Kindlin-1 drives early steps of breast cancer metastasis
Abbreviations
-
- 2D
-
- two-dimension
-
- 3D
-
- three-dimension
-
- AAkind1
-
- mutant form of kindlin-1 protein carrying the mutation QW611/612AA
-
- D
-
- displacement
-
- ECM
-
- extracellular matrix
-
- EEA1
-
- early endosomal antigen 1
-
- Kind1
-
- Kindlin-1
-
- qRT-PCR
-
- quantitative real-time polymerase chain reaction
-
- T
-
- distance
Kindlin-1 plays a major role in the activation of integrins [1]. Kindlin-1 is often overexpressed in cancers. Kindlin-1 overexpression has been associated with a higher propensity of breast cancer patients to metastasize to the lungs [2, 3]. Furthermore, Kindlin-1 depletion in mouse models was found to inhibit tumor growth and lung metastasis [3, 4]. However, the underlying mechanisms remain poorly explored.
We used the 4T1 mouse model to unravel the causal role of Kindlin-1 in breast cancer. This model comprises of four syngeneic tumor cell lines, including the nonmetastatic 168FARN and the highly metastatic 4T1 cells [5]. We previously demonstrated that Kindlin-1 was only expressed in the 4T1 cells [3]. We now investigated whether Kindlin-1 expression in the 168FARN cells is sufficient to enhance their metastatic capacities (Supplementary Materials).
We ectopically expressed Kindlin-1 in 168FARN cells and determined the status of β1-integrin activity. Consistent with previous studies [4, 6, 7], adhesion to extracellular matrix substrates was enhanced in Kindlin-1 expressing cells (hereafter cited as Kind1-cells) as compared to control cells (a three-fold increase on laminin, P < 0.001) (Figure 1A). We then evaluated the binding of Kind1-cells to a fibronectin fragment (FN7-10), harboring the integrin-binding RGD motif. We observed that Kindlin-1 enhanced integrin binding to the fibronectin fragment (Figure 1B). Moreover, Kindlin-1 induced the activation of β1-integrin as shown by increased levels of the active form of the protein specifically detected by the 9EG7 antibody on the cell surface (Figure 1C,D). In agreement with previous works [7], Kindlin-1 enhanced cell spreading as measured by the area of the cells (1240 versus 556 μm2, P < 0.001), and Kind1-cells exhibited a reorganized actin cytoskeleton into stress fibers (Supplementary Figure S1A-B). Furthermore, Kindlin-1 triggered the phosphorylation of Src at Tyr416 and the autophosphorylation of FAK at Tyr397, indicating that integrin signaling was enhanced (Figure 1E).
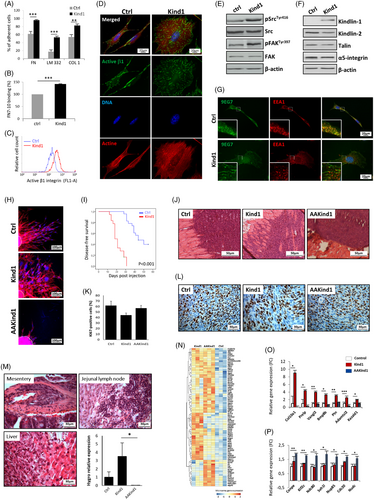
We then verified that altered expression of other integrins and regulators was not the reason for the changes in cell adhesion and spreading. Talin, α5-integrin and Kindlin-2 levels were found to be similar to those of the control cells (Figure 1F). Further, the levels of mature β1-integrin were increased in Kind1-cells, whereas precursor β1 was reduced, suggesting that Kindlin-1 regulated integrin processing and/or turnover (Supplementary Figure S1C). Quantitative real-time polymerase chain reaction (qRT-PCR) analysis showed similar ITGB1 mRNA levels in Kind1 and control cells, confirming that the difference was not due to a Kindlin-1-mediated transcriptional regulation (Supplementary Figure S1D). In addition, total β1-integrin, α5-integrin and α5β1 integrin levels were increased at the surface of Kind1-cells, whereas other integrins such as β3-integrins were less affected (Supplementary Figure S1E).
Kindlin-1 was shown to regulate integrin dynamics and adhesion turnover in keratinocytes [7]. We, therefore, investigated whether Kindlin-1 could influence integrin trafficking in cancer cells. We performed an antibody-based recycling assay in which cell-surface active β1-integrins were internalized and recycled back to the membrane for 15 min. We found that most active β1-integrins rapidly returned to the plasma membrane in Kind1-cells, whereas in control cells, they remained in the endosomes, as shown by the colocalization with the EEA1 (early endosomal antigen 1) marker (Figure 1G). These results indicate that Kindlin-1 promotes β1 recycling leading to increased active β1-integrins on cancer cells’ surfaces.
We then examined the effect of Kindlin-1 on cell migration and invasion. Kind1-cells exhibited higher migratory and invasive capacities in two-dimensional (2D) (Supplementary Figure S2A-B) and three-dimensional (3D) environments (Figure 1H and Supplementary Figure S2C). Interestingly, while control cells migrated straight ahead, Kind1-cells exhibited a capacity for randomly changing directions (Figure 1H). Time-lapse imaging showed that the persistence (displacement [D]-over-traveled distance [T] directionality ratio) of individual cells was reduced in Kind1-cells (P = 0.007), accompanied by a 3.8-fold increase in the velocity of cell migration (P < 0.001) (Supplementary Figure S2C-E). Thus, Kind1-cells had an increased ability to migrate in multiple directions while traveling a similar distance in a 3D environment.
Next, we ectopically expressed an integrin-binding-defective mutant of Kindlin-1 (QW611/612AA, hereafter cited as “AAKind1”) in 168FARN cells (Supplementary Figure S2A) [3, 4, 8]. We showed that AAKind1 did not trigger integrin activation and cell proliferation (Supplementary Figure S2F-H). Consistent with previous reports [4, 9], integrin-binding was necessary for Kindlin-1 to promote migration. More strikingly, we showed that AAKind1 drastically reduced the invasive capacities of 168FARN cells through 2D and 3D matrices (Figure 1H and Supplementary Figure S2B-E). The replacement of two amino acids in Kindlin-1 generated a mutant protein that acts as a dominant-negative in preventing cell invasion (Figure 1H and Supplementary Figure S2B-E).
We then performed orthotopic injection of control, Kind1 or AAKind1-cells into the mammary fat pad of syngeneic BALB/c mice. 168FARN cells were previously described as highly tumorigenic, rarely metastasizing but spreading through the lymphatics [5]. In our study, while 40% of control mice remained disease-free, all Kind1 or AAKind1-mice formed mammary tumors. Yet, Kind1 and AAKind1 mice exhibited distinct phenotypes. Kind1-mice presented tumors with restricted growth at the primary site but increased spreading to distant organs (Figure 1I-J). In contrast, AAKind1-mice displayed tumors rapidly reaching ethical size (< 24 days) without any indication of metastasis (Figure 1J). The pathological examination revealed that Kind1 and AAKind1-tumors displayed a similar proliferation rate as quantified by Ki67 staining, suggesting that AAKind1 did not trigger proliferation, in accordance with our in vitro experiments (Figure 1K-L). On the other hand, Kind1-tumors exhibited increased local invasion of the surrounding normal tissue, whereas AAKind1-tumors grew focally as encapsulated masses maintaining a distinct margin with the neighboring healthy muscles (Figure 1J and Supplementary Figure S3A-B). These observations suggest that Kind1-cells are highly invasive and rapidly escape from the mammary gland to colonize distant organs. Conversely, AAKind1-tumors did not invade but grew locally, leading to increased primary tumor size. An additional in vivo experiment confirmed our observations in earlier stages of the disease. Mice sacrificed on days 6, 10, 14 and 18 showed an early tumor onset in both Kind1 and AAKind1-mice. Kind1-tumors were already highly invasive on day 14, whereas AAKind1 remained focalized (Supplementary Figure S3C).
We collected distant organs from all mice. The qRT-PCR amplification of the hygromycin-resistance gene showed an increased metastatic spread of Kind1-tumors (Figure 1M and Supplementary Figure S3D). Conversely, AAKind1-tumors exhibited no metastatic spread (Figure 1M and Supplementary Figure S3E-F), indicating that Kindlin-1 promoted metastasis in vivo, and this capacity was abrogated by the mutation of the integrin-binding domain. Our results complement a recent study demonstrating that Kindlin-1 enabled pulmonary colonization by promoting tumor cell arrest within the lungs [4]. Here, we show that Kindlin-1 also triggered early steps of metastasis by enhancing the local invasion of breast tumors.
Lastly, we performed a transcriptomic analysis of ctrl, Kind1 and AAKind1-tumors (3 mice for each group; Figure 1N and Supplementary Table S1 and S2). Interestingly, Kind1-tumors activated pathways related to cell-matrix adhesion and cell invasions, like matrix reorganization or Wnt signaling, while AAKind1-tumors displayed pathways involved in the mitotic spindle assembly, another known function of Kindlin-1[10] (Supplementary Figure S4). Representative examples of upregulated genes are highlighted in Figures 1O and 1P.
In conclusion, Kindlin-1 is an essential regulator of integrin activation and trafficking with important implications for tumor progression. Kindlin-1 might be considered a major player in cell malignancy and a promising drug target with clinical utility in a broad range of cancers.
ACKNOWLEDGEMENTS
We thank Philippe Chavrier and Marina Glukhova for fruitful scientific advice and Marie Irondelle for her excellent technical assistance (UMR 144, CNRS-Institut Curie). This work was supported by a grant from the Breast Cancer Research Foundation (BCRF, USA) [grant number BCRF-20-097] and a grant from La Ligue contre le Cancer (Allocation de recherche Doctoral 4ème année – 2020).
CONFLICT OF INTEREST
The authors declare no competing financial interests
AUTHOR CONTRIBUTIONS
FB, PA, and SN have conducted experiments involving in vitro cells. FB and AC have conducted experiments involving in vivo mouse models. ZT performed the bioinformatics data analyses. FB, PA, ZT, SN, RL, and KD participated in data interpretation and statistical analysis. KD provided supervision and suggestions for the implementation of the study. KD wrote the manuscript, which was revised by FB, AC, SN, ZT and RL. All authors have read and approved the final manuscript.
CONSENT FOR PUBLICATION
Not applicable
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethics approval was not mandatory for this study. All mouse experiments were performed by competent researchers in accredited facilities (license B75-05-18 and B75-05-17 given by the National Authority) and following the French and European regulation in force on the protection of animals used for scientific purposes and to the Institut Curie recommendation on animal care, including 3R principle.
Open Research
DATA AVAILABILITY STATEMENT
The data generated in this study are available within the article. Expression profile data from mice tumors analyzed in this study are available from the corresponding author upon reasonable request.




