Resistance to antibody-drug conjugates in breast cancer: mechanisms and solutions
Abstract
Antibody-drug conjugates (ADCs) are a rapidly developing therapeutic approach in cancer treatment that has shown remarkable activity in breast cancer. Currently, there are two ADCs approved for the treatment of human epidermal growth factor receptor 2-positive breast cancer, one for triple-negative breast cancer, and multiple investigational ADCs in clinical trials. However, drug resistance has been noticed in clinical use, especially in trastuzumab emtansine. Here, the mechanisms of ADC resistance are summarized into four categories: antibody-mediated resistance, impaired drug trafficking, disrupted lysosomal function, and payload-related resistance. To overcome or prevent resistance to ADCs, innovative development strategies and combination therapy options are being investigated. Analyzing predictive biomarkers for optimal therapy selection may also help to prevent drug resistance.
List of abbreviations
-
- ADC
-
- antibody-drug conjugates
-
- ADCC
-
- antibody-dependent cell-mediated cytotoxicity
-
- BCRP
-
- breast cancer resistance protein
-
- CDK
-
- cyclin-dependent kinases
-
- ctDNA
-
- circulating tumor DNA
-
- DAR
-
- drug-antibody ratio
-
- EFS
-
- event-free survival
-
- EMA
-
- European Medicines Agency
-
- Endo II
-
- endophilin A2
-
- ESMO
-
- European Society for Medical Oncology
-
- FcRn
-
- neonatal Fc receptor
-
- FDA
-
- U.S. Food and Drug Administration
-
- FISH
-
- fluorescence in situ hybridization
-
- HER
-
- human epidermal growth factor
-
- HRR
-
- homologous recombinational repair
-
- HSP90
-
- heat shock protein 90
-
- IDFS
-
- invasive disease-free survival
-
- IHC
-
- immunohistochemistry
-
- ISH
-
- in situ hybridization
-
- mAb
-
- monoclonal antibody
-
- MDR
-
- multidrug-resistance protein
-
- MMAE
-
- monomethyl auristatin E
-
- MMAF
-
- monomethyl auristatin F
-
- mOS
-
- median overall survival
-
- mPFS
-
- median progression-free survival
-
- MRP
-
- multidrug-resistance-associated protein
-
- MUC4
-
- mucin 4
-
- ORR
-
- objective response rate
-
- PARP1
-
- poly ADP-ribose polymerase 1
-
- pCR
-
- pathological complete response
-
- PDX
-
- patient-derived xenograft
-
- PI3K
-
- phosphoinositide 3-kinase
-
- PIK3CA
-
- phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha
-
- PLK1
-
- polo-like kinase 1
-
- PTK7
-
- protein tyrosine kinase 7
-
- ROR1
-
- receptor tyrosine kinase-like orphan receptor
-
- SLC
-
- solute carrier
-
- TACSTD2
-
- tumor-associated calcium signal transducer 2
-
- TKI
-
- tyrosine kinase inhibitors
-
- TNBC
-
- triple-negative breast cancer
-
- TOPI
-
- topoisomerase I
-
- TROP2
-
- trophoblast cell surface antigen 2
1 BACKGROUND
According to the most recent data obtained from GLOBOCAN online database, breast cancer has now become the most diagnosed malignant disease worldwide among women; breast cancer is also the primary cause of cancer death among 20- to 59-year-old women [1]. Breast cancer can be categorized into five molecular subtypes based on the expression status of hormone receptors, including estrogen and progesterone receptors (ER and PR), human epidermal growth factor 2 (HER2), and levels of Ki-67: luminal A with ER+/HER2-, Ki-67 low, and PR high; luminal B with ER+/HER2-, and either Ki-67 high or PR low; luminal B-like with ER+, HER2 overexpression or amplification, any Ki-67, and any PR; and triple-negative breast cancer (TNBC) with ER-, PR-, and HER2- [2]. Among these subtypes, hormone receptor+/HER2- breast cancer is the most common, and TNBC is associated with the worst prognosis [3]. Treatments for breast cancer have profoundly developed over the past few decades. Thanks to the improved therapeutic approaches, most patients with early-stage breast cancer are now curable. Even though various emerging therapies with promising efficacy and safety profiles have improved the survival of patients with advanced breast cancer to a great extent, it is still incurable.
Antibody-drug conjugates (ADCs) are a novel class of targeted therapy in cancer treatment and have shown promising efficacy with tolerable systematic toxicity. ADCs for breast cancer are now under rapid development (Table ;1). ADCs consist of a monoclonal antibody (mAb) conjugated to a cytotoxic agent (referred to as payload) via a chemical linker. This unique structure allows specific cytotoxicity against tumor cells. Trastuzumab emtansine (T-DM1, Kadcyla), the first ADC that obtained the US Food and Drug Administration (FDA) approval for breast cancer, has proven effective in HER2+ metastatic breast cancer. It has also profoundly changed the treatment paradigm and now become a standard second-line option for these patients [4]. The US FDA then granted accelerated approval to trastuzumab deruxtecan (DS-8201a, T-DXd, Enhertu) for the treatment of HER2+ advanced breast cancer 6 years after the approval of T-DM1. Remarkably, in an ongoing head-to-head trial, this novel ADC acheived a superior clinical response over T-DM1 [5]. Sacituzumab govitecan (IMMU-132, Trodelvy) is the first ADC that received the US FDA approval for TNBC and was also approved as a second-line treatment for TNBC in Europe. Furthermore, with trastuzumab duocarmazine (SYD985) and ARX788 on the US FDA fast track, this novel therapeutic approach shows a rather promising future. However, a substantial amount of patients with distinct genetic features progress on ADC treatment. Resistance to ADCs has gained more attention since it was applied in clinical use, and a more in-depth understanding of the underlying mechanisms is crucial. Multiple approaches have been launched to address resistance to ADC treatments.
| Target | ADC | Disease status | Combination | Trial phase | Trial number |
|---|---|---|---|---|---|
| HER2 | T-DM1 | Adjuvant HER2+ BC | None | Approved | NCT01772472 |
| Adjuvant HER2+ BC | Atezolizumab | Phase III | NCT04873362 | ||
| Second-line advanced HER2+ BC | None | Approved | NCT00829166 | ||
| Second-line advanced HER2+ and PD-L1+ BC | Atezolizumab | Phase II | NCT04740918 | ||
| DS-8201a | Adjuvant HER2+ BC | None | Phase III | NCT04622319 | |
| Neoadjuvant HER2+ BC | -/Followed by THP | Phase III | NCT05113251 | ||
| First-line advanced HER2+ BC | -/+Pertuzumab | Phase III | NCT04784715 | ||
| Second-line advanced HER2+ BC | None | Phase II | NCT03529110 | ||
| Third-line advanced HER2+ BC | None | Approved | NCT03523585 | ||
| Third-line advanced HER2-low BC, post chemo | None | Phase III | NCT03734029 | ||
| Third-line advanced HER2-low BC, Chemo naive | None | Phase III | NCT04494425 | ||
| SYD985 | Neoadjuvant HER2-low BC | None | Phase II | NCT01042379 | |
| Third-line advanced HER2+ BC | None | Phase III | NCT03262935 | ||
| R/R Metastatic cancer | Paclitaxel | Phase I | NCT04602117 | ||
| ARX788 | Neoadjuvant HER2+ BC | Pyrotinib maleate | Phase II | NCT04983121 | |
| R/R Advanced HER2+ BC | None | Phase II | NCT04829604 | ||
| R/R Advanced HER2-low BC | None | Phase II | NCT05018676 | ||
| RC48 | R/R Advanced HER2-low BC | None | Phase III | NCT04400695 | |
| MRG002 | R/R Advanced HER2+ BC | None | Phase II | NCT04924699 | |
| R/R Advanced HER2 low BC | None | Phase II | NCT04742153 | ||
| A166 | R/R Advanced HER2-expressing cancer | None | Phase II | NCT03602079 | |
| BDC-1001 | R/R Advanced HER2-expressing cancer | -/+Nivolumab | Phase II | NCT04278144 | |
| ALT-P7 | R/R Advanced HER2+ BC | None | Phase I | NCT03281824 | |
| PF-06804103 | R/R Advanced BC | -/+ Palbociclib and Letrozole | Phase I | NCT03284723 | |
| ZW49 | R/R HER2+ cancer | None | Phase I | NCT03821233 | |
| DHES0815A | R/R HER2+ cancer | None | Phase I | NCT03451162 | |
| GQ1001 | R/R HER2+ cancer | None | Phase I | NCT04450732 | |
| XMT-1522 | R/R HER2+ cancer | None | Phase I | NCT02952729 | |
| Non-HER2 | IMMU-132 | Postneoadjuvant HER2- BC | None | Phase III | NCT04595565 |
| Neoadjuvant TNBC | -/+Pembrolizumab | Phase II | NCT04230109 | ||
| First-line mTNBC | -/+Pembrolizumab | Phase II | NCT04468061 | ||
| Second-line mTNBC | None | Approved | NCT02574455 | ||
| Third-line advanced hormone receptor+/HER2- BC | None | Phase III | NCT03901339 | ||
| U3-1402 | Preoperative hormone receptor+/HER2- BC | None | Phase I | NCT04610528 | |
| R/R mBC | None | Phase II | NCT04699630 | ||
| R/R HER3+ mBC | None | Phase II | NCT02980341 | ||
| SGN-LIV1 | First line mTNBC | -/+Pembrolizumab | Phase II | NCT03310957 | |
| R/R mBC | -/+Trastuzumab | Phase I | NCT01969643 | ||
| DS-1062 | Second-line advanced hormone receptor+/HER2- BC | None | Phase III | NCT05104866 | |
| MORAb-202 | R/R Advanced solid tumors | None | Phase II | NCT04300556 | |
| SKB264 | R/R Advanced solid tumors | None | Phase II | NCT04152499 | |
| CX-2009 | R/R Advanced HER2- BC | CX-072 | Phase II | NCT04596150 | |
| R/R Advanced solid tumors | None | Phase II | NCT03149549 | ||
| ASG-22ME | R/R Advanced solid tumors | None | Phase II | NCT04225117 | |
| BA3021 | R/R Advanced solid tumors | -/+PD-1 inhibitor | Phase II | NCT03504488 | |
| PF-06647020 | R/R Advanced solid tumors | None | Phase I | NCT02222922 | |
| NBE-002 | R/R Advanced solid tumors | None | Phase I | NCT04441099 | |
| ASN004 | R/R Advanced solid tumors | None | Phase I | NCT04410224 |
- Abbreviations: ADC, antibody-drug conjugate; BC, breast cancer; HER2, human epidermal growth factor receptor 2; HER3, human epidermal growth factor receptor 3; mBC, metastatic breast cancer; mTNBC, metastatic triple-negative breast cancer; PD-L1, Programmed cell death protein ligand 1; R/R, relapse/refractory; THP, paclitaxel, trastuzumab and pertuzumab
This article briefly introduces the mechanisms of action of ADC with a summary of the efficacy profile of ADCs in breast cancer. This review also focus on drug resistance noticed in clinical application and discussed the potential strategies for overcoming emerging resistance and improving the clinical activity of ADCs in breast cancer.
2 STRUCTURE OF ADCS
ADCs contain three major parts: a mAb, a chemical linker, and cytotoxic payloads (Figure 1A).

2.1 Antigen and mAb
An ideal tumor antigen should be expressed abundantly and homogeneously on the surface of tumor cells with minimal shedding and high specificity [6]. The endocytic properties of the target antigen are also important, since internalization is generally considered essential for the activity of ADCs; in some cases, the potency of some ADCs is directly associated with receptor internalization [7]. Optimal mAbs for ADCs require high tumor specificity to reduce off-target toxicity, a long half-life in the bloodstream, and low immunogenicity [6, 8]. The affinity of antibodies needs to balance between tumor penetration and internalization rate. High affinity of antibodies facilitates receptor internalization and compromises penetration of solid tumors [9]. It is also desirable for mAbs to retain their intrinsic antitumor activity after linking to a cytotoxic payload. Except for binding to the target antigen via the Fab portion, mAbs can also mediate antibody-dependent antitumor immune responses via the Fc portion. The IgG isotypes commonly used in therapeutic mAbs are IgG1, IgG2, and IgG4. Most ADCs utilize IgG1 due to its ability to mediate antitumor immunity, while IgG2 is harder to develop and is used only when the antigen-binding activity is required, and IgG4 undergoes Fab-arm exchange and becomes functionally monovalent in vivo [10].
2.2 Linker
Linkers connect the cytotoxic payload to mAbs and stabilize the structure of ADCs. Their ability to remain stable in the bloodstream and to be cleaved in target cells is important to prevent potential systemic toxicity caused by premature cleavage. The linker chemistry affects the stability of the ADC and the amount of payload release in the plasma, hence the nature of the linker being the main cause of toxicity [11]. It also requires high water solubility to assist adequate release of the cytotoxic payload-linker metabolite [12]. Based on their drug release mechanisms, linkers in ADCs are categorized into cleavable and non-cleavable linkers. Considering the different conditions for the cleavage of cleavable linkers, they can be subcategorized into chemically cleavable and enzyme cleavable linkers [13]. Non-cleavable linkers remain intact in the bloodstream and in the intracellular catabolism process [14]. ADCs with non-cleavable linkers require efficient lysosomal degradation of the mAb portion to release the active cytotoxic complex [15]. Compared to cleavable linkers, non-cleavable linkers have superior plasma stability and a relatively longer half-life, thus milder systematic toxicity [8, 16].
However, it was noticed that ADCs with non-cleavable linkers release membrane-impermeable cytotoxic complexes and exhibit a reduced bystander effect compared to those with cleavable linkers [17]. Bystander effect is that cytotoxic payloads escape from the cell or are released extracellularly and kill neighboring cells, including non-antigen-expressing tumor cells [18]. This effect can be used to enhance therapeutic effects in heterogeneous or low target antigen levels [19]. ADCs with non-cleavable linkers, however, are more effective for hematological cancers or high target-expressing solid tumors [13]. For example, T-DM1 with non-cleavable linkers is cleaved in the lysosome and releases Lys-SMCC-DM1, which shows low membrane permeability compared to DXd, the cytotoxic compound released by DS-8201a with cleavable linkers. Lys-SMCC-DM1 has a permeability coefficient (Peff) value lower than 0.1 at both neutral and acidic pH, while Dxd released by DS-8201a has a Peff value over 10. This suggested that Lys-SMCC-DM1 could not penetrate neither lysosomal membrane nor cytoplasm membrane without facilitation [20]. This kind of amino acid-linker-payload released by ADCs with noncleavable linkers require membrane transporters to move across biological membranes, such as SLC46A3 for the transportation of Lys-SMCC-DM1 released by T-DM1 [21].
2.3 Payload
An ideal cytotoxic agent in ADCs requires high tumor specificity and a balance between cytotoxic potency and tolerability. Typical targets for payloads include DNA, microtubule/tubulin, and topoisomerase I (TOPI). Even though highly potent payloads are usually preferable, this is not always the case. For instance, IMMU-132 uses a moderately toxic agent that is more tolerable and allows a more favorable therapeutic window [22]. Drug-antibody ratio (DAR) is an important indicator of ADC, which means the number of cytotoxic molecules attached to one mAb. Low DAR indicates lower activity but a higher therapeutic index, while high DAR can improve the efficacy but lead to faster drug clearance and increased toxicity. The compositions of ADCs in breast cancer are summarized in Table 2.
| ADC | Target | Antibody | IgG isotype | Payload | Linker | DAR | Development phase | Ref |
|---|---|---|---|---|---|---|---|---|
| A166* | HER2 | Trastuzumab | IgG1 | Anti-microtuble agent, Duo-5 | Cleavable | 2 | Phase II clinical trial | [146] |
| ALT-P7* | HER2 | Trastuzumab biobetter HM2 | IgG1 | Microtubule polymerization inhibitor, auristatin analogue MMAE | Cleavable | 2 | Phase I clinical trial | [147] |
| ARX788* | HER2 | Trastuzumab | Engineered IgG1 | Microtubule polymerization inhibitor, auristatin analogue MMAF | Non-cleavable | 1.9 | Phase II clinical trial | [148] |
| ASG-22ME | Nectin-4 | Enfortumab | IgG1 | Microtubule polymerization inhibitor, auristatin analogue MMAE | Cleavable | 3-4 | Phase I clinical trial | [149] |
| BA3021 | ROR2 | Ozuriftamab | IgG1 | Microtubule polymerization inhibitor, auristatin analogue MMAE | Cleavable | NA | Phase II clinical trial | [150] |
| CX-2009 | CD166 | CX-191 | IgG1 | Microtubule polymerization inhibitor, maytansinoid derivative DM4 | Cleavable | 3.5 | Phase II clinical trial | [151] |
| DS-1062 | TROP2 | hTINA1 | IgG1 | TOPI inhibitor, camptothecin analogue DXd | Cleavable | 4 | Phase III clinical trial | [152] |
| DS-8201a | HER2 | Trastuzumab | IgG1 | TOPI inhibitor, camptothecin analogue DXd | Cleavable | 8 | Approved | [47] |
| IMMU-132* | TROP1 | hRS7 | IgG1 | TOPI inhibitor, camptothecin analogues SN-38 | Cleavable | 7.6 | Approved | [22] |
| MORAb-202 | FRα | Farletuzumab | IgG1 | Microtubule inhibitor, eribulin | Cleavable | 4 | Phase II clinical trial | [153] |
| MRG002 | HER2 | MAB802 | IgG1 | Microtubule polymerization inhibitor, auristatin analogue MMAE | Cleavable | 3.8 | Phase II clinical trial | [154] |
| NBE-002* | ROR1 | XBR1-402 | IgG1 | Highly potent anthracycline derivative PNU-159682 | Non-cleavable | NA | Phase I clinical trial | [155] |
| PF-06647020 | PTK7 | hu6M024 | IgG1 | Microtubule polymerization inhibitor, auristatin-based payload Aur0101 | Cleavable | 4 | Phase I clinical trial | [156] |
| PF-06804103* | HER2 | T-(ĸkK183C+K290C) | IgG1 | Microtubule polymerization inhibitor, auristatin analogue Aur0101 | Cleavable | 4 | Phase I clinical trial | [157] |
| RC48 | HER2 | Hertuzumab | IgG1 | Microtubule polymerization inhibitor, auristatin analogue MMAE | Cleavable | 4 | Phase III clinical trial | [36] |
| SGN–-LIV1 | LIV-1 | hLIV22 | IgG1 | Microtubule polymerization inhibitor, auristatin analogue MMAE | Cleavable | 4 | Phase II clinical trial | [158] |
| SKB264* | TROP2 | NA | IgG1 | TOPI inhibitor, belotecan-derived payload | Cleavable | 7.4 | Phase II clinical trial | [159] |
| SYD985 | HER2 | Trastuzumab | IgG1 | DNA-alkylating agent, duocarmycin | Cleavable | 2.8 | Phase III clinical trial | [160] |
| T-DM1 | HER2 | Trastuzumab | IgG1 | Microtubule polymerization inhibitor, maytansinoid derivative DM1 | Non-cleavable | 3.5 | Approved | [161] |
| U3-1402 | HER3 | U3-1287 | IgG1 | TOPI inhibitor, camptothecin analogue DXd | Cleavable | 8 | Phase II clinical trial | [162] |
| XMT-1522 | HER2 | HT-19 | IgG1 | Microtubule polymerization inhibitor, AF-HPA moiety | Cleavable | 12 | Phase I clinical trial | [80] |
| ZW49 | HER2 | ZW25 | Bispecific IgG1 | Microtubule polymerization inhibitor, auristatin-based payload | Cleavable | NA | Phase I clinical trial | [115] |
- *: site-specific conjugation
- Abbreviations: ADC, antibody-drug conjugate; AF-HPA, auristatin F-hydroxypropylamide; CD166, cluster of differentiation 166; DAR, drug-antibody ratio; DM, derivative of maytansines; FRα, folate receptor alpha; HER, human epidermal growth factor receptor; MMAE, monomethyl auristatin E; MMAF, monomethyl auristatin F; NA, not acquired; PTK7, protein tyrosine kinase 7; ROR, receptor tyrosine kinase orphan receptor; TLR, toll-like receptor 7/8; TOP, topoisomerase; TROP2, trophoblast cell surface antigen 2
3 MECHANISMS OF ACTIONS
Upon being given intravenously, the circulating ADCs bind to tumor targets and initiate endocytosis. So far the mostly used ADCs in breast cancer are anti-HER2 ADCs, where HER2 is targeted by the mAb portion by recognizing the corresponding epitope. Natural antibodies are monospecific, which have two identical antigen-binding sites on both heavy and light chain variable domains to bind to specific targets. By antibody engineering, bispecific antibodies are developed, which can bind to either two distinct targets or two different epitopes on one target, those targeting two different epitopes on one target are referred as biparatopic antibodies [23]. Biparatopic antibodies are also equipped in ADCs, such as MEDI4276 that binds to two distinct HER2 epitopes, which leads to enhanced internalization and increased lysosomal trafficking [24]. Endocytosis can be manifested either by clathrin-dependent or clathrin-independent pathways [25]. Clathrin-mediated endocytosis is the most prominent pathway reported for ADC internalization, which is specific and requires receptor binding [25, 26]. Clathrin-independent pathways such as caveolae-mediated endocytosis and macroscale endocytosis also play a role. Caveolae is the essential invagination of the plasma membrane that is composed of lipids and caveolin proteins. Caveolins and other additional proteins facilitate the budding of caveolae, among which caveolin-1 is vital in this process [27, 28]. Macroscale endocytosis includes phagocytosis and macropinocytosis, both of which are non-specific. After binding to the target antigen, the endocytic process starts and leads to the formation of early endosomes. Notably, a small fraction of ADCs bind to neonatal Fc receptor (FcRn) in endosomes and return to the extracellular environment [6]. The vascular endothelium is the major site of recycling [29]. This recycling mechanism plays a critical role in long half-lives of IgGs and performs as a protective approach for normal cells in case of misdelivery. However, excess recycling in cancer cells might also contribute to resistance. The majority of endosomes then fuse with lysosomes, where ADCs undergo lysosomal degradation and free cytotoxic payloads into the cytosol ensues. The intracellular cytotoxic payloads thereby induce apoptosis, and the bystander effect expands apoptosis to neighboring target-negative cells.
Aside from the cytotoxic effects of payloads, mAbs can also exert their intrinsic antitumor activity such as blocking target antigens and triggering antibody-dependent immune responses. As mentioned above, IgG1 is the dominant IgG isotype in ADCs due to its advantage in inducing antitumor immunity. IgG1 supports immune responses, including antibody-dependent cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP), while IgG2 and IgG4 induce weak immune responses. In ADCC and ADCP, effector cells such as natural killer cells and macrophages bind to target tumor cells by binding Fc fragment and FcR. CDCs are activated upon the binding of mAbs and their targets, followed by the assembling of a membrane attack complex. These immune responses constitute ADC antitumor activity. T-DM1 even showed slightly stronger ADCC activity than trastuzumab in vivo, which indicated that the function of the Fc portion remains unaffected after the engineering of trastuzumab in T-DM1 [30]. Moreover, payloads also contribute to the immune activity of ADCs. For instance, DS-8201a increased tumor-infiltrating dendritic cells and CD8+ T cells. The expression of dendritic cell marker, CD86, was also increased, which resembled the immune effect of Dxd treatment. Programmed cell death 1 ligand 1 (PD-L1) and major histocompatibility complex class I were also upregulated on tumor cells [31]. The mechanisms of actions of ADCs are demonstrated in Figure 1B.
4 EFFICACY OF ADCS IN BREAST CANCER
4.1 HER2-targeted ADCs
HER2 is a member of the epidermal growth factor receptor family of receptor tyrosine kinases. HER2 overexpression is known to associate with more malignant tumor behaviors and unfavorable prognosis. HER2 serves as an ideal therapeutic target for HER2+ breast cancer due to the significantly high levels of HER2 expression compared to normal tissues [32]. HER2+ is defined as immunohistochemistry (IHC) assay 3+ or in situ hybridization (ISH) test positive, while HER2-low is described as IHC 2+/1+ with ISH test negative. It has been noticed that the addition of trastuzumab failed to improve invasive disease-free survival (IDFS) in patients with HER2-low breast cancer [33]. Considering that HER2-low breast cancer is insensitive to traditional HER2-targeted therapies, new approaches need to be developed. Under this circumstance, ADCs are a potential therapeutic option for these patients [34]. The efficacy results of HER2-targeted ADCs are summarized in Table 3.
| Registration number | Trial name | ADC | Phase | Patients | Interventions vs. control | Efficacy results (interventions vs. control) | Adverse events | Ref |
|---|---|---|---|---|---|---|---|---|
| In advanced disease setting | ||||||||
| NCT00829166 | EMILIA | T-DM1 | Phase III | HER2+ advanced breast cancer (previously treated) | T-DM1 vs. lapatinib plus capecitabine | PFS: 9.6 months vs. 6.4 months (HR = 0.65, 95% CI = 0.55-0.77; P < 0.001) | The most commonly reported grade 3/3+ events | [37, 38] |
| OS: 29.9 months, 95% CI = 26.3-34.1 vs. 25.9 months, 95% CI = 22.7-28.3 (HR = 0.75, 95% CI = 0.64–0.88) | T-DM1: thrombocytopenia (12.9%), increased AST (4.3%), increased ALT (2.9%) | |||||||
| ORR: 43.6% vs. 30.8% (P < 0.001) | Lapatinib plus capecitabine: diarrhea (20.7%), palmar-plantar erythrodysesthesia (16.4%) | |||||||
| NCT01419197 | TH3RESA | T-DM1 | Phase III | HER2+ advanced breast cancer (previously treated) | T-DM1 vs. TPC | PFS: 6.2 months, 95% CI = 5.59–6.87 vs. 3.3 months, 95% CI = 2.89–4.14 (HR = 0.528, 95% CI = 0.422–0.661; P < 0.0001) | The most common grade 3/3+ events | [73, 163] |
| OS: 22.7 months, 95% CI = 19.4-27.5 vs. 15.8 months, 95% CI = 13.5-18.7 (HR = 0.68 95% CI = 0.54–0.85; P = 0.0007) | T-DM1: Thrombocytopenia (5%), anemia (4%) | |||||||
| ORR: 31% vs. 9% (P < 0.0001) | Physician's choice: Neutropenia (16%), diarrhea (4%), febrile neutropenia (4%) | |||||||
| NCT01120184 | MARIANNE | T-DM1 | Phase III | HER2+ advanced breast cancer(previously untreated) | T-DM1 +/- P vs. HT | PFS: T-DM1 vs. HT: 14.1 months vs. 13.7 months (HR = 0.91; 97.5% CI = 0.73 to 1.13; P = 0.31) | The most commonly reported grade 3/3+ events | [39, 40] |
| T-DM1+P vs. HT: 15.2 months vs. 13.7 months (HR = 0.87; 97.5% CI = 0.69 to 1.08; P = 0.14) | T-DM1: increased AST (6.9%), thrombocytopenia (6.6%), and anemia (5.0%) | |||||||
| OS: T-DM1 vs. HT: 53.7 months vs. 50.9 months (HR = 0.93; 97.5% CI = 0.73-1.20) | T-DM1+P: thrombocytopenia (9.0%), anemia (7.1%),, increased AST (6.0%) | |||||||
| T-DM1+P vs. HT: 51.8 months vs. 50.9 months (HR = 0.86; 97.5% CI = 0.67-1.11) | HT: neutropenia (19.3%), febrile neutropenia (6.5%), diarrhea (4.2%). | |||||||
| ORR: HT: 67.9%, 95% CI = 62.3-73.3 | ||||||||
| T-DM1: 59.7%, 95% CI = 54.1-65.3 | ||||||||
| T-DM1+P: 64.2%, 95% CI = 58.6 -69.7 | ||||||||
| NCT02924883 | KATE2 | T-DM1 | Phase II | HER2+ advanced breast cancer | T-DM1 plus atezolizumab vs. T-DM1 | PFS: 8.2 months vs. 6.8 months (HR = 0.82, 95% CI = 0.55-1.23; P = 0.33) | The most commonly reported grade 3/3+ events | [140] |
| thrombocytopenia (13% vs. 4%), increased AST (8% vs. 3%), anaemia (5% vs. 0), neutropenia (5% vs three 4%), and increased ALT (5% vs 3%) | ||||||||
| NCT01702571 | KAMILLA | T-DM1 | Phase III | HER2+ advanced breast cancer | T-DM1 (single arm) | PFS: 6.9 months, 95% CI = 6.0-7.6 | The most commonly reported grade 3/3+ events | [164, 165] |
| OS: 27.2 months, 95% CI = 25.5-28.7 | Anaemia (3.0%), thrombocytopaenia (2.7%), fatigue (2.5%) | |||||||
| ORR: 29.3%, 95% CI = 27.1-31.6 | ||||||||
| The final results of the control group were provided former in this table | ||||||||
| NCT02564900Δ | DS8201-A-J101 | DS-8201a | Phase I | HER2+ advanced breast cancer, previously treated with T-DM1 | DS-8201a (single arm) | ORR: 59.5%, 95% CI = 49.7-68.7 | The most commonly reported TEAEs | [49] |
| DOR: 20.7 months (95% CI not estimable) | gastrointestinal and hematological events | |||||||
| DCR: 93.7%, 95% CI = 87.4-97.4 | The most commonly reported grade 3/3+ events | |||||||
| PFS: 22.1 months (95% CI not estimable) | Anemia (17%), decreased neutrophil (14%), decreased white blood cell (9%), decreased platelet (8%) counts | |||||||
| The final results of the control group were provided former in this table | ||||||||
| HER2-low advanced breast cancer | DS-8201a (single arm) | ORR: 37.0%, 95% CI = 24.3% - 51.3%] | Common grade 3/3+ TRAEs | [52] | ||||
| DOR: 10.4 months, 95% CI = 8.8 - NE | Decreased neutrophil, platelet, and WBC counts; anemia; hypokalemia; AST increase; decreased appetite; and diarrhea | |||||||
| PFS: 11.1 months, 95% CI not estimable | ||||||||
| NCT03248492 | DESTINY-Breast01 | DS-8201a | Phase II | HER2+ advanced breast cancer, previously treated with T-DM1 | DS-8201a (single arm) | ORR: 61.4%, 95% CI = 54.0-68.5] | The most commonly reported grade 3/3+ events | [50] |
| DOR: 20.8 month, 95% CI = 15.0-NE | Decreased neutrophil count (20.7%), anemia (8.7%), and nausea (7.6%). | |||||||
| DCR: 97.3%, 95% CI = 93.8-99.1 | ||||||||
| PFS: 19.4 months, 95% CI = 14.1-NE | ||||||||
| NCT03529110 | DESTINY-Breast03 | DS-8201a | Phase III | HER2+ advanced breast cancer, previously treated with trastuzumab and taxane | DS-8201a vs. T-DM1 | ORR: 79.7%, 95% CI = 74.3-84.4 vs. 34.2% 95% CI = 28.5-40.3 (P < 0.0001) | The most commonly reported grade 3/3+ events | [53] |
| PFS: Not reached 95% CI = 18.5-NE vs. 6.8 months, 95% CI = 5.6-8.2 (HR = 0.2840, 95% CI = 0.2165-0.3727; P = 7.8*10−22) | DS-8201a: neutropenia (19.1%), thrombocytopenia (7.0%), leukopenia (6.6%), nausea (6.6%) | |||||||
| T-DM1: thrombocytopenia (24.9%), increased AST (5.0%), increased ALT (4.6%) | ||||||||
| NCT02277717Δ | / | SYD985 | Phase I | Advanced solid tumor with variable HER2 status who were refractory to standard cancer treatment | SYD985 (single arm) | ORR: HER2+: 33%, 95% CI = 20.4-48.4 (all PR) | The most common TRAE (grades 1-4) | [166] |
| HER2-low, hormone receptor+: 28%, 95% CI = 13.8-46.8 (all PR) | Fatigue (33%), conjunctivitis (31%), and dry eye (31%) | |||||||
| HER2-low, hormone receptor-: 40%, 95% CI = 16.3-67.6 (all PR) | ||||||||
| PFS: HER2+: 7.6 months, 95% CI = 4.2-10.9 | ||||||||
| HER2-low, hormone receptor+: 4.1 months, 95% CI = 2.4-5.4 | ||||||||
| HER2-low, hormone receptor+: 4.9 months 95% CI = 1.2–NE | ||||||||
| NCT03262935 | TULIP | SYD985 | Phase III | HER2+ advanced breast cancer, with ≥ 2 previous MBC regimens or previous MBC treatment with T-DM1 | SYD985 vs. TPC | PFS: 7.0 months, 95% CI = 5.4-7.2 vs. 4.9 months, 95% CI = 4.0-5.5 (HR = 0.64, 95% CI = 0.49-0.84; P = 0.002) | The most commonly reported events | [167] |
| SYD985: conjunctivitis (38.2%), keratitis (38.2%) and fatigue (33.3%) | ||||||||
| PC: diarrhea (35.8%), nausea (31.4%) and fatigue (29.9%) | ||||||||
| NCT02881190 | / | RC48 | Phase I | HER2+ advanced solid tumors | RC48 (single arm) | ORR: 21% | The most commonly reported grade 3/3+ events | [168] |
| DCR: 49.1% | Neutropenia (19.3%), leukopenia (17.5%), hypoesthesia (14.0%), and increased conjugated blood bilirubin (8.8%) | |||||||
| PFS: 3.5month, 95% CI = 1.9–5.3 | ||||||||
| NCT03052634Δ | C003 CANCER | RC48 | Phase Ib | Advanced Breast Cancer, HER2+ or HER2-low | RC48 (single arm) | ORR: HER2+: 31.4% | the most common treatment-related adverse events (TRAEs) were AST increased (62.9%), ALT increased (61.4%), leukopenia (51.4%), hypoesthesia (51.4%) and neutropenia (51.4%) | [169] |
| HER2-low: 39.6% 95% CI = 25.8-54.7 | The most commonly reported grade 3/3+ events | |||||||
| PFS: HER2+: 5.8 months. | Neutropenia (21.4%), asthenia (15.7%), and leukopenia (10.0%) | |||||||
| HER2-low: 5.7 months, 95% CI = 4.1-8.3 | the most common treatment-related SAE was ileus (in 2 patients, 2.9%). | |||||||
| CTR20181301Δ | / | A166 | Phase I | Advanced Breast Cancer, HER2+ or HER2-low | A166 (single arm) | ORR: HER2+: 63.9% (23/36) | The most commonly reported grade 3/3+ events | [146] |
| HER2-low: 25%(1/4) (PR) | Corneal epitheliopathy (17.5%), hypophosphatemia (5.3%), and dry eye (5.3%) | |||||||
| CTR20171162 | / | ARX788 | Phase I | HER2+ metastatic breast cancer, heavily pretreated (median 5 prior treatments) | ARX788 (single arm) | ORR: 74%(14/19) | Adverse events that required special attention were ocular and pulmonary toxicity | [170] |
| DCR: 100% | ||||||||
| NCT03284723 | / | PF-06804103 | Phase I | HER2+/- solid tumor | PF-06804103 (single arm) | ORR: 52.4%(11/21) | The most commonly reported TRAEs (any grade) alopecia (48.6%), fatigue (42.9%), and neuropathy (25.7%) | [171] |
| CTR20181778 | / | MRG002 | Phase I | HER2+ advanced solid tumor | MRG002 (single arm) | (in breast cancer) ORR: 55%, 95% CI = 40.3-68.9 | The most commonly reported grade 3/3+ TRAEs | [172] |
| PFS: 8 months | Decreased neutrophil count, increased triglycerides | |||||||
| CTR20210235Δ | / | MRG002 | Phase II | HER2-low advanced breast cancer | MRG002 (single arm) | ORR: 5/18, all PR | NA | NP |
| NCT03281824 | / | ALT-P7 | Phase I | HER2+ metastatic breast cancer (refractory on based therapy) | ALT-P7 (single arm) | ORR: 77.3% (17/22) | The most common grade 3/4 event | [147] |
| PFS: 6.2 months, 95% CI = 2.5-9.9 | neutropenia (n = 4). | |||||||
| In early disease setting | ||||||||
| NCT01772472 | KATHERINE | T-DM1 | Phase III | HER2+ early breast cancer, residual cancer after neoadjuvant therapy (post-neoadjuvant) | T-DM1 vs. trastuzumab | 3-year IDFS event-free rate: 77.02%, 95% CI = 73.78-80.26 vs. 88.27%, 95% CI = 85.81-90.72 (HR = 0.50, 95% CI = 0.39–0.64, P < 0.001) | The most commonly reported grade 3/3+ events | [41] |
| T-DM1: decreased platelet count (5.7%) and hypertension (2.0%) | ||||||||
| Trastuzumab: hypertension (1.2%) and radiation-related skin injury (1.0%) | ||||||||
| NCT01966471 | KAITLIN | T-DM1 | Phase III | High risk (LN+/-, hormone receptor-, and tumor size > 2.0cm, without neoadjuvant therapy) HER2+ early breast cancer (in adjuvant setting) | AC-KP vs. AC-THP | 3-year IDFS event-free rate: 93.1% vs. 94.2% (HR = 0.98, 95% CI = 0.72–1.32) | Similar incidence of grade ≥3 events between groups (55.4% vs 51.8%) | [173] |
| NCT02131064 | KRISTINE | T-DM1 | Phase III | HER2+ early breast cancer (in neoadjuvant setting) | T-DM1 plus pertuzumab vs. TCH plus pertuzumab | 3-year IDFS event-free rate: 93.0%, 95% CI = 89.4-96.7 vs. 92.0%, 95% CI = 86.7-97.3 (HR = 1.11, 95% CI = 0.52-2.40) | The most commonly reported grade 3/3+ events | [42, 43] |
| 3-year EFS event-free rate: 85.3%, 95% CI = 80.5-90.1 vs. 94.2%, 95% CI = 91.0-97.4 (HR = 2.61, 95% CI = 1.36-4.98) | T-DM1+P: anemia (5.8%), neutropenia (3.6%), peripheral neuropathy (3.1%), and decreased platelet count (2.2%) | |||||||
| pCR: 44.5% vs. 55.7% (absolute difference = −11.3%, 95% CI = −20.5- −2.0; P = 0.016) | TCH+P: neutropenia (25.1%), diarrhea (15.5%), febrile neutropenia (15.1%), and anemia (11%) | |||||||
| NCT02568839 | PREDIX | T-DM1 | Phase II | HER2+ early breast cancer (in neoadjuvant setting) | T-DM1 vs. Trastuzumab, Pertuzumab, and Docetaxel | pCR: 43.9%, 95% CI = 33.9-54.3 vs. 45.5%, 95% CI = 35.4-55.8, P = 0.82 | The most commonly reported grade 3/3+ events | [45] |
| Trastuzumab, Pertuzumab, and Docetaxel: diarrhea, mucositis, exanthema, and sensory neuropathy | ||||||||
| T-DM1: headache, mucositis, sensory neuropathy, and increase of liver transaminases | ||||||||
- Δ clinical trials recruiting HER2-low patients
- AC-KP, Anthracycline Followed by Trastuzumab Emtansine and Pertuzumab; AC-THP, Anthracycline Followed by Trastuzumab, Pertuzumab, and Taxane; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; DCR, disease control rate; DOR, duration of response; EFS, event-free survival; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; HT, trastuzumab plus taxane; IDFS, invasive disease-free survival; NA, not acquired; NE, not reached; NP, not published; ORR, objective response rate; OS, overall survival; P, pertuzumab; pCR, pathologic complete response; PFS, progression-free survival; PR, partial response; SAE, serious adverse events; TCH, docetaxel, carboplatin, and trastuzumab; TPC, treatment of physician's choice; TRAE, treatment-related adverse events.
T-DM1 received the US FDA approval in 2013 for the treatment of patients with HER2+ advanced breast cancer who were previously treated with trastuzumab and a taxane [35]. It is composed of anti-HER2 mAb trastuzumab conjugated to the microtubule polymerization inhibitor DM1 via a non-cleavable thioether linker, with an average DAR of 3.5 [36]. The efficacy and safety profile of T-DM1 has been demonstrated in multiple phase III trials, and T-DM1 is now a standard second-line treatment for advanced HER2+ breast cancer. The US FDA approval of T-DM1 was based on the EMILIA trial (NCT00829166), which showed favored median progression-free survival (mPFS), objective response rate (ORR) and median overall survival (mOS) of T-DM1 over lapatinib plus capecitabine [37, 38]. T-DM1 was then investigated as the first-line treatment for advanced HER2+ breast cancer in the MARIANNE trial (NCT01120184), where T-DM1 +/- pertuzumab was compared to the standard first-line therapy. The T-DM1-containing therapies showed a non-inferior but not superior mPFS and OS along with an improved safety profile [39, 40]. This result indicated the possibility of T-DM1 being used as the first-line therapy in certain patients.
Six years after its first approval, T-DM1 received new approval to expand its use to HER2+ breast cancer patients with residual invasive cancer following neoadjuvant therapy based on the results of the KATHERINE trial in May 2019. The KATHERINE trial (NCT01772472) showed a significantly longer IDFS with adjuvant T-DM1 compared to trastuzumab [41]. The efficacy of T-DM1 in neoadjuvant setting was evaluated in the KRISTINE trial (NCT02131064), which compared T-DM1 plus pertuzumab to the standard of care in early HER2+ breast cancer. However, the control arm showed a higher pathological complete response (pCR), a lower risk of an event-free survival (EFS) event, and an equivalent risk of an IDFS event. An EFS event is defined as disease progression or disease recurrence (local, regional, distant, or contralateral, invasive or non-invasive), or death from any cause; and an IDFS event is defined as ipsilateral, ipsilateral locoregional, contralateral invasive breast tumor recurrence, distant recurrence, and death from any cause [42, 43]. Even so, the recent results from the neoadjuvant I-SPY2 trial (NCT01042379) indicated that this combination could serve as a safe strategy for de-escalate therapy [44]. A comparable conclusion was drawn in a phase I trial (NCT02568839) which compared neoadjuvant T-DM1 monotherapy to the current standard treatment in HER2+ breast cancer. Similar pCR rates (43.9% vs. 45.5%) were reached in both arms [45]. More studies of T-DM1-containing therapies are ongoing.
DS-8201a has been granted the US FDA accelerated approval in 2019 for unresectable or metastatic HER2+ breast cancer patients who have received at least two prior anti-HER2-based regimens in the metastatic setting [46]. DS-8201a is comprised of a humanized anti-HER2 mAb and a membrane-permeable TOPI inhibitor conjugated by an enzyme-cleavable linker with an average DAR of 8. DS-8201a showed a more vigorous anti-proliferation activity than T-DM1 and promising antitumor activity in both HER2+ T-DM1-refractory and HER2-low patient-derived xenograft (PDX) models [47, 48]. The US FDA approval was based on the results of the DS8201-A-J101 trial (NCT02564900) and DESTINY-Breast01 trial (NCT03248492), both of which showed a promising ORR of DS-8021a in heavily pretreated patients with HER2+ advanced breast cancer [49-51]. DS-8201a was also studied separately in HER2-low breast cancer in the J101 trial, whose efficacy results supported DS-8201a as a treatment option for HER2-low patients who exhausted meaningful treatment [52]. In the DESTINY-Breast03 trial (NCT03529110), where DS-8201a was compared to T-DM1, patients with HER2+ advanced breast cancer achieved a significantly longer PFS using DS-8201a than T-DM1 [53].
Trastuzumab duocarmazine (SYD985) and ARX788 are two investigational anti-HER2 ADCs that have received fast-track designation by the US FDA for HER2+ advanced breast cancer. Both have demonstrated clinical benefits in both HER2+ and HER2-low breast cancer, even though ARX788 was designed with limited bystander effect [54]. SYD985 and ARX788 are currently on phase III trials, and their outcomes are awaiting.
Other anti-HER2 ADCs such as disitamab vedotin (RC48), PF-06804103, MRG002, and A166 are being investigated in both HER2+ and HER2-low breast cancer in ongoing phase I trials, and their promising efficacy results are reported in multiple international conferences. The encouraging clinical data from ADCs shed light on the future of the treatment of HER2-low breast cancer. More anti-HER2 ADCs are now under investigation, such as XMT-1522 (NCT02952729) incorporated with a mAb which binds to a distinct epitope from trastuzumab, ZW49 (NCT03821233) with biparatopic antibody, and FS-1502 (NCT03944499), all demonstrated antitumor activity in preclinical studies.
4.2 Non-HER2-targeted ADCs
TNBC lacks hormone receptor and HER2 expression and is related to more aggressive tumor biology than other subtypes of breast cancer. TNBC is heterogeneous at the molecular level and absent of distinct molecular targets. Thus, the development of targeted therapy in TNBC is in urgent need. The efficacy results of non-HER2-targeted ADCs are summarized in Table 4.
| Registration number | Trial name | ADC | ADC target | Payloads | Phase | Indications | Interventions vs. control | Efficacy | Adverse events | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT02574455 | ASCENT | IMMU-132 | TROP2 | SN-38 | Phase III | Locally advanced or metastatic TNBC, ≥2 prior treatments | IMMU-132 vs. TPC | PFS: 5.6 months, 95% CI = 4.3-6.3 vs. 1.7 months, 95% CI = 1.5-2.6 (HR = 0.41, 95% CI = 0.32 - 0.52, P < 0.001) | The most commonly reported TRAE (any grade) | [58] |
| OS: 12.1 months, 95% CI = 10.7-14.0 vs. 6.7 months, 95% CI = 5.8-7.7, HR = 0.48, 95% CI = 0.38-0.59, P < 0.001. | Neutropenia (63% vs. 43%), diarrhea (59% vs. 12%), nausea (57% vs. 26%), alopecia (46% vs. 16%), fatigue (45% vs. 30%), and anemia (34% vs. 24%) | |||||||||
| ORR: 35% vs. 5% |
The most commonly reported grade3/3+ TRAE |
|||||||||
| neutropenia (51% vs. 33%), leukopenia (10% vs. 5%), diarrhea (10% vs. <1%), anemia (8% vs. 5%), and febrile neutropenia (6% vs. 2%). | ||||||||||
| NCT01631552 | / | IMMU-132 | TROP2 | SN-38 | Phase I/II | Hormone receptor+/HER2- metastatic breast cancer, prior endocrine-based therapy and at least one chemotherapy | IMMU-132 (single arm) | ORR: 31.5%, 95% CI = 19.5-45.6 | The most commonly reported grade 3/3+ TRAEs | [59] |
| PFS: 5.5 months, 95% CI = 3.6-7.6 | neutropenia (50.0%), anemia (11.1%), and diarrhea (7.4%). | |||||||||
| OS: 12 months, 95% CI = 9.0-18.2 | ||||||||||
| NCT04152499 | / | SKB264 | TROP2 | TOPI inhibitor, belotecan-derived | Phase I/II | Locally advanced/metastatic solid tumors, no available standard therapies | SKB264 (single arm) | ORR: overall: 35.3% (6/17) | The most commonly reported TEAEs | [174] |
| 2 TNBC achieved PR (40%, 2/5) | Nausea (72.2%) and alopecia (66.7%) (grade 1-2) | |||||||||
| 1 HER2+breast cancer achieved PR (100%, 1/1) | The most commonly reported grade 3/3+ TEAEs | |||||||||
| DCR: 70.6% (12/17) | Decreased neutrophil (27.8%), white blood cell (22.2%) count, and anemia (16.7%) | |||||||||
| NCT03401385 | TROPION-PanTumor01 | DS-1062 | TROP2 | DXd | Phase I | Advanced solid tumors, no available standard treatment | DS-1062 | (in TNBC) |
The most commonly reported TEAEs |
[175] |
| ORR: 43% | Nausea (50%), stomatitis (44%), alopecia (40%), and fatigue (33%) | |||||||||
| DCR: 95% | ||||||||||
| NCT01969643 | SGNLVA-001 | SGN-LIV1 | LIV-1 | MMAE | Phase I | Second line mTNBC | SGN-LIV1 (single arm) | ORR: 28%, 95% CI = 13-47 | The most common TEAEs | [176] |
| nausea (60%), fatigue (58%), peripheral sensory neuropathy (54%), decreased appetite (44%), and constipation (39%) | ||||||||||
| The most common grade 3/3+ TEAEs | ||||||||||
| neutropenia (21%), fatigue (14%), hyperglycemia (12%), hypokalemia (12%), and hypophosphatemia (12%) | ||||||||||
| The most common serious adverse events | ||||||||||
| pneumonia (6%) and abdominal pain (4%) | ||||||||||
| NCT03310957 | SGNLVA-002 | SGN-LIV1 | LIV-1 | MMAE | Phase Ib/II | First line locally advanced or metastasis TNBC | SGN-LIV1 plus pembrolizumab (single arm) | ORR: 54%, 95% CI = 33.4-73.4 |
The most commonly reported TEAEs |
[177] |
| Nausea (53%), fatigue (45%), diarrhea (43%), alopecia (33%); constipation(29%), hypokalemia (29%), vomiting (27%), decreased appetite (25%); abdominal pain (24%); decreased weight (22%) | ||||||||||
| The most commonly reported grade 3/3+ events | ||||||||||
| Neutropenia (16%); diarrhea, fatigue, hypokalemia, and maculopapular rash (8% each); and abdominal pain, increased ALT, and urinary tract infection (6% each) | ||||||||||
| The most common SAEs | ||||||||||
| Abdominal pain and constipation (6% each). | ||||||||||
| NCT02222922 | / | PF-06647020 | PTK7 | Aur0101 | Phase I | Locally advanced or metastatic solid tumors, no available standard therapy | PF-06647020 (single arm) | (in TNBC) | The most common TEAEs | [156] |
| PFS: 1.5moths, 95% CI = 1.4–4.3 | nausea, alopecia, fatigue, headache, neutropenia, and vomiting | |||||||||
| ORR: 21%, 95% CI = 8-40 | The most commonly reported grade 3/3+ events neutropenia | |||||||||
| NCT02980341 | / | U3-1402 | HER3 | DXd | PhaseI/II | HER3+ metastatic breast cancer | U3-1402 (single arm) | ORR: HER3-high: 20.3% (13/64), all PR | The most commonly reported grade ≥3 TEAEs | [178] |
| HER3-low: 29% (6/21) all PR | Decreased neutrophil count (25%), decreased platelet count (23%), decreased white blood cell count (16%), and anemia (18%) | |||||||||
| CBR: HER3-high: 31.3% (20/64) | ||||||||||
| HER3-low: 33% (7/21) | ||||||||||
| NCT03386942 | / | MORAb-202 | FRα | eribulin | Phase I | FRα-positive solid tumors, no available standard therapy | MORAb-202 (single arm) | ORR: 45.5% (1CR+9PR /22) | The most commonly reported TEAEs | [179] |
| DCR: 81.2% (1CR+9PR+8SD /22) | Leukopenia and neutropenia (45% each), increased ALT (32%), anemia and increased AST (27% each) | |||||||||
| NCT03149549 | / | CX-2009 | CD166 | DM4 | Phase I/II | Locally advanced/metastatic solid tumors | CX-2009 (single arm) | ORR: Hormone receptor+/HER2-: 11% (n = 18) | NA | [180] |
| TNBC: 38% (n = 8) | ||||||||||
| CBR (at 24 weeks): 35% |
- Abbreviations: CBR, clinical benefit rate; CR, complete response; DCR, disease control rate; FRα, folate receptor alpha; HER, human epidermal growth factor receptor; HR, hazard ratio; NA, not acquired; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; SD, stable response; TEAE, Treatment-emergent adverse events; TNBC, triple-negative breast cancer; TPC, treatment of physician's choice.
Aside from IMMU-132, other TROP2-targeting ADCs such as datopotamab deruxtecan (Dato-DXd; DS-1062) and SKB264 also demonstrated preliminary clinical activity in TNBC. Multiple ADCs targeting different antigens are currently under investigation in TNBC as well, including ladiratuzumab vedotin (SGN-LIV1) targeting the zinc transporter SLC39A6, patritumab deruxtecan (U3-1402) targeting HER3, and cofetuzumab pelidotin (PF-06647020) targeting protein tyrosine kinase 7.
5 RESISTANCE
As the first ADC approved for breast cancer, T-DM1 has been widely used in HER2+ breast cancer patients. However, some patients experience relapse or progress on T-DM1 treatment. Such resistance may develop after responding to the treatment (acquired resistance) or exist from the beginning (de novo resistance). The antitumor activity of ADCs depends on both the antibody and the payload, while the drug release process is also necessary for payloads to exert cytotoxic effects. Thus, we categorized the resistance mechanisms into 1) antibody-mediated resistance, 2) impaired drug trafficking, 3) disrupted lysosome functions, and 4) payload-related resistance (Figure 2A).
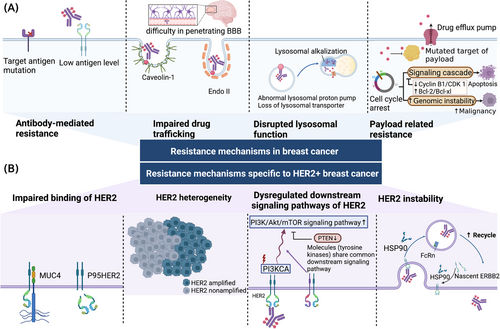
5.1 Resistance mechanisms in breast cancer
5.1.1 Antibody-mediated resistance
Reduced target expression is a common mechanism for inadequate antigen-antibody binding. For instance, the correlation between higher HER2 levels and greater T-DM1 efficacy was observed in a phase II trial [61]. Decreased HER2 expression and reduced binding were noticed in T-DM1-resistant cell lines from separate studies, which indicated HER2 loss as a mechanism of T-DM1 resistance [62, 63]. The absence of target expression has also been noticed in a TNBC patient with de novo resistance to IMMU-132, who showed undetectable expression of TROP2. In the same study, mutated TACSTD2 (encoding TROP2) was detected in another patient with acquired resistance to IMMU-132, which led to reduced binding due to an altered subcellular localization of TROP2 [64]. Interestingly, although the appearance of TROP2 is necessary, higher level of TROP2 does not necessarily result in a better response [65]. Low levels of antigen expression were also noticed in other cancer types. Downregulation of CD30 induced brentuximab vedotin-resistant in Hodgkin lymphoma [66], and reduced CD33 expression level on leukemic cells was associated with worse outcomes of gemtuzumab ozogamicin in acute myeloid leukemia [67].
5.1.2 Impaired drug trafficking
Compromised endocytosis is considered another mechanism of ADC resistance. Many proteins are essential in the ADC cellular internalization process. Endophilin A2 (Endo II) is a scaffolding protein and is involved in clathrin-independent endocytosis. Impaired Endo II expression is related to decreased HER2 internalization and reduced response to T-DM1 in HER2+ breast cancer models [68]. Aberrant caveolae-mediated endocytosis has also been noticed in T-DM1-resistant cell lines. Enhanced caveolae-mediated endocytosis was noticed in a gastric cancer N87 cell line with acquired T-DM1 resistance, possibly due to caveolae not favoring drug trafficking to lysosomes [69]. However, knockdown of caveolin-1 did not appear to restore the sensitivity to T-DM1. In another study, upregulated caveolin-1 appeared to improve T-DM1 activity in a different cell line (BT-474) [70]. Furthermore, hypoxia-induced translocation of caveolin-1 from vesicles to the plasma membrane was suggested to be the possible mechanism of reduced trastuzumab internalization in the hypoxia microenvironment in breast cancer SKBR3 cells [71]. In the study conducted by Sung et al. [69], they noticed that the ADC:CAV1 ratio differs in different cell lines, which indicates the caveolae-mediated endocytosis weighs differently, and the impact of CAV-1 level. In conclusion, the impact of caveolae-mediated endocytosis in resistance to T-DM1 remains debatable possibly related to the leading endocytosis pathway of the target antigen in a certain cell line.
Except for impaired intracellular trafficking, the way of ADCs trafficking in the circulation system also contribute to drug resistance. T-DM1 is a large hydrophilic molecule and is difficult to diffuse through the blood-brain barrier, which shares a similar tissue distribution with trastuzumab. However, in preclinical studies, T-DM1 demonstrated active in trastuzumab-resistant mouse models with breast cancer brain lesions due to the cytotoxicy of the payload component, DM1 [72]. However, patients with brain metastases did not achieve improved mPFS in T-DM1 group compared to the control group in the TH3RESA trial [73]. Comparable results were also reached in the KATHin ERINE trial, where similar central nervous system recurrence rates were noticed with or without T-DM1 [41]. Notably, breast cancer with brain metastases appears to be better managed using DS-8201a. In the DESTINY-Breast03 trial, patients with brain metastases benefited more with DS-8201a than T-DM1 [53]. Encouraging results were also yielded in the phase II TUXEDO-1 trial, 11 out of 15 patients with brain metastases respond to DS-8201a treatment, including 70% had received prior T-DM1 treatment (NCT04752059) [74].
5.1.3 Disrupted lysosomal function
The lysosomal degradation of ADCs depends on the acidic lysosomal environment and active lysosomal enzymes. Lysosomal alkalization and impaired lysosomal proteolytic enzyme activity were identified in a T-DM1-resistant breast cancer cell line [75]. The cleavage of non-cleavable ADCs depends on the activity of lysosomal enzymes which require a highly acidic environment. The acidic environment is ensured by V-ATPase, a proton pump that regulates lysosomal acidification [76]. Aberrant V-ATPase activity was tested in T-DM1-resistant N87 gastric cancer cell line [77]. The inhibition of V-ATPase by bafilomycin A1 reduced the production of active metabolites of T-DM1 and thus the cytotoxicity of T-DM1 in N87 gastric cancer cells but not in T-DM1-resistant N87 cells [77]. A comparable situation was also noticed in a T-DM1-resistant breast cancer cell line, where lysosomal alkalization and impaired lysosomal proteolytic enzyme activity were observed in BT474 cells [75].
Cytotoxic payloads need to be transported across the lysosomal membrane to exert their cytotoxic effect. As for ADCs incorporated with membrane-impermeable payloads, transporters are required for payload release. SLC46A3 belongs to the solute carrier (SLC) transporter family and serves as a transporter on the lysosomal membrane of maytansine-based catabolites. Decreased levels of SLC46A3 lead to an accumulation of maytansine-based catabolites in lysosomes, while ADCs carrying auristatin-based monomethylauristatin F (MMAF) remain unaffected [21]. Loss of SLC46A3 conferring resistance to T-DM1 was also confirmed in a different study using T-DM1-resistant BT-474M1 cells [63]. A similar mechanism was also noticed in brentuximab vedotin for the treatment of lymphoma. Lysosomal multidrug-resistance protein 1 (MDR1) mediates the efflux of monomethyl auristatin E (MMAE) across the lysosomal membrane. It was inferred that the inhibition of lysosomal MDR1 enhanced the cytotoxicity of brentuximab vedotin in the Hodgkin lymphoma KM-H2 cell line [78].
5.1.4 Payload-related resistance
Payloads are the main agent for the antitumor activity of ADCs. Some cells may develop resistance by upregulating drug efflux pumps and interrupting drug deposition. In a study of a non-Hodgkin lymphoma cell line that was made resistant to anti-CD22-vc-MMAE and anti-CD79b-vc-MMAE, upregulated MDR1 (encoded by ABCB1) expression was identified to be responsible for resistance to vc-MMAE-based conjugates [79]. Likewise, since vc-MMAE-based conjugates are utilized in breast cancer, the overexpression of MDR1 might also be a resistance factor for ADCs in breast cancer. Maytansinoids are another class of substrates for drug efflux transporters. Overexpression of multidrug resistance-associated protein 1 (MRP1, encoded by ABCC1), MRP2 (ABCC2), and MDR1 was demonstrated in different T-DM1-resistant cell lines, and their sensitivity could be restored by the concomitant use of MRP1, MRP2, and MDR1 inhibitors, respectively [62, 63, 80]. Similar mechanisms are also noticed in IMMU-132. Overexpression of breast cancer resistance protein (BCRP, encoded by ABCG2) was verified in IMMU-132-insensitive breast cancer cell lines, and the inhibition of ABCG2 helped to overcome resistance [81].
Tumor cells can also avoid cytotoxic effects via altered targets of payloads. A specific point mutation in TOPI was identified in TNBC patients who were resistant to IMMU-132, which has been previously described and is known to induce resistance to clinical TOPI inhibitors [64]. Other than TOPI mutations that directly disrupt the target of SN-38, the proficiency of the homologous recombinational repair (HRR) pathway is also related to IMMU-132 resistance by compensating for DNA damage caused by SN-38 [82]. Regarding ADCs with anti-mitotic agents like T-DM1, modifications in the microtubule/tubulin complex were found in T-DM1-resistant MDA-MB-361 cells [83].
Anti-mitotic agents arrest target cells at the G2/M phase of the cell cycle and lead to apoptosis. Cyclin B1/cyclin-dependent kinase 1 (CDK1) complex is essential for cell mitosis and mitotic catastrophe [84]. Some T-DM1-resistant cells manage to escape mitotic catastrophe and apoptosis through defective cyclin B1. This mechanism was verified in HER2+ breast cancer cell lines with various levels of acquired T-DM1 resistance, where T-DM1 failed to induce the upregulation of cyclin B1 and the consequent CDK1 activation. In addition, cyclin B1 knockdown induced T-DM1 resistance, while upregulation of cyclin B1 partially sensitized the resistant cells [85]. Moreover, the inability to eliminate genetically unstable cells might further increase tumor malignancy [86]. Besides cyclin B1, Bcl-2/Bcl-xl also participate in cell cycle regulation. Overexpression of Bcl-2/Bcl-xl is associated with resistance to gemtuzumab ozogamicin in acute myeloid leukemia and anti-CD79b-vc-MMAE in NHL cell lines [87, 88]. The inhibition of polo-like kinase 1 (PLK1) can rescue T-DM1 resistance via CDK1-dependent Bcl-2 phosphorylation [89], and the inhibition of Bcl-2/Bcl-xl significantly enhanced the antitumor activity of T-DM1 in PDX models that are either T-DM1-sensitive or T-DM1-resistant [90].
5.2 Resistance mechanisms specific to HER2+ breast cancer
The specificity of the ADC resistance mechanisms in breast cancer mainly lies in the special traits of HER2. Here we categorized HER2-related resistance mechanisms into 1) impaired binding of HER2, 2) heterogeneous HER2 expression, 3) dysregulated downstream signaling pathways, 4) HER2 instability (Figure 2B).
5.2.1 Impaired binding of HER2
The binding of mAbs to HER2 is related to multiple factors. HER2 shedding generates soluble truncated extracellular domain of HER2 molecules and leaves the 95-kDa intracellular domain, p95HER2 [91]. High level of p95HER2 is associated with resistance to trastuzumab and is also expected to confer to T-DM1 resistance. Furthermore, a membrane-bound glycoprotein mucin 4 (MUC4) was also considered a source of T-DM1 resistance. The tumor necrosis factor α (TNFα)-induced upregulation of MUC4 impaired binding and inhibited ADCC by masking the epitope of trastuzumab in HER2, while silencing MUC4 can restore sensitivity to T-DM1. This clinical relevance was proved in patients that the MUC4-positive tumors were associated with unfavorable DFS [92].
5.2.2 HER2 heterogeneity
HER2 intratumoral heterogeneity is referred to as different HER2 expression or amplification status within the same tumor, which can be identified by an area with HER2 amplification in >5% but <50% of tumor cells, or a HER2-negative area by fluorescence in situ hybridization (FISH) [93, 94]. Intratumoral HER2 heterogeneity presents more in breast cancers with low-grade HER2 amplification and equivocal HER2 expression [95].
T-DM1 showed limited efficacy when encountering tumors with heterogeneous HER2 expression. As noticed in the KRISTINE trial, none of the patients with HER2 intratumoral heterogeneity responded to T-DM1 therapy; and a lower pCR was more prevalent in tumors with heterogeneous HER2 expression than homogenous in the exploratory biomarker analysis [96]. This underlying association was also investigated in a phase II trial in early breast cancer patients, based on which HER2 heterogeneity is suggested to be related to T-DM1 resistance, where the fraction of HER2-nonamplified cells was the critical factor [93]. This mechanism was studied in vitro, where HER2 heterogeneity was mimicked by coculturing HER2+ KPL-4 cells and HER2- MDA-MB-468 cells. T-DM1 failed to suppress HER2- cells, while DS-8201a induced cytotoxicity to HER2- cells which neighbor HER2+ cells due to its effective bystander effect, which is also confirmed using phosphor-integrated dots imaging [97]. According to a recent retrospective clinical study, patients with various statuses of HER2 (heterogenous, reduced, and loss) benefited from DS-8201a [98].
It was reported that HER2 heterogeneity was related to centromere 17 copy number gain, possibly associated with chromosomal aneuploidy [99], whereas chromosomal aneuploidy was also noticed in T-DM1-resistant cells [83]. As a consequence of chromosomal instability, chromosomal aneuploidy is a known cause of multidrug resistance in cancer and an indication of poor prognosis [100]. Altogether, chromosomal aneuploidy might also contribute to T-DM1 resistance.
5.2.3 Dysregulated downstream signaling pathways of HER2
mAbs in ADCs also exert intrinsic cytotoxic effects apart from binding to the target antigen. The phosphatidylinositol-3-kinase (PI3K)/Akt and the mammalian target of rapamycin (mTOR) signaling pathway is an important HER2 downstream pathway that is related to cancer development. Upregulation of this pathway is a known mechanism of resistance to anti-HER2 therapy. The tumor suppressor gene, phosphatase and tensin homolog deleted on chromosome ten (PTEN), negatively regulates the PI3K signaling pathway. Decreased PTEN expression in T-DM1-resistant BT-474M1 cells was also noticed, and adding pan-PI3K inhibitor, GDC-0941, resensitized T-DM1-resistant BT-474M1 cells [63]. In the EMILIA trial, patients with absent or decreased tumor PTEN expression benefited more from T-DM1 than capecitabine and lapatinib compared to patients with normal or increased tumor PTEN expression [101].
In the WSG ADAPT TP trial (NCT01779206), phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (PIK3CA) mutation was significantly associated with poor prognosis in early HER2+/hormone receptor+ breast cancer [102]. With respect to advanced HER2+ breast cancer, PIK3CA mutation status was also considered a prognostic factor for PFS in the MARIANNE trial; however, this impact was contradictory to the results from both the EMILIA trial and TH3RESA trial, where the clinical efficacy of T-DM1 treatment is irrelevant to PIK3CA mutation status [101, 103]. Given the conflicting results among trials, the investigators hypothesized that due to the co-occurrence of HER2 amplification and PIK3CA mutation following prior treatments, a relatively smaller scale of tumor cells with both HER2- and PIK3CA mutation were presented in pretreated tumors than treatment-naive tumors, even though these tumors were all identified as HER2+ and PIK3CA-mutated as a whole. Hence the impact of PIK3CA mutation status was insignificance in the EMILIA trial and the TH3RESA trial where T-DM1 was used as a second- or later-line treatment [104]. The relationship between PIK3CA mutation and T-DM1 sensitivity still requires further exploration.
Moreover, since other tyrosine kinases share common downstream signaling molecules with HER2, their abnormalities also contribute to T-DM1 resistance. A study investigating the combination therapy of pertuzumab and T-DM1 suggested that the HER3 ligand, heregulin (NRG-1b), reduced cytotoxic activity of T-DM1 in some cell lines by inducing HER2-HER3 heterodimerization and activating downstream PI3K signaling pathways [105]. Receptor tyrosine kinase-like orphan receptor 1 (ROR1) is a receptor tyrosine kinase-like orphan receptor. It was reported that ROR1-positive cells showed increased T-DM1 resistance than ROR1-negative cells [106]. ROR1 overexpression induced by T-DM1 treatment, in turn, leads to T-DM1 resistance through Hippo/YAP pathway and the increase in stemness and self-renewal properties [106]. YES Proto-Oncogene 1 (YES1) is a non-receptor tyrosine kinase whose amplification leads to resistance to HER2-targeted drugs, including T-DM1. The addition of YES1 inhibitor dasatinib could resensitize the resistant cells [107].
5.2.4 HER2 instability
It is essential for ADCs to be transported to lysosomes, where they undergo lysosomal degradation and release cytotoxic payloads. HER2 is endocytosis-deficient with rapid recycling [108]. Insufficient lysosomal trafficking due to excess endosomal recycling has been noticed in T-DM1-resistant JIMT-1 cells [62]. Fewer ADCs were transported to lysosomes since more were shunted to the recycling pathway and traveled back to the plasma membrane.
The membrane stability and signal transduction of HER2 are also in close association with the heat shock protein 90 (HSP90) chaperone machinery. Hyperactive HER2 on HER2+ breast cancer cells require chaperoning by HSP90 to maintain their stability, and the inhibition of HSP90 promotes HER2 to undergo ubiquitin-dependent degradation [109]. The irreversible tyrosine kinase inhibitor neratinib has shown to induce HER2 endosomal-lysosomal endocytosis by dissociating HSP90 from HER2 and triggering ubiquitylation [110]. This mechanism was further applied in improving ADC potency, where irreversible pan-HER kinase inhibitors such as neratinib and afatinib were used in combination with T-DM1. This combination enhanced T-DM1 activity in vitro and was also verified in a patient with HER2-amplified breast cancer enrolled in a clinical trial (NCT01494662) who experienced partial response upon T-DM1 progression [111].
6 SOLUTIONS
6.1 New drug development
A variety of novel strategies have been developed for ADCs to improve drug efficacy and overcome resistance (Figure 3A). ADCs with non-cleavable linkers, such as T-DM1 with thioether linker and ARX788 with maleimide linker, utilize an active cytotoxic complex that includes a single amino acid (lysine or cysteine) attached to the linker and the payload. The cytotoxic complex has limited membrane permeability and insufficient bystander effect [15]. This design limits drug potency in tumors with low or heterogeneous HER2 expression. Some newly developed ADCs have overcome this obstacle and have shown promising efficacy in this population of patients. For instance, DS-8201a showed superior antitumor activity in patients with low HER2 expression and HER2 intratumoral heterogeneity compared to T-DM1, owing to its cleavable linker, a higher DAR, and more potent payload [97]. Cleavable linkers now remain dominant in the ADC market due to their high feasibility and compatibility.
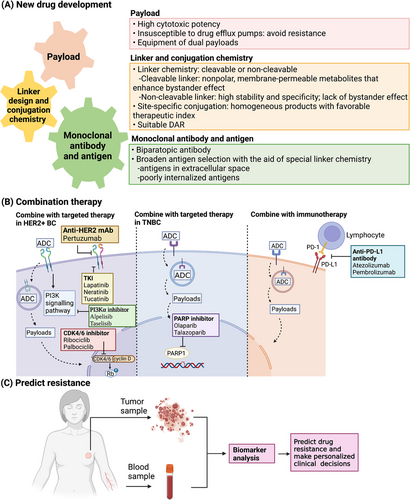
However, unlike T-DM1, ARX788 showed activity in HER2-low breast cancer PDX models because of its site-specific conjugation manner, implying that efficient drug delivery could compensate for the low levels of antigen expression [54]. The conventional conjugation procedure yields heterogeneous products with different DARs and conjugation sites. In contrast, the site-specific conjugation method selectively yields ADCs with a moderate DAR, which endows the ADC products with higher homogeneity and more favorable therapeutic index [112]. Since the site-specific conjugation is based on engineered cysteines at specific sites in antibodies and restrained to one payload per cysteine, this technology met its limitation when producing ADCs with a DAR greater than 2. Currently, this conjugation technology is being revised for the production of site-specific ADCs with higher DARs. Refinements such as engineering multiple unpaired cysteines in the antibody or utilizing branched linkers are under development [113, 114].
Aside from optimizing linker and conjugation chemistry, antibody engineering is also important for ADC production. Biparatopic antibodies appear to be an inspiring approach for drug resistance, which allows increased binding, more rapid HER2 internalization, and enhanced lysosomal degradation. ZW49 utilizes an anti-HER2 biparatopic antibody, ZW25, which shares the same domains as trastuzumab and pertuzumab. ZW49 has shown growth inhibitory activity in both low and high HER2-expressing breast cancer models [115]. This biparatopic ADC has also demonstrated manageable tolerability and preliminary efficacy in a phase I dose-escalating study (NCT03821233) in patients with HER2+ advanced breast and gastric cancers who are refractory to all standard treatments, including T-DM1. However, dose-limiting toxicity is a concern for biparatopic ADCs. Another biparatopic ADC with site-specific conjugation, MEDI4276, showed limited clinical activity and unfavorable toxicity in the phase I trial (NCT02576548) [116].
Strengthening antitumor immunity is another possible way of improving ADC efficacy and tackling resistance. Fc-mediated immune responses can be strengthened through mAb engineering. Glycolengineering and amino acid substitution were developed to enhance the ADCC activity of mAbs [117]. However, such engineered mAbs have not been applied in ADCs yet.
Although cellular internalization is considered necessary for its function, a non-internalizing mechanism is under investigation. For instance, cathepsin B is a lysosomal enzyme that exists in extracellular spaces due to its overproduction by tumor cells and tumor-associated cells; therefore the cathepsin B-cleavable linker can be cleaved both intracellularly and extracellularly. ADCs equipped with cathepsin B-cleavable linkers, such as SYD985, are expected to exert cytotoxic effects in tumors with low antigen expression or defective internalization pathways. In this way, ADCs can use poorly internalized antigens on tumor cells or even extracellular antigens as targets, which has broadened the landscape of potential target antigens [18, 118, 119].
As an important component of ADCs and part of the reason for resistance, innovative payload selection is another aspect of addressing drug resistance. Asides from suitable cytotoxic potency, lacking susceptibility to drug efflux pump-mediated resistance is also crucial [120]. An ADC with dual payloads was produced to combat HER2 heterogeneity and drug resistance. This ADC is equipped with MMAE and MMAF, the complementary properties of which allow the ADC to overcome resistance while remaining effective. This dual drug ADC has exerted prominent efficacy in animal models of refractory breast cancer with heterogeneous HER2 expression and is ready to proceed into the clinical phase [121].
6.2 Combination therapy
Combining ADCs with other targeted therapies with distinct action mechanisms and minimal overlapping toxic effects seems to be an effective approach to overcome or prevent resistance; while combining ADCs with immunotherapies can enhance antitumor immunity and exert a prolonged clinical benefit (Figure 3).
6.2.1 Combine with chemotherapeutics/targeted therapies
The inability of ADCs to elicit antitumor effects in tumors with target loss or target heterogeneity implies the importance of systemic chemotherapy that targets all tumor cells irrespective of target expression [122]. The combination of T-DM1 and docetaxel was efficacious, yet toxic and induced severe adverse events occur in nearly half of the patients [123].
Dual HER2 blockade appears to be more effective than monotherapy and can overcome resistance possibly by inducing efficient internalization and degradation of HER2 [124, 125]. Pertuzumab binds to HER2 and inhibits HER2 dimerization, particularly HER2-HER3 heterodimerization. The co-administration of pertuzumab and T-DM1 resulted in synergistic inhibition in HER2+ tumor models [105]. However, a pooled analysis of the efficacy of adding pertuzumab to T-DM1 showed a non-inferior but not superior clinical outcome [126].
Tyrosine kinase inhibitors (TKIs) bind to the intracellular domain of HER and are thus expected to overcome resistance related to HER2 shedding. Lapatinib is a dual TKI that binds to EGFR/HER2 reversibly. Concomitant use of lapatinib plus T-DM1 and chemotherapy yielded significant efficacy results in both early and advanced HER2+ breast cancer [127, 128]. Neratinib is a second-generation TKI that irreversibly binds to EGFR, HER2, and HER4. The addition of neratinib to T-DM1 has shown preliminary clinical efficacy in a dose-escalation study (NCT02236000) [129]. Tucatinib, a selective HER2 inhibitor, is also being studied in combination with T-DM1 in a phase III trial (HER2CLIMB-02 [NCT03975647]) in patients with pretreated metastatic HER2+ breast cancer.
Cyclin D1 and CDK4/6 are essential for tumor cell proliferation in HER2+ breast cancer. Other than suppressing Rb phosphorylation and inducing cell cycle arrest, CDK4/6 inhibitors also cause suppression of HER downstream mTORC1/S6K/S6RP signaling pathways, thereby disinhibiting HER family phosphorylation and resensitizing HER2-resistant cells to HER2 blockade [130]. The addition of CDK4/6 inhibitors can aid anti-HER2 therapies and induce suppression of HER2+ tumor cells that are refractory to T-DM1 in a preclinical study [131]. The combination of ribociclib and T-DM1 has acheived promising PFS results independent of the prior use of T-DM1 in heavily pretreated patients with HER2+ breast cancer [132]. A phase I/Ib study (NCT01976169) also verified that the addition of palbociclib resensitized patients who were resistant to T-DM1 and other anti-HER2 treatments [133].
Cotreatment of T-DM1 and PI3Kα inhibitors such as alpelisib and taselisib may enhance the activity of T-DM1 by working synergistically to inhibit the PI3K pathway. In a phase I trial investigating alpelisib plus T-DM1 in HER2+ metastatic breast cancer patients (NCT02038010), this combinational therapy appeared to be able to overcome T-DM1 resistance [134].
More potential combinational therapies containing HER2-targeted ADCs have been studied in cell lines and animal models, including co-administration with TNF-α inhibitor, drug efflux pump inhibitors, PLK1 inhibitor volasertib, and HSP90 inhibitor geldanamycin. These inhibitors can help restore sensitivity to T-DM1 by counteracting the corresponding resistance mechanisms, but more investigations are needed before clinical application.
ADC-containing combination therapies are also being researched in TNBC. Poly(ADP-ribose) polymerase 1 (PARP1) inhibitors specifically target PARP which is involved in base excision repair for DNA single-chain breaks. PARP1 inhibitors are indicated for patients with BRCA1/2-mutated breast cancer [135]. The combination of IMMU-132 and PARP inhibitors has been confirmed to have enhanced and synergistic effects in IMMU-132-resistant TNBC tumor models compared to monotherapies [136]. The combination of IMMU-132 and talazoparib is currently being studied in a phase Ib/II study in patients with metastatic TNBC (NCT04039230).
6.2.2 Combine with Immunotherapy
Immune checkpoint inhibitors, such as antibodies against immune inhibitory receptors cytotoxic T-lymphocyte antigen-4, programmed death-1 (PD-1), and programmed death ligand 1 (PD-L1), unleash the inhibition of T cells and activate antitumor immunity; while ADCs mediate antitumor immunity via activation of antigen-presenting cells and mediating intrinsic immunogenic tumor cell death [137]. It was noticed that stromal tumor-infiltrating lymphocytes increased in response to T-DM1, which promoted the augmentation of tumor-specific immunity. The potential for ADCs to act synergistically with immune checkpoint inhibitors to overcome or prevent resistance is rather inspiring [138, 139]. The combination of T-DM1 and anti-PD-1 mAbs appeared to be more efficacious than monotherapies in preclinical models. However, the addition of atezolizumab did not improve PFS and was related to more severe toxicity than the monotherapy with T-DM1 in the KATE2 trial (NCT02924883) [140]; even so, a possible OS benefit was noticed in PD-L1+ patients. This combination is now being explored specifically in patients with HER2+ and PD-L1+ metastatic breast cancer [141]. Another anti-PD-L1 antibody, pembrolizumab, is also being investigated with T-DM1 and DS-8201a.
The combinations of ADCs and immune checkpoint inhibitors are also being studied in TNBC. IMMU-132 and anti-PD-1 antibodies, such as atezolizumab and pembrolizumab, are now being investigated in multiple clinical trials (NCT04468061, NCT04434040), which are expected to optimize the treatment of TNBC.
6.3 Resistance prediction
Except for switching to new drugs or combination therapies when acquired resistance develops, we can also predict insensitivity to ADC therapies using predictive biomarkers (Figure 3). Target antigen expression levels are always the primary biomarker for ADC sensitivity. For instance, low HER2 expression is a known biomarker of T-DM1 resistance. In addition to IHC and ISH, a study showed that HER2 amplification levels can be measured by analyzing circulating tumor DNA (ctDNA) in the plasma. Negative HER2 gene amplification in ctDNA was associated with the occurrence of progressive disease. This study supported predicting de novo resistance of T-DM1 by analyzing ctDNA, as well as hormone receptor status [142]. MUC4 expression can also serve as an independent predictor for T-DM1 sensitivity. With regard to IMMU-132, besides the expression of TROP2 as the primary biomarker, other secondary biomarkers such as HRR proficiency should also be considered when predicting the clinical benefit of IMMU-132 [82].
RAB5A (RAB5A, member RAS oncogene family) regulates clathrin-mediated endocytosis and serves as an early endosome biomarker. Overexpression of RAB5A was identified as a predictive factor of the aggressive manner of breast cancer [143]. A recent study discovered that T-DM1 sensitivity was associated with RAB5A levels in five HER2-expressing cell lines. This association was further verified in the neoadjuvant I-SPY2 trial and KAMILLA trial (NCT01702571), where patients with higher levels of RAB5A tended to have more significant clinical benefits. Moreover, this study proposed that the combination of RAB5A and HER2 expression levels can serve as a better predictive biomarker compared to using either one separately [144]. As mentioned before, SLC46A3 is a lysosomal drug efflux transporter and is related to the resistance of maytansine-based ADCs. A study evaluating SLC46A3 in PDX in vitro models suggested that SLC46A3 can be examined as a potential patient selection biomarker with immediate relevance to maytansine-based ADCs such as T-DM1 [145]. Evaluations of more prospective biomarkers for ADCs in breast cancer are ongoing.
7 CONCLUSIONS
To date, T-DM1, DS-8201a, and IMMU-132 have been approved by the US FDA, SYD985 and ARX788 are on the US FDA fast track for the treatment of breast cancer, and more investigational ADCs are on the way. ADCs combine mAbs with cytotoxic agents, which allows effective tumor cell killing with high specificity. This novel class of drugs has exhibited great efficacy in both early and advanced breast cancer, even in HER2-low breast cancer and TNBC, which are considered treatment refractory. However, it is emphasized that the emerging resistance to ADC treatment is still troublesome. Understanding the mechanisms of resistance is crucial for optimizing new drug development, as well as providing predictive biomarkers for ADC treatment. More investigations are still necessary for more in-depth knowledge of addressing resistance. To overcome the limitations of ADCs in clinical use, modifications of the target, payload, and linker, as well as the discovery of conjugation chemistries, are priorities in ADC development. Furthermore, inspired by the results from studies in cell and animal models, combining ADCs with other targeted therapies or immunotherapies can synergistically improve clinical outcomes with minimal toxicity. Multiple clinical trials are ongoing to investigate the efficacy and safety profiles of combination therapies.
DECLARATIONS: ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable
CONSENT FOR PUBLICATION
Not applicable
AVAILABILITY OF DATA AND MATERIAL
Not applicable
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
Supported by grants from the National Natural Science Foundation of China (82072916), the 2018 Shanghai Youth Excellent Academic Leader, the Fudan ZHUOSHI Project, Chinese Young Breast Experts Research project (CYBER-2021-A01).
AUTHOR CONTRIBUTIONS
All authors wrote, read, and approved the final manuscript.
ACKNOWLEDGEMENTS
The figure is created with Biorender.com.




