Harnessing SARS-CoV-2-specific CD8+ T cells to kill target tumor cells for cancer immunotherapy
Keunok Jung and Deok-Han Ko have contributed equally to this work.
List of abbreviations
-
- CTL
-
- cytotoxic CD8+ T cell
-
- HLA
-
- human leukocyte antigen
-
- MHC-I
-
- major histocompatibility complex class I
-
- PBMC
-
- peripheral blood mononuclear cell
-
- pMHCI
-
- peptide–MHC-I complex
-
- SARS-CoV-2
-
- severe acute respiratory syndrome coronavirus 2
-
- TEDbody
-
- CD8+ T cell epitope-delivering antibody
Dear Editor,
During the coronavirus disease 2019 (COVID-19) pandemic era, many individuals were naturally infected with or vaccinated against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), resulting in the presence of cytotoxic CD8+ T cells (CTLs) specific to SARS-CoV-2 antigens, including the spike protein [1, 2]. Although these SARS-CoV-2-specific CTLs cannot eliminate cancer cells lacking the virus antigens on their surface, they can be repurposed to target and eliminate cancer cells by mimicking SARS-CoV-2-infected cells [3, 4]. However, the repurposing of SARS-CoV-2-specific CTLs for cancer immunotherapy remains unexplored. Here, we engineered CD8+ T cell epitope-delivering antibodies (TEDbodies) [5] to deliver spike antigen-derived CTL epitope peptides into the tumor cell cytosol. This process led to degradation of the antigenic peptide mediated by the cytosolic ubiquitin proteasome system, generating mature epitopes that bind to the major histocompatibility complex class I (MHC-I), with the epitope peptide-MHC-I complex (pMHCI) presented on the tumor cell surface. Consequently, tumor cells were recognized as SARS-CoV-2-infected cells and killed by the spike-specific CTLs (Figure 1A).
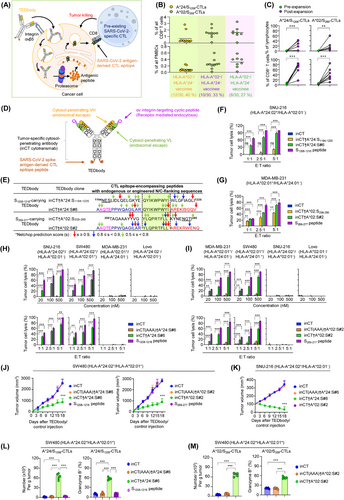
TEDbody-mediated delivery of MHC-I antigens derived from the spike protein of SARS-CoV-2 into the cytosol of integrin αv-expressing target tumor cells enables the repurposing of spike-specific CTLs to eliminate tumor cells.
(A) Schematic of the TEDbody-mediated delivery of SARS-CoV-2 antigen-derived CTL epitopes into the target tumor cell cytosol to harness pre-existing SARS-CoV-2-specific CTLs and kill tumor cells, representing a promising approach for cancer immunotherapy.
(B) Frequency of A*24/S1208- and A*02/S269-CTLs among PBMCs or their CD8+ T cells from vaccinated individuals (n = 30) with HLA-A*24 and/or HLA-A*02 alleles. See Supplementary Table S1 for details.
(C) Pairwise analysis depicting the prevalence of A*24/S1208- and A*02/S269-CTLs in PBMCs (n = 14) from vaccinated individuals before and after ex vivo expansion with the corresponding epitope of the synthetic S1208-1216 or S269-277 peptide (5 μg/mL) and cytokines for 14 days.
In (B-C), each symbol represents a value obtained from an individual donor.
(D) Schematic of the TEDbody, engineered for the delivery of an MHC-I-restricted CTL epitope peptide, derived from the spike protein of SARS-CoV-2, into the cytosol of target tumor cells.
(E) Design of TEDbodies carrying natural and modified N/C-terminal flanking sequences of S1208-1216 and S269-277 epitopes. S1208-1216 or S269–277-encompassing peptides with N/C-extended flanking sequences were fused to the C-terminus of the heavy chain of inCT via a 5-mer G4S linker. See Supplementary Figure S3-S4 for details. Color-coded arrows indicate the NetChop3.1 prediction score, between 0 and 1, at each residue. Values closer to 1 indicate a strong preference for the C-terminal cleavage of a specific amino acid residue, whereas values closer to 0 indicate the opposite.
(F-I) Percentage of TEDbody-mediated cytolysis of target tumor cells with different HLA-A*24:02 and HLA-A*02:01 haplotypes by ex vivo-expanded A*24/S1208-CTLs (F, H) and A*02/S269-CTLs (G, I). The indicated tumor cells were treated with the indicated synthetic peptide, TEDbody, or control antibody (500 nmol/L in [F, G] and the lower panels of [H, I] or the indicated concentrations in the upper panels of [H, I]) at 37°C for 12 h. The treated cells were then cocultured with the spike-specific CTLs at the indicated E:T ratio (5:1 in the upper panels of [H, I] or the indicated E:T ratios in [F, G] and the lower panels of [H, I]) for an additional 18 h. The extent of lysis was determined by measuring lactate dehydrogenase levels in the supernatant, and the bar graphs represent the mean ± SEM (n = 3).
(J-M) In vivo antitumor efficacy of the TEDbody compared to that of the control antibodies and the mechanism underlying these effects were evaluated in conjunction with the adoptive transfer of ex vivo-expanded spike-specific CTLs in immunodeficient NSG mice carrying pre-established SW480 and SNU-216 cell-derived subcutaneous tumor xenografts (100-120 mm3). A test TEDbody or the control antibody (20 mg/kg) plus interleukin-15/IL-15 receptor α chain-Fc protein (15 μg) was intraperitoneally injected every 3 d for a total of six doses; 18 h later, ex vivo-expanded spike-specific CTLs (107 cells) were injected intravenously once every 6 d for a total of three doses. See Supplementary Figure S6A for the treatment scheme. In (J, K), the tumor growth was measured as the average tumor volumes of SW480 (J) and SNU-216 xenografts (K) in response to the indicated treatment. Error bars: ± SEM (n = 6-7 per group for each tumor). Data were pooled from two independent experiments with at least three mice per group. In (L, M), the number of tumor-infiltrating A*24/S1208-CTLs (L) and A*02/S269-CTLs (M) per gram of tumor and the percentage of granzyme B-expressing tumor-infiltrating A*24/S1208-and A*02/S269-CTLs were determined in SW480 tumors excised from mice on day 3 after the last administration of the indicated treatment. Each symbol represents a value for one tumor from an individual mouse. Error bars denote ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 indicate a significant difference compared to the inCT group (F, G, H, I, J, K) or between the indicated groups (C, L, M), determined using a one-way ANOVA with Newman-Keuls post-hoc test. ns, not significant.
Abbreviations: TEDbody, CD8+ T cell epitope-delivering antibody; MHC-I, major histocompatibility complex class I; CTL, cytotoxic CD8+ T cell; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; HLA, human leukocyte antigen; PBMC, peripheral blood mononuclear cell; E:T, effector-to-target; ANOVA, analysis of variance.
As TEDbody payloads, we selected 2 immunodominant CTL epitope peptides, HLA-A*24:02-restricted 1208QYIKWPWYI1216 (S1208-1216) and HLA-A*02:01-restricted 269YLQPRTFLL277 (S269-277), from the SARS-CoV-2 spike protein [6]. These epitopes can be presented on the target tumor cell surface as S1208–1216-loaded HLA-A*24:02 (A*24/S1208 pMHCI) and S269–277-loaded HLA-A*02:01 (A*02/S269 pMHCI). From 30 COVID-19-vaccinated donors, we isolated A*24/S1208- and A*02/S269-CTLs (Supplementary Table S1). Among these individuals, 28 had detectable A*24/S1208- or A*02/S269-CTLs, representing 0.22%-3.20% of all CD8+ T cells in their blood (Figure 1B, Supplementary Table S1). The spike-specific CTLs, namely A*24/S1208- and A*02/S269-CTLs, were efficiently expanded ex vivo, through cognate epitope peptide stimulation, into a dominant effector memory subset with potent cytotoxicity (Figure 1C, Supplementary Table S2, Supplementary Figures S1-S2).
For cytosolic CTL epitope delivery, TEDbodies were designed by genetically fusing S1208–1216- and S269–277-encompassing peptides with natural N/C-terminal flanking residues to the heavy chain C-terminus of the human IgG1/κ antibody, inCT [5, 7], which can specifically penetrate the cytosol of integrin αv-expressing target tumor cells (Figure 1D-E, Supplementary Figure S3A). However, their ability to induce target tumor cell lysis, mediated by A*24/S1208- and A*02/S269-CTLs, was limited (Figure 1F-G, Supplementary Figure S3B-C). Therefore, we optimized the N/C-flanking sequences surrounding the mature CTL epitope using in silico proteasomal cleavage prediction tools [8, 9] (Supplementary Figure S4). This sequential N/C-flanking engineering produced optimized inCT†A*24:S#6 and inCT†A*02:S#2 TEDbodies (Figure 1E), which efficiently induced the lysis of HLA-matched target tumor cells mediated by A*24/S1208- and A*02/S269-CTLs, respectively (Figure 1F-G). These findings highlight the significant impact of N/C-flanking sequences on antigen processing and pMHCI presentation [10].
Upon tumor cell treatment with inCT†A*24:S#6, A*24/S1208-CTLs specifically lysed HLA-A*24:02+ SNU-216 and SW480 cells, while sparing HLA-A*24:02− MDA-MB-231 and LoVo cells (Figure 1H). Similarly, inCT†A*02:S#2 induced A*02/S269-CTL-mediated cytolysis, selectively killing HLA-A*02:01+ MDA-MB-231 and SW480 cells, while sparing HLA-A*02:01− SNU-216 and LoVo cells (Figure 1I). These findings demonstrated the HLA-type specificity of TEDbodies. The extent of tumor cell lysis was proportional to the TEDbody concentration, effector-to-target ratio (Figure 1H-I), and incubation time (Supplementary Figure S5). The cytosolically inaccessible inCT(AAA)-based fusion antibodies, inCT(AAA)†A*24:S#6 and inCT(AAA)†A*02:S#2, which could not deliver the epitope payload into the tumor cell cytosol [5], caused negligible lysis across all tested cells (Figure 1H-I). These results emphasize the crucial role of the TEDbody-mediated cytosolic delivery of engineered S1208–1216- and S269–277-encompassing peptides for the surface presentation of A*24/S1208 and A*02/S269 pMHCI antigens, enabling their recognition by cognate spike-specific CTLs.
The TEDbody demonstrated a conventional IgG1 antibody serum half-life and favorable intratumoral distribution in nonobese diabetic/severe combined immunodeficiency/interleukin-2γ-null (NSG) mouse models (Supplementary Figure S6). The in vivo antitumor efficacy of TEDbodies combined with the adoptive transfer of ex vivo-expanded spike-specific CTLs was evaluated using immunodeficient NSG mice carrying pre-established SW480 and SNU-216 cell-derived subcutaneous tumor xenografts (Supplementary Figure S7). Compared to that in the inCT control, inCT†A*24:S#6 and inCT†A*02:S#2 significantly delayed HLA-A*24:02+/HLA-A*02:01+ SW480 tumor growth, showing tumor growth inhibition rates of approximately 70% and 68%, respectively (Figure 1J, Supplementary Figure S8). Importantly, inCT†A*24:S#6, but not inCT†A*02:S#2, suppressed HLA-A*24:02+/HLA-A*02:01− SNU-216 tumor growth (Figure 1K), confirming the HLA-restricted in vivo antitumor activity. In contrast, the cytosolically inaccessible inCT(AAA)†A*24:S#6 and inCT(AAA)†A*02:S#2 controls exhibited no antitumor activity. The injection of S1208-1216 and S269-277 CTL epitopes in synthetic peptide form did not result in antitumor activity (Figure 1J-K), despite the substantial target tumor cell lysis in vitro, mediated by spike-specific CTLs through direct extracellular surface loading (Figure 1F-I). This suggests inefficient in vivo pMHCI formation through the extracellular surface loading of epitope peptides after systemic injection. Treatment with inCT†A*24:S#6 and inCT†A*02:S#2 led to a ∼60-fold increase in the total numbers of A*24/S1208- and A*02/S269-CTLs, respectively, within the tumor and a ∼3-fold increase in the percentages of cells expressing granzyme B, a cytotoxic effector of activated CTLs, compared to those in the control groups (Figure 1L-M). These findings indicate that TEDbody-mediated pMHCI presentation on target tumors likely induces sustained antigenic spike-specific CTL stimulation, resulting in enhanced tumor infiltration and cytotoxicity within the tumor microenvironment. The materials and methods used in this study were mentioned in Supplementary Materials.
In conclusion, we developed TEDbodies capable of delivering spike protein-derived CTL epitope peptides into the target tumor cell cytosol, repurposing spike-specific CTLs against tumors. We observed the functional activity of A*24/S1208- and A*02/S269-CTLs in COVID-19-vaccinated individuals, demonstrating their ability to eliminate target tumor cells presenting corresponding A*24/S1208 and A*02/S269 pMHCI antigens. Our results highlight the potential of TEDbody technology to deliver MHC-I-restricted SARS-CoV-2 epitope peptides into the target tumor cell cytosol, harnessing pre-existing SARS-CoV-2-specific CTLs from natural infection or vaccinations to combat cancer.
DECLARATIONS
AUTHOR CONTRIBUTIONS
Yong-Sung Kim conceived and designed the experiments. Keunok Jung, Deok-Han Ko, and Jeong-Yun Jang performed the experiments. Jung Yeon Heo and Young Rong Kim supervised the collection of blood samples from vaccinated donors. All authors analyzed and interpreted the data. Keunok Jung, Deok-Han Ko, and Yong-Sung Kim wrote the manuscript. All authors have read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We thank everyone who donated the blood samples for this study.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING INFORMATION
This work was supported by the Samsung Future Technology Center (SRFCMA1802-09) and the Korea Health Technology R&D Project (HR22C173402) through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health and Welfare.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
PBMCs from healthy donors were acquired using protocols approved by the Institutional Review Board of Ajou University (approval ID: AJOUIRB-SMP-2021-238). We obtained written informed consent from individual healthy donors who donated blood samples used in this study. All animal experiments were approved by the Animal and Ethics Review Committee of Woojung Bio Inc. (approval ID: IACUC2003-004) and performed in accordance with the guidelines established by the Institutional Animal Care and Use Committee.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The datasets used in the current study are available from the corresponding author upon reasonable request.