE) is an esterification product of PUFAs, mainly arachidonic acid (AA) and adrenoyl (AdA), which directly induce the development of ferroptosis after being oxidized. Ferroptosis requires acyl coenzyme synthetase long-chain family 4 (ACSL4) [52-55]. Peroxisomes are also sources of the synthesized lipids needed to initiate ferroptosis, and peroxisome-mediated plasmalogen enhances ferroptosis in tumor cells by synthesizing polyunsaturated ether phospholipids (PUFA-ePLs) in the endoplasmic reticulum, causing lipid peroxidation [56]. In contrast to PUFAs, MUFAs such as exogenous oleic acid (OA) and palmitoleic acid (POA) inhibit erastin- and RSL3-induced ferroptosis in tumor cells. Specifically, MUFAs inhibit the production of lipid ROS, especially in cells of the serous membrane, replacing PUFAs in cells [57].
As mentioned above, the abundance and localization of PUFAs determine the degree of lipid peroxidation in cells and, therefore, the degree of iron toxicity. Free PUFAs are substrates for the synthesis of lipid signaling mediator substrates, but they must be esterified to membrane phospholipids and undergo oxidation [54]. Thus, the formation of CoA derivatives of these PUFAs and the incorporation of these derivatives into phospholipids are critical for the generation of death signaling induced by iron overloading. The incorporation of newly synthesized fatty acids into phospholipids requires the conversion of the long-chain fatty acid stearyl-CoA catalyzed by acyl-coenzyme A synthase (ACS) and re-acylation catalyzed by lysophospholipid acyltransferases (LPLATs) [58, 59]. The acyl coenzyme A (acyl-CoA) synthetase long-chain family is expressed mainly in the cytoplasmic matrix and mitochondrial outer membrane and catalyzes the conversion of fatty acids to acyl-CoAs [60]. These acyl-CoAs are intermediate products of lipid metabolism and participate in fatty acid metabolism and biofilm modifications [61]. Among the ACSL family, the most closely related to ferroptosis are ACSL4 and ACSL3. ACSL4 attaches CoA to free fatty acids via esterification in an ATP-dependent manner, and its preferred substrates are AA and AdA, and its downregulation in tumors leads to the resistance of tumor cells to ferroptosis [5, 52, 62]. Elongation of very long-chain fatty acid protein 5 (ELOVL5) and fatty acid desaturase 1 (FADS1) may also increase the sensitivity of cells to ferroptosis by promoting the synthesis of PUFAs [63]. In contrast, ACSL3, for which the preferred substrate is OA, can activate MUFAs, which replace the PUFAs in the plasma membrane, thereby reducing the sensitivity of the plasma membrane to peroxidation and inhibiting PUFA-induced lipotoxicity [57, 62]. Familiar to ACSL3, stearoyl CoA desaturase (SCD/SCD1) is a multifunctional enzyme involved in lipid metabolism, and it can catalyze the further desaturation of unsaturated fatty acids and inhibit ferroptosis [64]. In addition, SCD1 also upregulates the expression of CoQ10 (an antioxidant that inhibits ferroptosis), and its anti-ferroptotic activity includes both monosaturated fatty acid-dependent and non-dependent functions [65]. In addition, a number of other factors can inhibit lipid peroxidation. Phospholipid-modifying enzymes (MBOAT1/2) can inhibit ferroptosis by remodeling the cellular phospholipid profile, and strikingly, their ferroptosis surveillance function is independent of GPX4 or FSP1 [66]. Ca2+ independent phospholipase A2β (iPLA2β) is a calcium-independent phospholipase, iPLA2β-mediated detoxification of peroxidized lipids is sufficient to suppress p53-driven ferroptosis upon ROS-induced stress, even in GPX4-null cells [67, 68].
Esterification is an important step in the peroxidation of phospholipids, carried out mainly by lysophosphatidyl transferases (LPCATs). LPCATs constitute a group of enzymes critical for phospholipid remodeling, mainly in the endoplasmic reticulum. They catalyze the attachment of the fatty acyl chain at the sn-2 site of phosphatidylcholine, thereby regulating the fatty acyl composition of phospholipids [48, 69]. After linking CoA via ACSL4, long-chain PUFAs esterify lysophospholipids via LPCAT; this in turn triggers peroxidation, leading to ferroptosis [51]. Lysophosphatidylcholine acyltransferase 3 (LPCAT3) regulates lipid metabolism mainly by regulating lipid uptake, lipoprotein secretion and de novo lipogenesis [48].
Peroxidation of PUFAs ultimately requires the incorporation of oxygen into lipids, and arachidonic acid lipoxygenases (ALOXs) are among these iron-containing dioxygenases [70]. ALOX5 is the key trigger of lipid peroxidation, and it can oxidize AA to form the unstable intermediate 5-hydrperoxyeicosatetraenoic acid (5-HPETE) [71]. In addition, ALOX5, in combination with the scaffolding protein PE-binding protein 1 (PEBP1), forms lipid peroxides and promotes ferroptosis [72, 73]. Cytochrome p450 oxidoreductase (POR) is an oxidoreductase that promotes the peroxidation of polyunsaturated phospholipids independent of ALOX action. It transfers an H molecule from NADPH to an O molecule to form H2O2, which induces the Fenton reaction with Fe2+ in cells to promote the peroxidation of PUFAs, thereby inducing ferroptosis [74, 75].
Lipids in cells are stored in the form of lipid droplets, and the outcome of this storage mechanism is the inhibition of ferroptosis by reducing the intracellular concentration of free unsaturated fatty acids [76, 77]. Tumor protein D52 (TD52) and Perilipin2 (PLIN2) promote the formation of lipid droplets and inhibit ferroptosis in tumor cells [78, 79]. In contrast, the degradation of lipid droplets promotes ferroptosis. Lipolysis and lipophagy are the two main modes of lipid droplet degradation, and RAS oncogene family member 7A (RAB7A)- and patatin like phospholipase domain containing 2 (PNPLA2)-mediated lipid droplet degradation promotes ferroptosis [79, 80].
Fatty acid metabolism is most closely related to ferroptosis, and peroxidation of PUFAs is the key factor in cellular ferroptosis; increased production of PUFAs and disruption of the PUFA regulatory system will lead to peroxidation of PUFAs, resulting in cellular ferroptosis. MUFAs, which are more stable than PUFAs, can replace PUFAs to reduce the occurrence of lipid peroxidation. Thus, for tumor cells, promoting the levels of intracellular PUFAs, increasing the activity of the PUFA production and regulation system, or attenuating the synthesis of MUFAs are all feasible solutions to promote ferroptosis and increase the efficacy of antitumor therapy.
2.3 Amino acid metabolism
Amino acids are essential nutrients for the human body and are the basic raw materials for protein synthesis. In addition, amino acids are involved in energy metabolism, the synthesis of biomolecules, signal transduction and the maintenance of intracellular redox homeostasis [81, 82]. Insufficient intake of amino acids can seriously endanger human health. Studies have shown that amino acid metabolism is closely related to tumor development, and abnormal amino acid metabolism has been found in many tumors [83]. Accumulating evidence suggests that the abnormal metabolism of amino acids is related to ferroptosis in tumor cells and the regulation of tumor sensitivity to ferroptosis inducers. Among those needed by the human body, some amino acids must be ingested through food; these are called essential amino acids; other amino acids can be produced intracellularly through a synthetic transformation pathway, and they are called nonessential amino acids. Both types of amino acids play important roles in regulating tumor cell ferroptosis (Figure 3) [84].
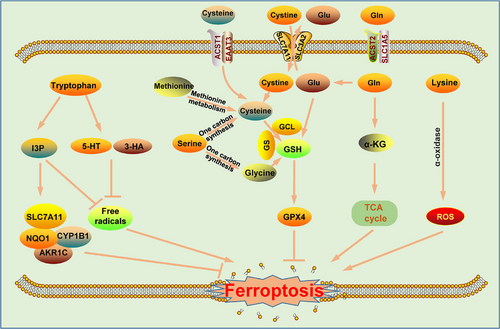
Cysteine is a nonessential amino acid that exists mainly in the form of cystine in the extracellular space. It is transported into cells by cystine transporters and, after being reduced to cysteine, participates in the synthesis of GSH, hydrogen sulfide (H2S), 3-thiol pyruvate, homocysteine, and other organic compounds, thereby regulating the growth, metastasis, and drug resistance of tumors [85]. In addition, cysteine is generated via the transsulfuration pathway [86]. In recent years, many studies have reported that cysteine deprivation not only inhibits the proliferation of tumor cells but also promotes ROS production by inhibiting GSH synthesis and reducing intracellular NADPH content, which ultimately induces tumor ferroptosis [5, 85]. Cysteine starvation therapy significantly inhibited the growth of tumors transplanted into mice [87]. Studies have shown that the anabolism of cysteine in tumor cells consists of 2 pathways: direct uptake of extracellular cysteine/cystine or generation of cysteine through other reactions (mainly via the transsulfuration pathway) [88]. Direct absorption of extracellular cysteine is mediated through the excitatory amino acid transporter 3 (EAAT3) and alanine/serine/cysteine/threonine transporter 1 (ASCT1), and the absorption of cystine into the cell is mediated through system Xc- (cystine/glutamate antiporter); in cells, cystine is reduced to yield cysteine [89–91]. The transsulfuration pathway involves the conversion of methionine to S-adenosylmethionine by the rate-limiting enzyme methionine adenylyltransferase 2A (MAT2A), and then a series of biochemical reactions culminates in the formation of cysteine [92]. In addition, cysteine can be formed by the autophagy-related breakdown of intracellular proteins and GSH [14]. We know that cysteine is a raw material in the production of reduced GSH, which is triggered in response to glutamate-cysteine ligase (GCL). GSH is an important intracellular reducing agent in cells that neutralizes intracellular ROS, thereby inhibiting cellular peroxidation. In cells, GSH exists in 2 forms: the reduced form (GSH) and the oxidized form (glutathione disulfide [GSSG]). The interconversion of the 2 forms maintains the redox balance in cells [93]. The abnormal metabolism of cysteine leads to the inhibition of GSH synthesis, causing disrupted intracellular oxidative homeostasis and tumor cell ferroptosis [94, 95].
Glutamine is a nonessential amino acid that is found in large quantities in tumor cells. It enters cells from the extracellular environment and can be produced in tumor cells through a series of reactions. After extracellular glutamine enters tumor cells through the transporter solute carrier family 1 member 5 (SLC1A5; known as ASCT2), it can be converted into glutamate under the catalytic effect of glutaminase. Glutamine contributes to the formation of GSH and NADPH together with Gly and Cys synthesis and regulates redox homeostasis in cells [96, 97]. Moreover, glutamine can be a raw material and intermediate metabolite in the synthesis of nucleic acids, other amino acids and biomolecules [98, 99]. Since the discovery of aerobic glycolysis in tumor cells, some tumor cells have been shown to produce energy independent of glycolysis; in addition, glucose deprivation does not cause cell death immediately. Studies have reported that excessive glutamine provides energy for tumor cells and thus maintains tumor cell survival [100]. Moreover, glutamine was shown to be consumed almost 10-fold faster in HCC and fibrosarcoma cells than in normal cells [84]. This rapid consumption is due to glutamine entering mitochondria and being converted into α-ketoglutarate (α-KG) under the action of glutaminase (GLS) and glutamate dehydrogenase (GDH), after which it enters the TCA cycle and produces ATP and TCA cycle intermediate metabolites needed for cell survival [99, 101, 102]. Glutamine metabolism is closely associated with ferroptosis, which may be related to the catabolic metabolism of glutamine [90]. Glutamine is converted into glutamate, aspartate, pyruvate, lactate, arginine, citrate, and other amino acids through a series of reactions catalyzed by metabolic enzymes such as GLS, GDH, and glutamic-oxaloacetic transaminase 2(GOT2), and these amino acids are subsequently consumed as raw materials in the TCA cycle and during lipid synthesis, thus reducing the ferroptosis rate [84] Furthermore, α-KG, a metabolite of glutamine, and a series of downstream α-KG products, including succinic acid, ferredoxin, and malic acid, can substitute for glutamine and promote the accumulation of lipid ROS [103]. Thus, inhibition of glutamine metabolic signaling, such as that caused by inhibition of glutamine transport carriers, GLS activity, or glutamic-oxaloacetic transaminase 1 (GOT1) activity, can profoundly inhibit ferroptosis [104-106]. However, due to the specificity of glutamine and its role as a nonessential amino acid, a compensatory mechanism is triggered in tumor cells, and the simple inhibition of glutamine uptake by inhibiting glutamine transport carriers is not sufficient to inhibit tumor growth [83]. Therefore, the combination of glutamine intake and catabolism inhibition is necessary to achieve a better therapeutic effect via glutamine-targeted tumor therapy.
Tryptophan is an essential amino acid that is involved mainly in the kynurenine (Kyn) signaling pathway and is a substrate in cells; in addition, the tryptophan metabolites serotonin (5-HT) and 3-hydroxyanthranilic acid (3-HA) markedly facilitate tumor cells, enabling them to escape ferroptosis [107, 108]. In addition, tryptophan can be metabolized to produce indole-3-pyruvate (I3P), a process catalyzed by interleukin 4 induction 1 (IL4i1), and it inhibits tumor ferroptosis. Mechanistically, I3P can upregulate the expression of ferroptosis-related genes such as the SLC7A11, NQO1, CYP1B1, and AKR1C family proteins at the transcriptional level; on the other hand, I3P shows the ability to scavenge free radicals efficiently, thereby inhibiting lipid peroxidation and inducing cell resistance to ferroptosis [109]. In addition, some other amino acids play important roles in the regulation of ferroptosis in tumor cells. Serine is an important component of the one-carbon synthesis pathway, and activation of the serine synthesis pathway is directly related to the synthesis of GSH. This may be because serine is directly involved in the synthesis of Cys, and Cys and Gly are important raw materials for GSH synthesis and are directly related to ferroptosis [84, 110]. This role played by serine implies that the metabolism of serine and glycine is likely to play an important role in ferroptosis in tumor cells. Recent studies have shown that lysine also plays an important role in the regulation of ferroptosis. L-lysine α-oxidase can activate ferroptosis signaling by catalyzing the oxidative decarboxylation of lysine and ROS production in triple-negative breast cancer cells [111].
Abnormal amino acid metabolism is an important feature of tumors. The current study suggests that amino acid metabolism affects ferroptosis mainly through the production of GSH and the induced expression of some ferroptosis-related genes, such as SLC7A11 and NQO1, by amino acid metabolites. Although reports on the regulation of ferroptosis by amino acids in tumor cells are rare, as research advances, the mechanism by which amino acid metabolism regulates ferroptosis is expected to become clearer.
2.4 Iron metabolism
Iron is an important trace element in the human body and an important component of blood cells. Furthermore, iron is a cofactor of various intracellular enzymes and participates in the regulation of various intracellular metabolic pathways. Disruptions of iron metabolism can lead to a variety of pathological processes, including iron-induced cell death, which is the basis for the term ferroptosis. The metabolism of iron in cells involves three main processes: import, transport, and storage/export. All of these processes exert a direct impact on tumor ferroptosis (Figure 4). Fe3+ binds to ferroportin (FPN) on the surface of the cell membrane and forms a complex with membrane protein TF receptor 1 (TFR1), which enables its transport into the cell through endocytosis [14, 112]. Fe3+ that enters a cell is reduced to Fe2+ in endosomes by six-transmembrane epithelial antigens of prostate 3 (STEAP3) and is subsequently transported to the cytoplasm by the divalent metal transporter 1 (DMT1) protein [113]. Fe2+ (establishing the labile iron pool [LIP]) in the cytosol as well as in mitochondria shows oxidative activity and induces ferroptosis by causing lipid peroxidation mediated through the Fenton reaction. In addition, LIP iron can bind to ferritin via poly-(rC)-binding protein 1 (PCBP1) and PCBP2, thereby reducing the number of intracellular free iron ions and inhibiting ferroptosis [114]. Intracellular iron can be transported outside the cell via the FPN protein, thus reducing the amount of intracellular iron accumulation and regulating intracellular iron homeostasis [115].

The influx, storage and efflux of intracellular iron affects the homeostasis of intracellular iron levels and regulates the sensitivity of tumor cells to ferroptosis. In glioma cells, the expression of transferrin receptor (TFRC) was increased by pseudolaric acid B, which promoted the uptake of Fe3+ and induced glioma cell ferroptosis [116]. Ferritin is an important protein that stores Fe2+. The autophagic degradation of ferritin, called ferritinophagy, causes the release of Fe2+, thereby increasing the content of free Fe2+ in cells and inducing ferroptosis [117]. Similarly, inhibition of ferroportin activity leads to the intracellular accumulation of iron in the LIP, leading to ferroptosis in tumor cells [118]. Recent studies have reported that prominin-2 plays an important role in promoting the transport of iron out of cells. Prominin-2 promotes the formation of multivesicular bodies, that is, exosomes containing ferritin, thereby enhancing the resistance of breast cancer cells to ferroptosis inducers [119].
Irons are important influences on ferroptosis, and the accumulation of intracellular Fe2+ causes lipid peroxidation through the Fenton reaction and induces ferroptosis. Thus, iron chelators can inhibit the occurrence of ferroptosis by reducing free Fe2+ in cells. However, not all iron accumulation leads to ferroptosis, and some tumor cells contained a large amount of iron but did not undergo ferroptosis [120], suggesting that iron-induced ferroptosis is tissue- and cell-specific, and further in-depth study of its mechanism is needed.
2.5 Selenium metabolism
Selenium is an essential trace element in the human body and plays an important role in regulating the redox balance in human cells under oxidative stimulation. Selenium in the human body is obtained mainly from the diet, including selenomethionine from plant food sources and selenocysteine from animal food sources. The metabolic pathways of selenomethionine in the body mainly include the following processes: (1) Selenomethionine is randomly inserted into the methionine site of a protein during protein synthesis. When the selenomethionine-carrying protein is degraded, selenomethionine re-enters the free selenomethionine pool for recycling. (2) Selenomethionine is metabolized by selenomethionine lyase to yield methyl selenite, which is processed by a demethylase to generate selenide that enters the normal selenium metabolism pathway. (3) Selenocysteine is formed via transsulfuration [121, 122]. However, the metabolism of intracellular selenocysteine is complex; specifically, selenocysteine can be decomposed into selenide and alanine via the action of selenocysteine lyase [123]. Through selenophosphate synthase, selenide is converted to monoselenophosphate, which mediates the generation of selenocysteine-specific transfer RNA (tRNAsec) and participates in the synthesis of selenoproteins that play important roles in the synthesis and biological function of selenoproteins. The specific pathway includes a combination of L-serine and tRNAsec in the presence of serine-tRNA synthase to form ser-tRNAsec. Then, ser-tRNAsec phosphorylation is catalyzed by phosphoseryl-tRNA kinase (PSTK) to yield p-ser-tRNAsec. Through an alternative pathway, selenide (HSe−) can be converted to monoselenophosphate (H2SePO3−) by selenophosphate synthetase 2 (SEPHS2) catalysis. and H2SePO3− functions as a donor in the formation of sec-tRNA by binding to p-ser-tRNAsec in the presence of selenocysteine synthase (SelA/SecS). In turn, tRNAsec recognizes a UGA codon and, through a complex series of processes, participates in selenoprotein synthesis (Figure 5) [124-126].
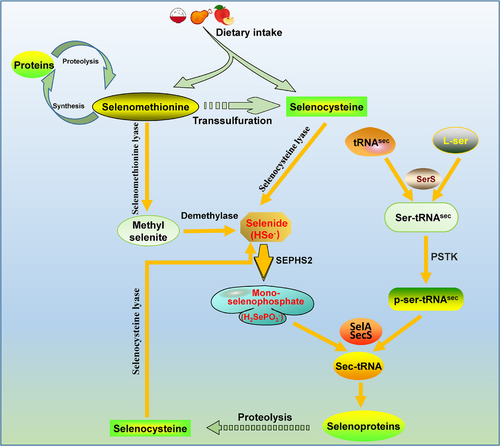
The selenoproteins involved in ferroptosis include mainly GSH peroxidase (GPX) family proteins and thioredoxin reductase (TrxR), with each of these proteins carrying a selenocysteine (sec, U, Se-Cys) [127]. GPX4, the role of which in tumor cell ferroptosis was previously mentioned, is an important antioxidant. TrxR1 also plays an important role in ferroptosis. In pancreatic cancer cells, diaphanous related dormin 3 (DIAPH3) activated the expression of selenoprotein TrxR1, thereby reducing the content of ROS, reducing the cellular lipid peroxidation rate, inhibiting the occurrence of ferroptosis, and ultimately promoting the malignant progression of pancreatic cancer [128].
Current research has shown that selenium is primarily involved in the synthesis of selenoproteins in the form of selenocysteine, which regulates the activity of selenoproteins. Hence, a normal rate of selenium metabolism may be an important factor in maintaining the normal physiological function of cells, and interfering with the selenium metabolism pathway or inducing abnormal selenium metabolism activity in tumor cells may be a new direction for cancer treatment.
3 FERROPTOSIS INDUCERS
Compared with normal cells, tumor cells demand a higher level of iron. In addition, tumor cells show abnormal metabolic activity and produce more ROS; therefore, they show a higher sensitivity to ferroptosis [15, 129]. Therefore, the induction of ferroptosis in tumor cells may be an effective strategy in cancer therapy. Ferroptosis inducers have shown great potential in the clinical treatment of cancer. In order to understand the progress of targeted ferroptosis in tumour therapy, we have summarised the inducers that are currently being used to regulate ferroptosis.
Glucose metabolism regulates cellular ferroptosis mainly by affecting the generation of intracellular reduction products (GSH, NADPH, ROS, NADH, etc.) [27, 35]. In addition, the induction of some ferroptosis-related proteins, such as FSP1, is also one of the important mechanisms by which glucose metabolism is regulated. FSP1, a GSH-independent ferroptosis inhibitor, was discovered in 2019 and inhibits the ferroptosis of tumor cells by reducing the level of CoQ10 [130]. In addition, FSP1 can also inhibit ferroptosis in an ESCRT-III-dependent membrane repair pathway or in a nonclassical redox cycle of the vitamin K pathway [131]. Recent studies suggest that FSP1 may also inhibit ferroptosis by eliminating lipid peroxidation through its intermediate metabolite 6-hydroxy-FAD [132]. Treatment with the specific small-molecule inhibitor FSP1 inhibitor (iFSP1) or FSP1-specific inhibitor (icFSP1) can specifically inhibit the activity of FSP1 and effectively induce ferroptosis [133, 134].
PUFAs increase membrane fluidity and are important protective factors that allow cells to adapt to their environment. However, abnormal expression of PUFA synthases (LPCAT3 and ACSL4) or lipid oxidases (LOXs) can lead to excessive lipid peroxidation, causing ferroptosis [135]. Bromelain induces the expression of ACSL4 and increases the lipid peroxidation rate, thereby inducing ferroptosis in tumor cells [136]. However, few agonists targeting PUFA synthases have been reported, and more need to be developed.
In amino acid metabolism, SystemXc- is the gateway for cystine entry into the cell, and its major subunit, SLC7A11, plays an important role in resistance to ferroptosis [91]. The SLC7A11-GPX4-GSH pathway is the most studied ferroptosis inhibitory signaling pathway, as it can prevent the depletion of intracellular GSH and protect cells from oxidative damage. Inhibition of this signaling pathway causes GSH depletion and induces ferroptosis, ultimately killing tumor cells [137]. System Xc- is an important amino acid transporter in the cell membrane that can import cystine to promote intracellular GSH synthesis and decrease intracellular oxidation levels; moreover, GSH is an essential cofactor of GPX4, facilitating GPX4 reduction of lipid peroxides into nontoxic lipid alcohols, thereby inhibiting ferroptosis in tumor cells [138]. Thus, inhibition of system Xc- activity effectively induces ferroptosis in tumor cells. SLC7A11 is an important component of system Xc-. Treatment with erastin, sorafenib or sulfasalazine effectively inhibits the activity of SLC7A11, leading to the depletion of intracellular GSH, which leads to the inactivation of GPX4 and induces ferroptosis in tumor cells [139-141]. Additionally, blocking GPX4 signaling with RSL3 or ML210 and promoting GPX4 degradation with FIN56 or PdPT induces ferroptosis [142-145]. Moreover, buthionine sulfoximine (BSO) promotes ferroptosis by inhibiting γ-glutamylcysteine synthase (γ-GCS), the rate-limiting enzyme in GSH synthesis, thereby reducing the level of reduced GSH and the activity of GPX4 [140].
For iron-sensitive tumors, iron overload in tumor cells is a significant contributor to ferroptosis. Inhibition of intracellular iron overload and ferrous ion accumulation effectively suppresses ferroptosis. Conversely, elevating intracellular iron levels facilitates ferroptosis [146]. Siramesine and lapatinib downregulate ferroportin activity and upregulate transferrin activity, leading to an increase in intracellular iron content and ultimately promoting ferroptosis [41, 147]. Artemisinins modulate the expression of iron metabolism-related genes, increase intracellular iron levels and promote tumor ferroptosis [148]. JQ1 and salinomycin induce ferritinophagy, leading to an increase in intracellular iron levels and triggering ferroptosis in tumor cells [26, 149]. Piperlongumine targets selenocysteine to inhibit TrxR1 activity and enhances tumor cell sensitivity to ferroptosis (Table 2) [150]. In addition, a number of other inhibitors of ferroptosis have been identified [151].
| Compound/drug | Target | Mechanism | Reference |
|---|---|---|---|
| iFSP1 | FSP1 | CoQ10 deletion | [133] |
| icFSP1 | FSP1 | Altered subcellular localization of FSP1 | [134] |
| Bromelain | ACSL4 | Increase lipid peroxidation | [136] |
| Erastin | SLC7A11 | cystine import inhibition, cysteine deprivation | [139] |
| Sorafenib | SLC7A11 | cystine import inhibition, cysteine deprivation | [140] |
| Sulfasalazine | SLC7A11 | cystine import inhibition, cysteine deprivation | [141] |
| RSL3 | GPX4 | GPX4 inactivation and GSH deprivation | [142] |
| ML210 | GPX4 | GPX4 inactivation and GSH deprivation | [143] |
| FIN56 | GPX4 and CoQ10 | GPX4 inactivation and CoQ10 deletion | [144] |
| PdPT | GPX4 | GPX4 degradation and GSH deprivation | [145] |
| BSO | γ-GCS | Inhibit GSH synthesis | [140] |
| Siramesine | Ferroportin-1 | Increase cellular irons | [41] |
| Lapatinib | Transferrin | Increase cellular irons | [147] |
| Artemisinins | Iron-related genes | Increase cellular irons | [148] |
| Salinomycin | Ferritinophagy | Ferritin degradation and increase cellular irons | [149] |
| JQ1 | Ferritinophagy | Ferritin degradation and increase cellular irons | [26] |
| Piperlongumine | Selenocysteine | TrxR1 inactivation | [150] |
- Abbreviations: γ-GCS, γ-glutamylcysteine synthas; ACSL4, acyl-CoA synthetase long-chain family member 4; CoQ10, coenzyme Q10; FSP1, ferroptosis suppressor protein 1; GPX4, glutathione peroxidase 4; SLC7A11, solute carrier family 7 member 11; TrxR1, thioredoxin reductase.
Theoretically, ferroptosis can be induced to eliminate tumor cells and thus achieve cancer treatment goals. However, no effective compounds targeting ferroptosis are currently in clinical use. This lack of suitable ferroptosis agonists may be partially attributed to the compounds that induce ferroptosis to induce other signaling pathways, leading to inadequate specificity in action and severe side effects. Another reason for this is the diverse regulatory pathways of ferroptosis in tumor cells and compensatory pathways that counteract these pathways. Therefore, the inhibition of only a single pathway does not lead to optimal results. It is believed that a more comprehensive understanding of the mechanism underlying ferroptosis and the discovery of compounds with highly specific affinity for these pathways are needed to address current deficiencies in tumor treatments targeting ferroptosis.
4 CONCLUSIONS AND PERSPECTIVE
Ferroptosis is a form of programmed cell death that is mediated via self-regulatory mechanisms in response to external stimuli or changes in the intracellular environment. The essence of ferroptosis is a redox imbalance that causes lipid peroxidation and damage to cell membranes, leading to cell death. Whether it is iron or other influences, the result is a perturbation of the intracellular redox microenvironment, causing lipid peroxidation, so that regulating the intracellular redox microenvironment can regulate cellular ferroptosis [152]. Cell metabolism is an important process through which cells maintain growth and self-renewal, providing the energy and various raw materials needed for nucleic acid synthesis and protein synthesis. Moreover, metabolic processes and metabolites in tumor cells influence the programmed regulation, including ferroptosis, of these cells. In this paper, we presented a comprehensive review of the impact of glucose, lipid, amino acid, iron and selenium metabolism on ferroptosis in tumor cells. We also explored the specific mechanisms regulating these metabolic pathways and provided a detailed summary of the compounds and clinical drugs that induce ferroptosis in tumor cells. It was found that glucose metabolism, in addition to providing energy and intermediate metabolites for nucleic acid and protein synthesis, plays an important role in regulating intracellular redox balance. Inhibition of aerobic glycolysis is considered an important strategy for the treatment of tumors. Inhibition of aerobic glycolysis inhibits the energy metabolism pathway in tumor cells and promotes the death of tumor cells by exhausting the energy supply. In addition, inhibition of aerobic glycolysis promotes a shift of glucose metabolism from glycolysis to OXPHOS mediated by the TCA cycle, enhancing the sensitivity of cancer cells to ferroptosis [153]. Inhibition of the PPP reduces NADPH production, inhibiting the expression of downstream reducing agents, such as GSH, Trx and CoQ10, which in turn promotes ferroptosis in tumor cells [130]. Sorafenib, a United States Food and Drug Administration-approved antitumor drug, has been recently shown to induce ferroptosis specifically in the presence of ACSL4. Notably, sorafenib enhances lipid ROS production in liver cancer cells by modulating metabolic pathway activity [154]. Artemisinin is a potent natural product used for the treatment of malaria, and it plays a crucial role in inducing ferroptosis in tumor cells by promoting ferritinophagy and increasing free iron levels, ultimately leading to tumor cell death [155]. Moreover, lapatinib sensitizes breast cancer cells to ferroptosis by decreasing the expression of ferroportin and ferritin and increasing the expression of transferrin [147]. BSO is a commonly used GSH inhibitor that effectively inhibits the synthesis of intracellular GSH and increases the level of intracellular ROS, thereby promoting ferroptosis sensitivity in breast cancer cells [156]. This evidence indicates that the combination of cell metabolism inhibitors and ferroptosis inducers can effectively kill tumor cells, which may be a new strategy for cancer treatment. Ferroptosis has a complex and highly context-dependent role in cancer biology and treatment. The development of translational anticancer strategies can be complex and is dependent on continued research to better understand the regulatory mechanisms and signaling pathways of ferroptosis. The search for biomarkers to facilitate the detection and tracking of ferroptosis in humans will be an area of active research in the coming years. Combinations of drugs are also an important direction of current clinical treatment, which can improve the efficacy of treatment while minimizing the toxic side effects of drugs. We anticipate that as research advancements are made, our understanding of ferroptosis will become more refined. Combining metabolic inhibitors and ferroptosis inducers to target tumor cell ferroptosis pathways is expected to be a crucial strategy for cancer therapy in the future.
DECLARATIONS
AUTHOR CONTRIBUTIONS
Xianjie Jiang, Qiu Peng, Mingjing Peng, Linda Oyang, Honghan Wang, Qiang Liu, Xuemeng Xu, Nayiyuan Wu, Shiming Tan, Wenjuan Yang, Yaqian Han, Jinguan Lin, Longzheng Xia, Yanyan Tang, and Xia Luo collected the related paper and drafted the manuscript. Jie Dai, Yujuan Zhou, and Qianjin Liao participated in the design of the review and draft the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors have nothing to report. This work was supported in part by grants from the following sources: the National Natural Science Foundation of China (82203233, 82202966, 82173142, 82302987, 82303534, and 81972636), the Natural Science Foundation of Hunan Province (2023JJ60469, 2023JJ40413, 2023JJ30372, 2023JJ30375, 2022JJ80078, and 2020JJ5336), the Research Project of Health Commission of Hunan Province (202203034978, 202109031837, and 20201020), Key Research and Development Program of Hunan Province (2022SK2051), Hunan Provincial Science and Technology Department (2020TP1018), the Changsha Science and Technology Board (kh2201054), the Changsha Municipal Natural Science Foundation (kq2014209), Ascend Foundation of National Cancer Center (NCC201909B06), Hunan Cancer Hospital Climb Plan (ZX2020001-3 and YF2020002), the Science and Technology Innovation Program of Hunan Province (2023RC3199, 2023SK4034 and 2023RC1073), and by China Postdoctoral Science Foundation (2022TQ0104 and 2022M721118).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




