A nanotherapeutic strategy to target drug-tolerant cells and overcome EGFR tyrosine kinase inhibitor resistance in lung cancer
List of abbreviations
-
- Axl-LP-VD-CTA091
-
- Axl targeted liposomes that are loaded with VD + CTA091
-
- Axl-LP-VD
-
- Axl targeted liposomes that are loaded only with VD
-
- Axl-LP
-
- Axl targeted empty liposomes that are not loaded with VD + CTA091
-
- CDH1
-
- cadherin 1
-
- CYP24A1
-
- cytochrome P450 family 24 subfamily A member 1
-
- Cy5-ODN
-
- Cy5 labeled oligodeoxynucleotide
-
- DTC
-
- drug tolerant cell
-
- EGFR
-
- epidermal growth factor receptor
-
- EMT
-
- epithelial mesenchymal transition
-
- IVIS
-
- in vivo imaging system
-
- LP
-
- liposome
-
- MMP2
-
- matrix metallopeptidase-2
-
- MRI
-
- magnetic resonance imaging
-
- NSCLC
-
- non-small cell lung cancer
-
- SRB
-
- sulforhodamine B
-
- TKI
-
- tyrosine kinase inhibitor
-
- US
-
- ultrasound
-
- VD
-
- 1,25-dihydroxyvitamin D3
For patients with epidermal growth factor receptor (EGFR) mutant non-small cell lung cancer (NSCLC), EGFR tyrosine kinase inhibitors (TKIs) are used as the first-line treatment [1, 2]. Despite initial therapeutic responses, patients invariably experience disease progression due to acquired drug resistance [3]. Resistance arises, in part, because a subset of cancer cells undergoes epithelial-mesenchymal transition (EMT) and remains viable despite exposure to EGFR TKI concentrations that eliminate the bulk population [4]. The surviving cells can be re-sensitized to treatment by prolonged culture in the absence of EGFR TKIs, indicating a transient, potentially reversible, tolerance to these drugs [4]. However, these drug-tolerant cells (DTCs) may regain proliferative potential, evolve, and give rise to diverse stable mechanisms of resistance in patients [5, 6]. To address this clinical challenge, we developed a novel liposomal nanodrug, Axl-LP-VD-CTA091, to inhibit DTCs, thus targeting the origin of diverse resistance mechanisms (Figure 1A). In this formulation, the pro-differentiation agents 1,25-dihydroxyvitamin D3 (VD) and CTA091 were co-encapsulated in liposomes (LP) (Figure 1B). VD was used to suppress EMT [7] that underlies drug tolerance. CTA091 prevented catabolic inactivation of VD by 24-hydroxylase [8]. Axl aptamers [9] enabled preferential targeting of nanodrug to DTCs, which are known to have increased Axl expression [10] (see Supplementary Materials for experimental details).
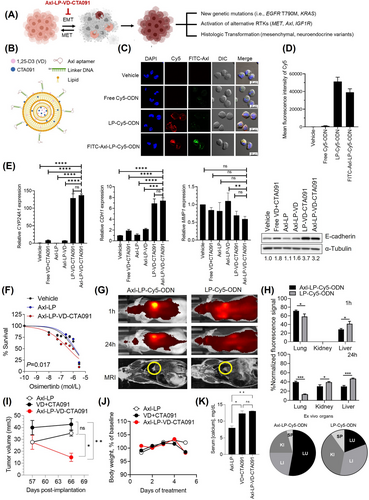
We prepared Axl-LP-VD-CTA091 by encapsulating VD and CTA091 in LP and then conjugated Axl aptamers on the LP surface (Supplementary Figure S1A). The size and zeta potential of Axl-LP-VD-CTA091 were 106 ± 3 nm and +30.9 ± 3.8 mV, respectively (morphology and size distribution in Supplementary Figure S1B). The encapsulation efficiency of VD and CTA091 was 75%. Axl-LP-VD-CTA091 were stable for 24 h ± serum (Supplementary Figure S1C).
We evaluated the targeting and uptake of Axl-LP-VD-CTA091 in H1975OR cells that developed EMT-associated osimertinib tolerance (Supplementary Figure S2A). Axl aptamers were labeled with FITC. LP were labeled by encapsulation of Cy5-oligodeoxynucleotide (Cy5-ODN). H1975OR cells were incubated with vehicle, free Cy5-ODN, untargeted LP-Cy5-ODN or FITC-Axl-LP-Cy5-ODN. Intact FITC-Axl-LP-Cy5-ODN were taken up by H1975OR cells, as evidenced by co-localization of green and red fluorescence in the cytoplasm (Figure 1C). Flow cytometric quantitation demonstrated strong cellular uptake of FITC-Axl-LP-Cy5-ODN with 816.5-fold higher uptake vs. vehicle control and 40.9-fold higher uptake vs. free Cy5-ODN (Figure 1D). However, Axl aptamers did not increase uptake beyond what was achieved with non-targeted LP.
Next, we tested the prediction that Axl-LP-VD-CTA091 promoted VD signaling, decreased EMT features and improved osimertinib sensitivity in H1975OR cells. Axl-LP-VD-CTA091 induced vitamin D target gene cytochrome P450 family 24 subfamily A member 1 (CYP24A1), increased expression of epithelial marker cadherin 1 (CDH1), and decreased expression of mesenchymal marker matrix metalloproteinase-2 (MMP2) (Figure 1E). Axl-LP-VD-CTA091 was superior to comparator treatments, including free VD + CTA091 (demonstrating the importance of drug encapsulation to activity), Axl-LP-VD (indicating the importance of using CTA091 to stabilize VD) and Axl-LP containing no VD and CTA091 (showing that Axl-aptamers and empty liposomes lack therapeutic activity). Gene expression changes induced by LP-VD-CTA091 and Axl-LP-VD-CTA091 were comparable and consistent with their similar in vitro uptake. Axl-LP-VD-CTA091 induced an 8-fold increase in CDH1 transcripts and a corresponding 3.2-fold increase in E-cadherin protein (Figure 1E). Osimertinib dose-response studies were used to test EGFR TKI sensitivity. Axl-LP-VD-CTA091 re-sensitized H1975OR cells to treatment, as evidenced by a 3.5-fold reduction in the osimertinib EC50 value compared with Axl-LP (P = 0.004). The osimertinib EC50 was comparable for vehicle control (HEPES) and Axl-LP (P = 0.625), indicating that empty liposomes did not affect EGFR TKI sensitivity (Figure 1F). Notably, Axl-LP-VD-CTA091 were unable to increase the EGFR TKI sensitivity of PC9ER cells that did not undergo EMT and had genetically fixed erlotinib resistance (Supplementary Figure S2B-C). These data further linked Axl-LP-VD-CTA091 activity to EMT control.
To investigate the in vivo activity of Axl-LP-VD-CTA091, we developed a novel orthotopic model of lung cancer based on ultrasound (US) guided trans-pleural injection of tumor cells into the lungs of mice (Supplementary Figure S3A-C). Nude mice were orthotopically implanted with parental H1975 cells (Supplementary Figure S3D-F) or H1975OR cells (Supplementary Figure S3G-I). Osimertinib treatment (5 mg/kg oral gavage, once daily, Mon-Fri) was initiated after tumor establishment (day 27 post-implantation) in H1975-bearing mice but within 1 week of implantation of H1975OR cells to maintain TKI tolerance. Osimertinib suppressed EGFR activation and reduced the volume of H1975 tumors (Supplementary Figure S3E-F). However, H1975OR tumors continued to grow over time despite receiving the same dose of osimertinib (Supplementary Figure S3G-H). H1975OR tumors expressed lower E-cadherin levels than parental H1975 tumors, indicating that they maintained their EMT phenotype in vivo (Supplementary Figure S3I). We concluded that osimertinib (5 mg/kg) allowed for continued growth of drug tolerant H1975OR cells with EMT phenotype in vivo.
We did not observe significant differences in cellular uptake and efficacy between Axl-LP-VD-CTA091 and untargeted LP-VD-CTA091 in vitro. One possible reason could be that in vitro culture conditions do not mimic in vivo conditions and lack blood flow, competing cells and off-target effects. To test for advantages of Axl-LP-VD-CTA091 over LP-VD-CTA091 in vivo, biodistribution studies were performed. Untargeted LP-Cy5-ODN or Axl-LP-Cy5-ODN were administered to mice harboring orthotopic H1975 xenografts at a Cy5-ODN dose of 5 mg/kg (Figure 1G-H). H1975 cells were used for biodistribution studies because they expressed Axl (Supplementary Figure S2A) and grew relatively rapidly. In vivo imaging system (IVIS) was used to quantify LP distribution over time. An intense fluorescent signal was detected within 1 h of administration in mice that received Axl-LP-Cy5-ODN (Figure 1G). The Cy5 signals were detected preferentially in the tumor-bearing lung. No specific localization was detected in mice that received LP-Cy5-ODN, despite the mice having comparable tumor burden. Quantitative in vivo analyses confirmed signal enrichment in the lungs of Axl-LP-Cy5-ODN treated mice at the 1 h timepoint, which was significantly higher than mice treated with LP-Cy5-ODN (71% vs. 58%, Figure 1H). After 24 h treatment, the majority of fluorescent signal (40%) was present in the lungs of Axl-LP-Cy5-ODN injected mice, while only 13% of fluorescent signal remained in the lungs of LP-Cy5-ODN treated mice (Figure 1G-H). In addition, the kidney and liver showed greater signal intensities than the lungs of LP-Cy5-ODN-treated mice. The relative enrichment of Axl-LP-Cy5-ODN in lung tissues was evident when isolated organs were subjected to IVIS imaging (Figure 1H, pie charts). The lung/kidney Cy5 signal intensity ratio was 2.58 and 0.5 in mice that received Axl-LP-Cy5-ODN versus LP-Cy5-ODN, respectively. Thus, Axl-targeting resulted in preferential localization and retention of LP within the lungs of tumor-bearing mice.
Based on these results, we conducted in vivo safety and efficacy tests of Axl-LP-VD-CTA091. H1975OR cells were orthotopically implanted. One week later, treatment with osimertinib was initiated. Once tumors were established (day 57 post-implantation), the mice were intravenously injected with empty Axl-LP, free VD + CTA091, or Axl-LP-VD-CTA091 at VD and CTA091 doses of 25 μg/kg and 14.5 μg/kg respectively, every 2 days, for a total of 4 injections. Magnetic Resonance Imaging (MRI)-based tumor volume measurements showed that Axl-LP-VD-CTA091 significantly suppressed the growth of H1975OR lung tumors in mice compared to treatment with Axl-LP or free VD + CTA091 (Figure 1I). However, the mice began to lose weight following treatment with Axl-LP-VD-CTA091 and free VD + CTA091, prompting us to terminate the study (Figure 1J). Weight loss was associated with an elevation in serum calcium, indicative of hypercalcemia (Figure 1K). Pathological assessments revealed no overt tissue toxicity (data not shown). Axl-LP-VD-CTA091 but not free VD + CTA091 reduced tumor burden; therefore, we anticipated that the therapeutic index of Axl-LP-VD-CTA091 might be improved using reduced drug doses and/or an alternative once-weekly dosing schedule.
Based on the ability of Axl-LP-VD-CTA091 to promote epithelial phenotype, distribute to tumor-bearing lungs in vivo, and increase EGFR TKI activity in vitro and in vivo, we contend that its continued additional evaluation as a novel strategy to inhibit DTC and promote EGFR TKI sensitivity is warranted.
AUTHORS CONTRIBUTIONS
Tatiana Shaurova: study design, model development, data generation, manuscript writing, and editing.
Lingyue Yan: study design, nanoparticle synthesis, data generation, manuscript writing, and editing.
Yafei Su: nanoparticle synthesis and data generation.
Laurie James Rich: co-development of orthotopic tumor models.
Vui King Vincent-Chong: immunohistochemistry.
Hannah Calkins: data generation, manuscript writing, and editing.
Saraswati Pokharel: pathologic assessment of tissues.
Martin Petkovich: synthesis and provision of CTA091, manuscript editing.
Mukund Seshadri: co-development of orthotopic tumor models, manuscript writing, and editing.
Yun Wu: study design, data generation, manuscript writing, and editing.
Pamela Anne Hershberger: study design, data generation, manuscript writing, and editing.
ACKNOWLEDGMENTS
We thank Dr. Min Gao and the TEM facility at the Advanced Materials and Liquid Crystal Institute at Kent State University for Cryo-TEM images.
COMPETING INTERESTS
Laurie JamesRich is currently an employee of Fujifilm-VisualSonics Corporation. The remaining authors declare no competing financial interest.
FUNDING
The authors gratefully acknowledge the following sources of funding support for our work: the Roswell Park Alliance Foundation (Taste of Life Award), the American Lung Association Lung Cancer Discovery Award LCD615335, S10OD010393-01, and the Roswell Park Cancer Center Support Grant P30 CA016056 that supports the Laboratory Animal Shared Resource and Translational Imaging Shared Resource utilized in this work. The Zeiss LSM 710 confocal microscope at University at Buffalo North Campus Imaging Facility was funded by National Science Foundation Major Research Instrumentation Grant # DBI 0923133.
AVAILABILITY OF DATA AND MATERIALS
Primary data will be made available upon request to the corresponding authors.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
In vivo studies were carried out under an IACUC-approved protocol (1213M) within the laboratory animal resource at Roswell Park Comprehensive Cancer Center.
CONSENT FOR PUBLICATION
Not applicable.




