Functions and clinical significance of mechanical tumor microenvironment: cancer cell sensing, mechanobiology and metastasis
Abstract
Dynamic and heterogeneous interaction between tumor cells and the surrounding microenvironment fuels the occurrence, progression, invasion, and metastasis of solid tumors. In this process, the tumor microenvironment (TME) fractures cellular and matrix architecture normality through biochemical and mechanical means, abetting tumorigenesis and treatment resistance. Tumor cells sense and respond to the strength, direction, and duration of mechanical cues in the TME by various mechanotransduction pathways. However, far less understood is the comprehensive perspective of the functions and mechanisms of mechanotransduction. Due to the great therapeutic difficulties brought by the mechanical changes in the TME, emerging studies have focused on targeting the adverse mechanical factors in the TME to attenuate disease rather than conventionally targeting tumor cells themselves, which has been proven to be a potential therapeutic approach. In this review, we discussed the origins and roles of mechanical factors in the TME, cell sensing, mechano-biological coupling and signal transduction, in vitro construction of the tumor mechanical microenvironment, applications and clinical significance in the TME.
ABBREVIATIONS
-
- TME
-
- tumor microenvironment
-
- ECM
-
- extracellular matrix
-
- CAFs
-
- cancer-associated fibroblasts
-
- CTGF
-
- connective tissue growth factor
-
- HGF
-
- hepatocyte growth factor
-
- TAMs
-
- tumor-associated macrophages
-
- NKs
-
- natural killer cells
-
- ROS
-
- reactive oxygen species
-
- IL
-
- interleukin
-
- HIF
-
- hypoxia-induced factor
-
- DDRs
-
- discoidin domain receptors
-
- GAGs
-
- glycosaminoglycans
-
- HS
-
- heparan sulfate
-
- CS
-
- chondroitin sulfate
-
- DS
-
- dermatan sulfate
-
- HA
-
- hyaluronic acid
-
- VEGF
-
- endothelial growth factor
-
- CTCs
-
- circulating tumor cells
-
- FAK
-
- focal adhesion kinase
-
- ERK
-
- extracellular signal-regulated kinase
-
- YAP
-
- Yes-associated protein
-
- LOX
-
- lysyl oxidase
-
- TGF-β
-
- transforming growth factor-β
-
- EMT
-
- epithelial-mesenchymal transition
-
- PA
-
- polyacrylamide
-
- μPACs
-
- micro-PA channels
-
- AFM
-
- atomic force microscopy
-
- 3D
-
- three-dimensional
-
- Bcl-2
-
- B-cell leukemia/lymphoma 2
-
- JNK
-
- c-Jun N-terminal kinase
-
- TFM
-
- traction force microscopy
-
- DRIE
-
- deep reactive ion etching
-
- PDMS
-
- polydimethylsiloxane
-
- RCP
-
- ruthenium-catalyzed photocrosslinking
-
- AMR
-
- active microrheology
-
- vECM
-
- vascular extracellular matrices
-
- ELP
-
- elastin-like protein
-
- RGD
-
- arginine-glycine-aspartate
-
- MCTSs
-
- multicellular tumor spheroids
-
- MGTD
-
- multicellular geometry-based tumor cell detection
-
- EBB
-
- extrusion-based bioprinting
-
- DBB
-
- droplet-based bioprinting
-
- LBB
-
- laser-based bioprinting
-
- IBB
-
- inkjet-based bioprinting
-
- PSI
-
- plexin-semaphorin-integrin
-
- EGF
-
- epidermal growth factor
-
- FAs
-
- focal adhesions
-
- Cav
-
- caveolin
-
- ROR1
-
- receptor tyrosine kinase like orphan receptor
-
- BFCOL1
-
- binding factor of a type-I collagen promoter
-
- GTPase
-
- guanosine triphosphatase
-
- GRAF1
-
- GTPase regulator associated with FAK-1
-
- PICK1
-
- protein interacting with protein kinase c alpha type-I
-
- TRP
-
- transient receptor potential
-
- P130CAS
-
- p130 CRK-associated substrate
-
- PAKs
-
- p21-activated protein kinases
-
- RAP1
-
- Ras-related protein1
-
- RIAM
-
- Rap1-GTP-interacting adaptor molecule
-
- F-actin
-
- filamentous actin
-
- G-actin
-
- globular actin
-
- TEAD
-
- TEA/ATTS domain
-
- ROCK
-
- Rho-associated protein kinase
-
- LIMK
-
- LIM kinases
-
- MLC2
-
- myosin regulatory light chain 2
-
- Rho-GEFs
-
- Rho-specific Guanine nucleotide exchange factors
-
- Rho-GAPs
-
- RhoGTPase-activating proteins
-
- Merlin
-
- moesin-ezrin-radixin-like protein
-
- FRMD
-
- FERM domain-containing protein
-
- MST
-
- mammalian STE20-like kinase
-
- SAV1
-
- salvador homolog-1
-
- LATS1/2
-
- large tumor suppressor homologs 1 and 2
-
- NF2
-
- neurofibromine 2
-
- GPCRs
-
- G-coupled receptors
-
- Shh
-
- Sonic hedgehog
-
- LINC
-
- Linker of nucleoskeleton and cytoskeleton
-
- KASH
-
- Klarischt/ANC-1/SYNE homology
-
- FHOD1
-
- Formin homology 2 domain containing 1
-
- HDAC
-
- histone deacetylase
-
- EPHA2
-
- EPH receptor A2
-
- LOXL2
-
- lysyl oxidase like 2
-
- CRAD
-
- capping protein inhibiting regulator of actin dynamics
-
- OS
-
- overall survival
-
- IFNγ
-
- interferon-γ
-
- cGAMP
-
- cyclic GMP-AMP
-
- ADRs
-
- adverse drug reactions
1 BACKGROUND
As the “Sanctuary of the devil”, the TME provides physical support for tumor cells to adhere and absorb nutrients, and escape from the immune system, which facilitates the emergence and development of tumors. The TME is mainly composed of three parts: the cellular components [1] (stromal cells [2], fibroblasts [3, 4], immune cells, pericytes [5], etc), the extracellular matrix (ECM) [5], and the vasculature (blood vessels and lymphatic vessels) [6]. Previous studies of the TME mostly focused on its biochemical cues, such as hypoxia, low pH, inflammation and immunosuppressive properties, but little on mechanical cues. In tumor tissues, the processes of growth and metastasis are tightly regulated by mechanical factors in the TME [7], such as solid stress, shear stress, increased matrix stiffness and topological changes in the ECM; conversely mechanical factors also affect the TME, for example, residual solid stress could squeeze the blood vessels and lymphatic vessels, resulting in the loss of function of blood vessels or lymphatic vessels, thus affecting the metabolic microenvironment [8].
Cells in the TME are subjected to external and internal forces, and in response to these mechanical forces, they experience mechanosensing and mechanotransduction [9, 10], which play important roles in regulating cell adhesion [11], morphology [12], motility, proliferation, differentiation, and migration [13]. Many studies focused on the cellular proteins involved in mechanosensing, such as integrins, focal adhesions (FAs), and their associated molecular mechanisms (e.g., cytoskeleton remodeling, integrin signaling, Rho signaling and Hippo signaling) [12]. Numerous mechanotransduction pathways converge in the nucleus, regulating momentous nuclear events such as DNA replication, transcription and cell cycle progression [14]. Thus, we devoted special attention to nuclear mechanotransduction. Based on this, a large number of in vitro models of the TME were constructed to further study the interaction between tumor cells and the ECM as well as the underlying mechanotransduction mechanisms. These changes in physical factors, such as increased matrix stiffness, compress the interstitial matrix, cause metabolic abnormalities and high interstitial pressure, and even increase the difficulty of drug delivery. In addition, due to the unique mechanical sensitivity of individual cell types that can promote pathological progression, mechano-based therapies for the TME mechanical factors represent an emerging clinical strategy [11].
In this review, we discussed the role of mechanical cues in the TME in influencing tumor cell behaviors as well as in vitro tumor mechanical microenvironment models and biomimetic technologies to provide targets for targeted mechano-based therapies, and assess the effectiveness of existing drugs and clinical trials. In addition, we presented the functions and regulatory mechanisms of cancer cell mechanosensing and mechanotransduction pathways. Finally, we proposed several current challenges and future perspectives in this field. We believe that mechanomedicine will become a new clinical treatment strategy for cancer.
2 TUMOR MICROENVIRONMENT (TME)
2.1 Tumor biochemical microenvironment
Biochemical and mechanical cues together constitute the dynamic and heterogeneous TME (Figure 1). The TME has many hallmarks that differ from the normal tissue microenvironment, thus influencing the “lifetime” of the tumor (Table 1). Biochemical signals, as the most well-known regulators, are involved in the regulation of tumor occurrence, development, and metastasis [15].
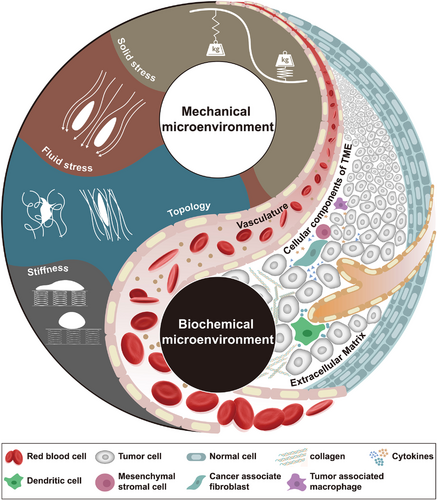
Properties of the TME. As a comprehensive environment, we suggest that the TME is mainly divided into two parts: (i) the biochemical microenvironment and (ii) the mechanical microenvironment. Due to the changes in biochemical components in the TME, the physical properties are dramatically changed. Uncontrolled proliferation of tumor cells constantly compresses adjacent tissues and tumor vasculature, generating internal high-pressure solid stress and further resulting in increased blood/interstitial pressure. The imbalance between pro- and antiangiogenic factors causes abnormal tumor vasculature that generates much pressure on the TME: this pressure is called fluid stress. Alterations in the composition and density of the ECM occur frequently in tumors. CAFs are constantly remodeling (by depositing or cross-linking) the ECM to cope with mechanical stress in the TME, leading to increased stiffness and altered topology. Stiffness (rigidity) refers to the resistance to deformation in response to applied force, which is measured as Young's elastic modulus. Topology (architecture) comprises ECM porosity, fibril orientation and other fibril characteristics, which wield important influences on cell polarity and function. The subtle and mutualistic relationship between tumor cells and their TME properties is formed early in solid tumor growth and evolves throughout the complete life cycle. Abbreviations: TME, tumor microenvironment; CAFs, cancer-associated fibroblasts; ECM, extracellular matrix
| Hallmarks | Mechanism | Main contributor | Consequence | Reference |
|---|---|---|---|---|
| Biochemical TME | ||||
| Hypoxia | The imbalance between increased oxygen consumption and insufficient oxygen supply | HIF-1α |
Tumor cell necrosis Drug resistance DNA instability |
[248] [19] |
| Low pH | The elevated interstitial fluid pressure, hypoxia, low glucose and high lactate concentration resulting from a predominantly anaerobic metabolism |
Glycolytic enzymes PDK1 PKM2 |
Enhanced tumor cell invasion DNA instability Radiation and drug resistance Immune escape |
[249] [250] |
| Inflammation | The production of inflammatory mediators |
NF-κB STAT3 HIF-1α |
Tumor angiogenesis Enhanced tumor cell proliferation Tumor apoptosis |
[131] [13] |
| Mechanical TME | ||||
| Solid stress | Cell proliferation, cell contraction, matrix deposition, and abnormal growth patterns |
Collagen MMPs Actomyosin |
Difficult drug delivery Enhanced tumor cell migration Mitosis suppression Tumor angiogenesis |
[34] [39] |
| Fluid stress | Abnormal/compressed vessels or nonfunctional lymphatics (fluid pressure), the velocity gradient of fluid flow and viscosity of blood (shear stress) |
VEGF Glycocalyx E-cadherin |
Tumor angiogenesis Enhanced tumor cell migration Cell cycle arrest Difficult drug delivery Cell intravasation |
[35] [42] |
| Stiffness | Matrix deposition and cross-linking |
Collagen MMPs |
Tumor invasion and metastasis Enhanced immune cell infiltration DNA methylation Directional cell migration Differentiation of tumor stem cells |
[4] [251] |
| Topology | Matrix deposition and cross-linking, and cell contraction |
Collagen MMPs Actomyosin |
Tumor invasion and metastasis DNA instability Directional cell migration Differentiation of tumor stem cells |
[58] [60] |
- Abbreviations: TME, tumor microenvironment; HIF-1α, hypoxia induced factor-1α; PDK1, pyruvate dehydrogenase kinase 1; PKM2, pyruvate kinase muscle isoform 2; NF-κB, nuclear factor -κB; STAT3, signal transducers and activators of transcription 3; MMPs, matrix metalloproteinases; VEGF, endothelial growth factor.
2.1.1 Cells and “products” in the TME
In addition to tumor cells, there are other tumor-associated stromal cells in the TME that can promote ECM remodeling, cell migration and drug resistance by producing growth factors and cytokines, mainly mesenchymal stromal cells, cancer-associated fibroblasts (CAFs), immune cells, tumor-associated endothelial cells, pericytes and even adipocytes. It has been proven that mesenchymal stromal cells and CAFs can promote solid tumor growth and metastasis by secreting soluble factors in different systems, such as connective tissue growth factor (CTGF) and hepatocyte growth factor (HGF) [2]. In addition, CAFs also promote desmoplasia and ECM remodeling by stimulating the secretion of various ECM proteins (e.g., hyaluronic acid), which suggests that CAFs could be potential targets for tumor therapies. Inflammation is a momentous determining component of tumor fate, and many cancers originating from sites of chronic inflammation can form an inflammatory TME [13]. Immune cells such as T cells, tumor-associated macrophages (TAMs) and natural killer cells (NKs) serve essential roles in solid tumorigenesis through a series of inflammatory reactions. They fuel the tumorigenic process by releasing cytokines or reactive oxygen species (ROS) [16], proangiogenic factors and extracellular proteases, in addition to killing tumor cells by activating interleukin (IL)-2, interferon, and IL-12. Clinical evidence indicates that the presence of NKs and NKTs in the TME predicts a relatively good prognosis for solid tumors [17].
2.1.2 Abnormal vasculature in the TME
To obtain nutrition and invade further distant organs, tumor cells always require the formation of new blood vessels, which are mainly generated in two ways: expansion from the existing vascular bed or the recruitment of stem cells from bone marrow. Unlike normal blood vessels, the tumor vascular system exhibits atypical morphology, including leaky, tortuous, saccular, and dilated characteristics [18]. The immaturity of this new vasculature leads to insufficient permeability, while the solid stress generated by tumor cell proliferation leads to further inadequate perfusion and increased hypoxia. As a typical feature of the tumor biochemical microenvironment, hypoxia arises because of the imbalance between increased oxygen consumption and insufficient oxygen supply, and its effects on tumor cells principally occur via the hypoxia-induced factor (HIF) family [19]. The “Warburg effect” indicates that tumor cells are able to absorb a large amount of glucose and produce high amounts of lactate via glycolysis, even in the presence of oxygen, thus causing TME acidification (another characteristic of the tumor biochemical microenvironment) [20]. Tumor angiogenesis also causes a low concentration of antitumor drugs and inhibits the infiltration of immune cells into the TME, resulting in immune escape, drug resistance and metastasis. Furthermore, surrounding the tumor vasculature, pericytes show low density and abnormal morphology, causing tumor hemorrhage and vessel wall instability [5], which makes it easier for various molecules in the TME to enter these blood vessels and further damage not only the blood flow but also the lymphatic flow. All of these abnormal situations increase the hydrostatic pressure outside the vasculature and drive TME hypoxia and acidosis which enormously increases the difficulty of clinical therapy of tumors.
2.1.3 The shelter (extracellular matrix) in the TME
The ECM is a highly dynamic but ordered meshwork encasing various cells and noncell components that are responsible for cell-cell and cell-matrix communication. Composed of water, growth factors, minerals, proteoglycans, and four major fibrous proteins (collagen, proteoglycans, laminin, and fibronectin) [21], the ECM is considered to be a key regulator of tumor growth and metastasis. Collagen, the most abundant and basic protein component of the ECM, has a unique triple helical structure that is composed of three collagenous peptide strands with typical Gly-X-Y motifs (here, X and Y are usually either proline or hydroxyproline) and maintains the structural stability of the ECM [22, 23]. Cells interact with collagen through their surface collagen receptors, including integrins and nonintegrin collagen receptors. The major functional motif GFOGER (O = 4-hydroxyproline) sequence in collagen is recognized by integrin αI domains [24]. The specific recognition between integrins and collagen enables cells to respond to the surrounding TME, triggering distinct intracellular signals to regulate their behavior. The nonintegrin collagen receptors mainly include discoidin domain receptors (DDRs), leukocyte receptor complexes and mannose receptors. Tyrosine kinase receptors DDR1 and DDR2 are activated by the triple helix of collagens I, II and III, followed by tyrosine autophosphorylation and signal transduction [25]. The content and distribution of the collagen network can be modified by tumor cells through changes in gene expression, signal transduction and receptor-ligand interactions [26].
As the essential constituents and functional modifiers of the ECM, proteoglycans are composed of one or more covalently bonded carbohydrate chains of negatively charged glycosaminoglycans (GAGs) that could be heparan sulfate (HS), chondroitin sulfate (CS), dermatan sulfate (DS) and hyaluronic acid (HA) [27]. Due to these structures, proteoglycans are able to interact with various ECM components and matrix-associated proteins. Specifically, proteoglycans influence ECM properties and cell growth by their GAG chains or their core proteins [28]. For example, GAG sulfation patterns often act as recognition motifs for many growth factors, chemokines and cytokines in the TME to regulate the properties of the ECM [29]. In addition, proteoglycans serve as coreceptors for promoting cell signal transduction and are involved in cell proliferation, migration and invasion [30]. Two additional major ECM components, laminin and fibronectin, act as a bridge to connect the extracellular space to cells. Laminins are trimeric glycoproteins composed of α, β, and γ chains that can establish a connection between cells and ECM by binding cell surface receptors, such as the dystroglycan and integrin families [31]. Fibronectin contains some important binding motifs that can be recognized by those cell surface receptors, which further facilitate the interaction between cells and ECM, mediating cell adhesion, differentiation and migration [32]. All of these ECM components create a complex network that fosters interactions between the components and the surroundings. Taken together, the ECM not only provides a shelter for tumor growth but also provides more sophisticated biochemical and biomechanical regulation for tumor cells.
2.2 Tumor mechanical microenvironment
In recent years, decades of studies have led to a focus on how mechanical cues in the TME regulate tumor cell morphology, behaviors and malignant evolution. As a critical component of the TME, the ECM is a complex system full of interlinked cell-scale fibrils and various macromolecules, which confers it with not only biochemical but also physical properties [33]. While providing physical support for the surrounding cells, the physical properties of the ECM, such as stiffness, topology, fluid stress and tumor cell solid stress, serve pivotal roles in many biological processes through ECM function and synergistic interaction with other components (Figure 1).
2.2.1 Solid stress
In the TME, tumor cells are the objects of mechanical cues as well as the producers of mechanical cues. As one of the most important mechanical cues of the TME, solid stress is derived from the structural components of the tumor, which can be classified into two parts [34, 35]. One part, known as cell proliferation-induced stress or residual stress, generated by the microscopic interactions among the structural components in the TME, remains in the tumor tissue even after the tumor has been separated from the surrounding tissues [36]. The other is described as externally applied stress from the host tissue to inhibit tumor expansion; in contrast to the former, it can be sharply diminished after the tumor tissue is removed. To date, many studies have proven that solid stress in the TME could result in detrimental phenomena: the compression and deformation of blood and lymphatic vessels, the elevation of fluid pressure, and even the remodeling of the ECM [37, 38]. In clinical pathology, the accumulation of solid stress leads to different consequences; in the brain, solid stress causes neuronal loss and neurological dysfunction [39]. In the breast, solid stress can also induce cell migration and invasion [40, 41].
2.2.2 Fluid stress
The role of biochemical cues in tumor angiogenesis has been fully studied, while little is known about the mechanical cues of the vascular system surrounding tumor cells. Recently, numerous studies have made it clear that abnormal tumor vasculature could generate much pressure on the TME, which is collectively referred to as fluid stress. As a general rule, fluid stress is applied by the blood and interstitial flow, which contains the microvascular fluid pressure, interstitial fluid pressure and shear stress [35]. Leaky and deformed characteristics of tumor vasculature cause the elevation of viscous and geometric resistance to blood flow, and the inadequate perfusion and insufficient supply of nutrients or oxygen [42], which also elevate the interstitial fluid pressure from near-zero in most normal tissue to 60 mmHg in neoplastic regions, and even as high as 130 mmHg in mouse pancreatic ductal adenocarcinomas [43]. Due to the elevated interstitial fluid pressure in the central tumor regions, interstitial fluid flows from the center to the periphery and conveys proangiogenic factors, such as vascular endothelial growth factor (VEGF), to promote tumor hemangiogenesis. These proangiogenic factors also lead to increased lymph node metastasis by promoting lymphangiogenesis [44]. Moreover, interstitial fluid pressure is reportedly correlated with the response to treatment or prognosis in various tumors, including cervical cancer, lymphoma, melanoma and lung cancer [45]. As a tangential stress exerted by the bloodstream on the vascular endothelial surface, shear stress is sensed by endothelial cells and determined by blood viscosity and shear rate [46]. Tumor cells are primarily subjected to shear stress in the process of metastasis to distant organs. During tumor cell metastasis, shear stress plays roles in tumor cell adhesion, motility [47] and invasion [48] through some important mechanotransduction pathways. Conversely, shear stress also eliminates circulating tumor cells (CTCs) [48]. It was reported that shear stress promotes liver cancer stem cell migration through the activation of the focal adhesion kinase (FAK) and extracellular signal-regulated kinase 1/2 (ERK1/2) signaling pathways [49] and induces autophagy to promote the migration and invasion of HepG2 cells via integrin/cytoskeleton pathways [47]. In addition, shear stress in the lymphatics has also been demonstrated to activate YAP1 (Yes-associated protein 1), and consequently drive cancer cell migration [48].
2.2.3 Stiffness
ECM stiffness, also known as ECM rigidity or elastic modulus, is an intrinsic characteristic of the TME. Different from solid or fluid mechanical stress, stiffness varies greatly in different tissues: several studies have shown that stiffness ranges from 1 kPa in brain tumors to 70 kPa in cholangiocarcinomas [35]. The heterogeneity of tumor ECM stiffness is also showed in different stages of tumor progression. One of the primary causes of matrix stiffening is excessive deposition and cross-linking of ECM proteins when the balance between matrix production and degradation is disturbed [50]. In addition, CAFs are the most important producers, with more actin stress fibers and FAs than nonactivated fibroblasts in the TME [51]. In addition to the quantity of fibers, the degree of cross-linking also determines the stiffness of ECM. The greater the cross-linking is, the stiffer the ECM becomes. For example, a high level of lysyl oxidase (LOX), an amine oxidase, leads to a higher degree of cross-linking and greater stiffness of the ECM [52, 53].
Stretching through cells, contraction and local expansion is the other cause of ECM stiffening [54]. These stresses increase the rigidity of the collagen network and can activate the focal adhesion contractility of nearby CAFs [55], leading to a vicious cycle of matrix deposition and stiffening. In addition, transforming growth factor-β (TGF-β) signaling increases the synthesis and deposition of ECM proteins by CAFs, triggering a feedback loop that stiffens the matrix [56]. Rigid ECM in turn promotes the malignant behaviors of tumor cells, such as tumor cell proliferation, migration, invasion, epithelial-mesenchymal transition (EMT), and drug resistance, which are also associated with increased tumor metastasis and poor clinical outcomes.
2.2.4 Topology
Structural remodeling of fibril was observed recurrently in the progression of breast cancer. During the period of ductal carcinoma in situ, epithelial cells proliferate irregularly, and collagen is cross-linked and arranged in bundles parallel to the tumor boundary. During the period of invasive ductal carcinoma, the epithelial cells fill almost the entire lumen, collagen undergoes further cross-linking and is arranged perpendicular to the tumor tissue boundary to provide a migration path for tumor cells [57], and the altered microarchitecture worsens the situation. Topology, also known as matrix architecture, is formed from the tension of tumor cells, excessive proliferation of tumor cells, and matrix remodeling including porosity, fibril orientation and other fibril characteristics. This matrix architecture alters matrix production and degradation [58] as well as yielding irregular accumulation of proteins (for example, collagen fibrils with diameters between 20-200 nm can form microscale collagen fibril structures with different hierarchies [59]) that can change the cell-matrix and cell-cell connections, mediating morphogenesis. Previous studies have demonstrated that the height, lateral spacing and diameter of nanoscale features in the ECM affect cell adhesion, and topological features of ECM such as geometry and dimension may cause changes in cellular morphology, alignment and even differentiation [60]. The nano to microscale architecture of collagen fibrils can affect cell polarity and promote migration by providing contact guidance clues [61]. Even more interesting is an observation suggesting that the height of nanotopography could modulate the nuclear size, thus regulating cell phenotype and function [62].
3 IN VITRO CONSTRUCTION OF THE TUMOR MECHANICAL MICROENVIRONMENT
3.1 Tumor mechanical microenvironment models
Over the past decades, many experimental data have supported the importance of mechanical cues during tumor growth and progression. However, the specific molecular mechanisms of tumor initiation and progression induced by mechanical forces are still not well understood. Therefore, it is necessary to develop and establish appropriate mechanical models to investigate cancer cell sensing, mechanobiology, drug response and metastasis behaviors [63], as summarized in Figure 2 and discussed below.
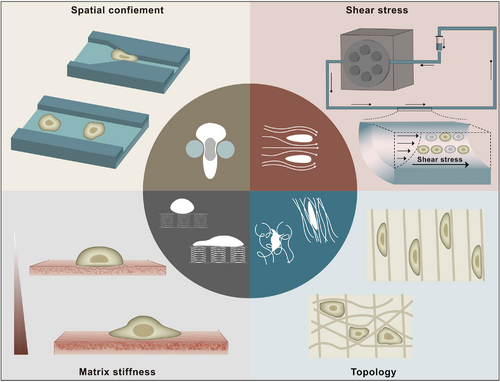
In vitro models of the TME. Eager to explore the specific interactions between mechanical cues and tumorigenesis in the TME, several in vitro research models have emerged as the times require, and we mainly summarize them into four categories:(i) confinement models, (ii) shear stress models, (iii) stiffness models, and (iv) topology models. Confinement models are used to simulate in vivo ductal structures and microchannels and provide confinement paths for cell invasion and migration. When entering the vascular system, CTCs become suspended cells and remain in blood vessels, where they experience considerable fluid shear stress. Considered to be the most common and recognized mechanical abnormality of the TME, increased tissue stiffness has widely been used as a diagnostic marker and prognostic factor. Specific topology formed by component changes and special arrangements in the TME are involved in cell morphogenesis, cell polarity and cell function. Abbreviations: TME, tumor microenvironment; CTCs, circulating tumor cells
3.1.1 Spatial confinement-cell interaction models
In native tumor tissues, there are many ductal structures formed by ECM remodeling, providing confinement for cell invasion and migration in the process of tumor metastasis [64]. To investigate the effects of ECM confinement on tumor cell migration, many strategies have been applied such as microchannels [65], parallel plates [66] and grooved substrates [67]. Here, we mainly discuss the most widely used microchannels. Several years ago, a microscale culture platform to study the effects of ECM stiffness and confinement on tumor cell migration attracted extensive attention. A team used photolithography techniques and tunable polyacrylamide (PA) hydrogel formulations to create micro-PA channels (μPACs) with specified stiffnesses and microchannel widths, whose stiffnesses ranged from 0.4 to 120 kPa and microchannel widths ranged from 10 to 40 μm. They used atomic force microscopy (AFM) to measure substrate stiffness, and observed that the enhanced cell traction polarization makes cells in narrow channels migrate faster than in wider channels and abrogates the dependence on substrate stiffness [67]. To mimic three-dimensional (3D) cell motility in tissues, Patteson et al. [68] designed microfluidic devices with channels of appropriate dimensions. These channels are wide enough to allow the cells to pass and narrow enough to constrict the vimentin network. To probe the cells' mechanical response in the channels, they performed AFM to assess the stiffness of the cell endoplasmic region and the perinuclear region, and found that the loss of vimentin decreases the cell stiffness and promotes 3D cell migration in small confining spaces.
In addition to the ductal structures formed by ECM remodeling, the microchannels with diameter less than 10 μm are also found in natural tissues such as in perineural tissues, providing spatial confinement for cell migration during tumor metastasis [69]. A hydrogel-based microchannel platform with tunable ECM stiffness (0.3-20 kPa) and tunable channel width (3-11 μm) was developed to investigate the type and the speed of cancer cell migration by combining the photolithography technique with collagen-alginate hydrogel. With this platform, the authors showed that the shift of movement modes between mesenchymal is strongly correlated with ECM stiffness, while the migration speed of the cancer cells is regulated synergistically by both the width and stiffness of the channels [64]. Techniques for simulating spatial constraints and physical environments in cell culture could provide powerful tools for studying tumor cell migration and the interaction between cancer cells and physical surroundings.
Since the nucleus is far more rigid than other structures in the cell, it is the main limitation factor for cell migration through tiny gaps. To study the relationship between channel size and nuclear passability, a microfluidic device consists of many microchannels whose channels are 150 μm in length and 5 μm in height and are arranged in groups with widths of 2 to 20 μm was manufactured. This study demonstrated that the increasing confinement of microchannels decreases the ability of cells to pass through small gaps, and that the cells cannot squeeze through tiny gaps below a threshold width [70]. With the advent of real-time imaging of cell and nuclear morphology during translocation, this microfluidic device is suitable for studying the adaptability of cells in a confined environment.
3.1.2 Shear stress-cell interaction models
After leaving the microenvironment of the primary tumor and entering the vascular system, CTCs become suspended cells and remain in the blood vessels, where they are easily affected by various factors in the blood circulation [71] and experience substantial fluid shear stress. Therefore, it is very important to reveal the survival mechanism of tumor cells in the blood circulation for the prevention of tumorigenesis and metastasis, and several in vitro models have been developed for this purpose. Typically, an in vitro circulation system consisting of a peristaltic pump, a silicone microtube and a syringe has been designed to mimic fluid shear stress in vivo by producing pulsating flow, which corresponds with shear stress in venous and arterial circulation of 0.5-4 dynes/cm2 and 4-30 dynes/cm2, respectively. With duration of CTCs in the vascular system of less than 12 h, it was found that fluid shear stress in blood circulation is able to eliminate most of the suspended CTCs [72]. However, low fluid shear stress also promotes the survival of remaining suspended CTCs through Puma and B-cell leukemia/lymphoma 2 (Bcl-2) by inducing c-Jun N-terminal kinase (JNK)-mediated EMT [73].
In addition to the plasma membrane, the nuclear lamina is a pivotal part of tumor cells with a certain mechanical strength, playing an important part in tumor progression and metastasis. Therefore, to determine whether lamin A/C contribute to CTCs against fluid shear stress in blood circulation, a device resembling a microscale conduit was developed that can be set at a flow rate of 14 mL/min. Its corresponding wall shear stress is calculated to be 5,920 dynes/cm2, and the fluid shear stress exposure time is strictly controlled at 1.08 ms to elevate fluid flow pulses to simulate the flow environment near the walls of large blood vessels. The efforts demonstrate that lamin A/C are key structural components of CTCs that could resist fluid shear stress-mediated death in the blood circulation and promote the survival and hematogenous metastasis of CTCs [74].
3.1.3 Matrix stiffness-cell interaction models
A tremendous amount of clinical data has shown that tumor growth is closely related to ECM stiffness [75]. In breast cancer, the increase in stiffness is one of the most important indicators for the clinical detection of breast cancer, but the mechanisms of the correlation between tissue stiffness and tumorigenesis are not fully clear. To investigate the effects of ECM stiffness on tumorigenesis as well as its specific molecular mechanisms, some models of matrix stiffness-cell interaction have been well established in vitro. For example, a bionic 3D Matrigel culture system was developed, in which the elastic modulus ranged from ∼150 Pa in normal breast tissue to ∼5700 Pa in breast tumor tissue, and it was found that the increasing matrix stiffness of ECM directly promoted EMT, invasion and metastasis by inducing the transcription factor Twist1 [76]. Because the degree of cross-linking and the density of collagen are the most important factors regulating ECM stiffness, an in vitro model in which collagen stiffness can be modulated was established. In this model, we can determine the effects of cross-linked collagen and increased matrix stiffness on angiogenesis and vascular growth in tumors by increasing the density of collagen gels from 1.5 to 10 mg/mL or within a given density, and increasing the extent of glycation from 0 to 100 mmol/L (the elastic modulus of the gels increases from 180 to 1,200 Pa) [77]. Almost all studies have shown that the stiffness of the ECM plays a significant role in promoting angiogenesis and tumor-like angiogenesis; even worse, it may impair therapeutic delivery and efficacy [77].
To reveal the regulatory role of mechanical cues in the matrix stiffness-mediated tumor progression, some new technologies have been applied. Since cells interact with substrates in vitro by exerting and sensing forces, traction force microscopy (TFM), magnetic tweezers and micropillars are fundamental tools for probing the mechanical forces between cell-substrate interactions [78]. Kourouklis et al. [79] combined cell microarrays on tunable stiffness substrates with TFM to assess the cell-generated traction stress and the cell phenotype in response to substrate cues. They found that cholangiocyte differentiation was regulated by matrix proteins and stiffness properties, and revealed the roles of ERK and Rho-associated protein kinase (ROCK) during the differentiation process. In another study, Tian et al. [80] mimicked the matrix stiffness of different tissues from soft brain to stiff bone using PA substrates and probed the mechanical responses of breast cancer cells against diverse stiffnesses using magnetic tweezers and advanced imaging techniques. To overcome the inherent limitations of a continuous elastic substrate, substrates consisting of micropillars were developed. This method has been widely used in measuring cell-generated forces as well as analyzing the interaction between matrix stiffness and cellular responses [81]. By using photolithographic [82] or deep reactive ion etching (DRIE) techniques to mold polydimethylsiloxane (PDMS) [83], adjustable micropatterned pillar array substrates can be obtained. The forces on the micropillar can be inferred directly from its displacement; for instance, the microforce sensing micropillars measure tens of nanometers of deflection and convert it to the actual force value at the nanonewton level [81].
3.1.4 Surface topology-cell interaction models
Some of the main steps in desmoplasia are cross-linking of collagens, fibril realignment and fibril elongation, which are closely related to poor survival in cancer patients [84]. Cancer cells respond to these topological cues in the TME by “contact guidance”, which means that through restructuring the cytoskeleton and various signal transduction pathways, the mechanical signals are translated into biochemical signals, further altering gene expression.
To explore the impact of fibril arrangement on tumor cells, an electrospun fibrous scaffolds with similar dimensions and different orientations (aligned and random fibrils) was created to mimic the 3D structure of the natural ECM random fibrils by a stationary collector (a part of the electrospun apparatus), and the well-oriented fibrils was obtained by a rotated collecting mandrel. It was found that the cytoskeleton and nucleus align along the fibril axes and that transforming growth factor beta 1 (TGF-β1) is upregulated in the cells cultured on aligned fibrils: these cells also underwent EMT in response to aligned fibril in the polymer scaffold [85]. Besides, Jagiello et al. [86] presented a 3D fibrous hydrogel with tunable local stiffness and fibril anisotropy to investigate the response of cancer cells to mechanical cues by using a cell-safe method of patterned photocrosslinking which is termed ruthenium-catalyzed photocrosslinking (RCP), and assessed the relationships between fibril alignment and stiffness by using multi-axes optical tweezers active microrheology (AMR) [87].
In addition, native vascular extracellular matrices (vECM) are made up of elastic fibrils with various topographical properties. To simulate these characteristics of the vECM and study the mechanism of the resulting effects on cell behavior, Mascharak et al. [88] engineered an electrospun elastin-like protein (ELP) system with independent tunability. The ELP polymer produced through this system contains multiple repeats of an elastin derived structural sequence, and the arginine-glycine-aspartate (RGD) ligand disperses in its elastin-like amino acid sequence, which enables cell-ECM interactions. The fibril width ranges from 0.8 to 2.0 μm and can be tuned by the ELP mass fraction in the electrospinning solution. The primary conclusions are that the increased topographical variation results in the loss of endothelial cell-cell junction organization, and the use of topology as a design parameter for implantable biomaterials to investigate cell migration and invasion was supported [88].
3.2 Bionic technology of 3D tumor microenvironment
Without hesitation, 2D in vitro models can simulate the TME to a certain extent. However, they only represent a simplified version of in vivo conditions, and many physiological questions remain unaddressed. Therefore, 3D reconstruction of the complex microenvironment is of great significance in modern tumor biology. Some approaches have been created for 3D modeling of the TME, mainly including spheroid cultures, cancer-on-a-chip platforms and 3D bioprinting.
3.2.1 Spheroid cultures
For in vitro 3D cell culture models, multicellular tumor spheroids (MCTSs) formed by the accumulation of multiple cells are one of the simplest but most effective methods. The methods of 3D cell spheroid formation mainly include hanging-drop approaches [89, 90], spinner flask approaches, micropatterned plates and magnetic levitation approaches [91]. Recently, an interesting work involving a rapid and efficient method for the formation of 3D cell culture spheroids was described [92]. Based on the phenomenon of interfacial reactions between two immiscible liquid solutions with different densities and surface tensions, spherical wells in elastomeric PDMS can be formed and adjusted by the density and surface tension of the dropped solutions. Specifically, conventional Petri dishes were covered by a certain amount of PDMS. Before solidification, various volumes of liquid droplets with different concentrations were added into PDMS to adjust the pore size and curvature to form spheroids. Different shapes, such as ellipsoidal, spherical, and sediment shapes, can be obtained by different solutions, such as ethanol, water, and ethylene glycol. To reflect the complexity of the TME, 3D tumor spheroids were formed by coculturing human kidney carcinoma cells (A498) and fibroblasts (NIH/3T3) at a variety of ratios after PDMS solidification, and single tumor spheroids were successfully formed with high efficiency (up to 97%) in each well. The results of in situ drug testing and monitoring of ROS levels were performed using 3D tumor spheroids located in PDMS wells, demonstrating that this platform can be used to study the responses of tumor cells to drugs. Furthermore, the system can also be used as a general tool for in situ drug screening of cancer and is easily implemented in practical applications [92]. In addition, Li et al. [63] successfully constructed a mechanical cue-based simultaneous 2D and 3D multicellular culture system. Different from hydrogel-based cell cultures, insert scaffold-based cellular cultures and 3D non-scaffold-based cellular aggregates, this 3D spheroids arrays showed the various multicellular architecture which could be used for cell type classification. This novel system would be useful in manufacturing of 3D spheroids, bridging the gap between 2D and 3D cellular studies, multicellular geometry-based tumor cell detection (MGTD) and tumor microenvironment reconstruction with heterogeneous cell types.
3.2.2 Cancer-on-a-chip platforms
Tumor cells do not reside in the void microenvironment, but exist in complex extracellular matrix scaffolds. Compared to spheroid cultures, the construction of multiaperture scaffolds offers much stronger points, such as the realization of a tumor vasculature system in vitro, minimal sample requirements and high throughput. The cancer-on-a-chip consists of some basic components: a microfluidic chip, matrix material, flow control equipment and cell components [93]. To date, cancer-on-a-chip technology has made remarkable contributions to tumor biology, including cancer motility, EMT, cancer metastasis and drug screening. To this end, several approaches have been proposed, mainly including soft lithography and microfluidic devices.
3.2.2.1 Soft lithography
Since 1988, soft lithography has been improved to nanocontact printing, which enables the fabrication of nanostructures of scaffolds. To obtain the lithographically made master mold, the first step is soft lithography. Then, the master mold is filled with PDMS precursor and degassed in vacuum to remove the remaining bubbles since PDMS has low toxicity and biocompatibility. Finally, the PDMS precursor solution is cured by baking to cross-link it with the sample [94]. Lee et al. [95] developed a 7-channel microchannel plate that was prepared using PDMS by soft lithography to illustrate that it can serve as a useful model for studying drug resistance and EMT.
3.2.2.2 Microfluidic devices
With the foundation of soft lithography technology and microfluidic chips, cancer-on-a-chip models have attracted a large amount of interest in the construction of biological scaffolds. However, there are many types of cancer-on-a-chips, including lumen chips, membrane chips, Y chips and membrane chips. These microfluidic devices are able to control the generation of chemical gradients via two channels with inlets: one provides the target chemical channel, and the other provides a buffer, while tumor cells can be seeded in the midzone [96]. Based on this technique, Acosta et al. [97] created a compartmentalized chip with a collagen ECM and a vessel-mimicking channel, and Gokce et al. [98] also proposed a 3D tri-culture in lab-on-a-chip devices for drug screening and testing. MDA-MB-231 carcinoma cells were seeded on a lumen chip, and the cell aggregates could be affected by the interstitial flow pressure gradient [99].
3.2.3 3D bioprinting
With the capability to accurately control the location and organization of intricate components in the TME, the use of 3D bioprinting technology to reconstruct the 3D TME, not only in cells but also in tissues and even organs, has gradually entered the field of vision. 3D bioprinting, also named rapid prototyping, is an additive manufacturing technique that is used to design 3D microenvironment structures layer-by-layer [100]. Materials (such as polymer hydrogels) used for 3D bioprinting are known as “bioinks”, which consist of living cells with or without biomaterials. So far, several techniques have been proven to realize 3D printing, including extrusion-based bioprinting (EBB), droplet-based bioprinting (DBB), and laser-based bioprinting (LBB).
3.2.3.1 EBB
EBB is a combination of a fluid distribution system and an automatic robotic system for extrusion and bioprinting, and the three most commonly used methods of EBB are pneumatic, piezoelectric, and screw-driven. Under the computer-controlled distribution system, the bioink is dispensed to precisely deposit those cells in the cylindrical filaments of desired 3D structures [101], a process that is more much biocompatible, with little resultant cell injury and damage compared to other techniques. Grolman et al. [102] created a coextrusion 3D bioprinting construction to study the connection between macrophages in the TME and MDA-MB-231 breast cancer cells.
3.2.3.2 DBB
DBB, comprising an inkjet, is a technology of acoustic-droplet-ejection and microvalve bioprinting with the capacity for better cell space repartition and cell density [103]. With different approaches and the same results, the DBB modality is based on the deposition of droplets under thermal, piezoelectric, acoustic waves or a solenoid pump to eject droplets [100]. An acoustic droplet printing method was introduced to construct a TME that consists of a tumor spheroid surrounded by CAFs, thus enabling exploration of the dynamic regulation of the interaction between tumor cells and CAFs with respect to tumor cell invasion. Furthermore, a droplet-based microfluidic device was presented to produce large-scale generation of tumor cell spheroids in a uniform way [104].
3.2.3.3 LBB
Like IBB, LBB produces 3D constructs or directly writes on the tissue by using a pulsed laser to deposit less viscous bioinks with the ability to create high-spatial resolution matrix patterning (more refined geometric features); however, it is expensive, complicated and non-trivial. Kingsley et al. [105] demonstrated that using a laser-based 3D bioprinting technique enables the development of spatial pattern size-controlled tumor spheroids.
4 TUMOR CELL SENSING AND MECHANOBIOLOGY
4.1 Mechanosensors
Mechanosensing, an approach for cells to sense mechanical cues in the TME, is vital for cells to react to these surrounding signals [106]. By activating mechanosensors on the surface of cells, such as integrins, FAs and caveolin-1(Cav-1), mechanical signals are sensed and transmitted into the cells to trigger a series of mechanotransductions [107] (Figure 3).
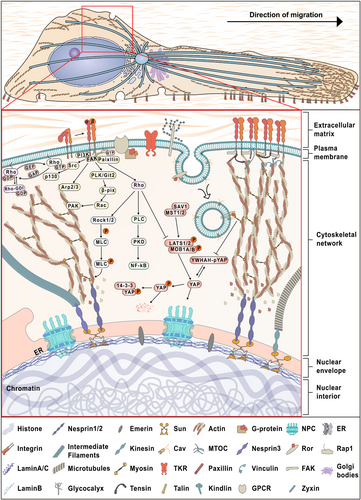
Polarized migrating tumor cells and related mechanotransduction pathways. As an iterative process, mechanotransduction touches upon multiple rounds of mechanosensing, transduction and response. Force-induced activation of mechanosensors, such as integrins, FAs and caveolin-1, transmits the force in the TME to intracellular locations by the anchored cytoskeleton (abundant studies have confirmed that it is directly linked to many mechanosensors) or other signaling pathways, such as integrin-FAK, Rho, and Hippo signaling. Physically connected with the internal cytoskeleton, the nucleus responds to mechanical factors in the TME throughLINC complexes and lamins, often in the form of changes in chromosomal reorganization and gene expression, thus promoting tumor cell migration. Abbreviations: FAs, focal adhesions; TME, tumor microenvironment; LINC, linker of nucleoskeleton and cytoskeleton; NPC, nuclear pore complex; MTOC, microtubule organizing center; TKR, tyrosine kinase receptor; FAK, focal adhesion kinase; GPCRs, G-coupled receptors; ER, endoplasmic reticulum
4.1.1 Integrins
Known as a bridge between the ECM and cell cytoskeleton, integrins not only serve as cell adhesion receptors to transmit biochemical cues to cells [108] but also respond to mechanical cues of the TME by altering their own protein conformation and function [109, 110]. Integrins are α and β heterodimers, in which 18 types of α-subunits and 8 types of β-subunits combine to form different integrin heterodimers through noncovalent interactions, including an extracellular ligand-binding head (with a β-propeller and thigh domains of the α-subunit and a βA domain, hybrid and plexin-semaphorin-integrin [PSI] domains of the β-subunit) and two multidomain legs (with calf1 and calf2 domains of the α-subunit and epidermal growth factor [EGF] repeats and the β-tail domain of the β-subunit) [111]. Among them, the binding site of ligands is between the propeller domain and βA domain. Several studies have shown that integrins exist in different ligand affinity states, including low, intermediate and high affinity [112], and mechanotransduction depends on the activation of integrins. One pathway is from inside by binding of proteins to the cytoplasmic tails, and the other is from the outside through binding of multivalent ligands [113].
Surrounded by the mechanical microenvironment, once force is applied, integrins are pulled open, triggering their activation and forming feedback between ligand-receptor binding dynamics and mechanical regulation of integrin conformation [114]. Specifically, any force applied to ECM fibrils enables them to pull on ligand-bound integrins. Integrins transmit those forces from the cell membrane to the actin cytoskeleton by binding with actin-binding adaptor proteins [115], such as vinculin, talin [116], zyxin and actinin, instead of binding with actin directly. Conversely, forces directly applied to actin, including those of actin polymerization and myosin contraction, can be transmitted to the ECM by actin-binding adaptor proteins and integrins [117]. As an important factor of the mechanical microenvironment, ECM stiffness is a passive mechanical parameter that cannot be directly sensed by cells. However, the stiffness can be detected by the deformation of ECM surrounding the cells via the interactions of actin cytoskeleton and integrins, since a given actomyosin contraction exerted by cells could cause different degrees of forces, which is determined by whether the surrounding matrix is soft or stiff [118, 119].
4.1.2 Focal adhesions
As complex cell membrane-associated macromolecular assemblies, FAs are highly dynamic structures that combine with ECM via integrin receptors and recruit generous FA-associated proteins to establish physical connections with the actin cytoskeleton [120]. Induced by biochemical or mechanical cues in the TME, FAs undergo maturation, and as reported, mature FAs are composed of approximately 180 proteins to make up integrin adhesives, including vinculin, paxillin, talin, zyxin, tensin, actinin and others [121-123]. The assembly of FAs is a mechanosensitive process that is initiated by receptor-ECM binding at the cell leading edge. These precursors of FAs, termed “nascent adhesions” [124], can initially attach to actin filaments through several adaptor proteins. When subjected to mechanical stress such as cell contractility, the FA abundance of actin-bundling proteins such as α-actinin [125] and actin cytoskeletal LIM domain-containing adaptors such as zyxin are found to be enhanced, and nascent adhesions mature to FAs that enable probing and responding to the mechanical cues of the TME by modulating their adhesions [126, 127].
A large amount of previous experimental data demonstrates that FAs act as the middle bridge that combines tumor cells with the surrounding ECM by outside-in signaling and engage in ECM remodeling by inside-out signaling. The localization, size, spatiotemporal distribution and maturation states of FAs can be modulated by the physical properties of the ECM, such as stiffness and topology [128]. FAs remodel the actin cytoskeleton via integrin-mediated mechanotransduction, regulate some features of tumor cells, cell morphology, proliferation, migration and drug resistance, and even engage in programing gene expression. In addition, forces change the conformation of FAs to modulate the related enzymatic activities and induce fresh binding interactions [129]. Wang et al. [130] found that the conformational changes of FAs caused the activation of tyrosine kinases to mediate mechanotransduction.
4.1.3 Caveolins
Related to a variety of human diseases, caveolae, cell-surface structures with 50 to 100 nm omega-shaped invaginations, are widely described as mechanical sensors and are involved in many cell signaling pathways [131, 132]. Previous studies have shown that one of the main differences between caveolae and lipid rafts is that caveolae include membrane proteins termed caveolins, which consist of Cav-1, Cav-2, and Cav-3 [133]. Cav-1 and Cav-2 are highly expressed in many cell types, whereas Cav-3 is predominantly found in skeletal and muscle cells [134]. In addition, caveolins and transmembrane receptor tyrosine kinase like orphan receptor (ROR1) also contribute to caveolae formation [135].
As mechanosensors, caveolins respond to mechanical cues in the TME by flattening disassembly and Cav-1 phosphorylation (pY14Cav-1). Sinha et al. [136] expounded that flattening and disassembly of caveolae is an actin- and ATP-independent cellular response during mechanical stress. After the flattening of caveolae mediated by membrane tension, Cav-1 is released, and its mobility increases at the plasma membrane. Caveolar endocytosis can be triggered by changes in membrane tension to then regulate anchorage-dependent signaling. Once the caveolae disintegrate, the protein subunit is activated. It was reported that dissociated Cav-1 combines with a binding factor of a type-I collagen promoter (BFCOL1), promoting ECM deposition [137]. Cell area is also a crucial mechanical factor affecting cell behavior, which can be controlled by mechanical cues that regulate pY14Cav-1 levels [138]. Western blotting data demonstrated that focus adhesion organization and signaling could be affected by adhesion-dependent stimulation of pY14Cav-1 levels [139, 140]. Cav-1 can regulate YAP activity by controlling actin polymerization in response to changes in ECM stiffness and other mechanical cues [141]. In addition, mechanosensitive Cav-1 plays critical role in the tumor cells vascular invasion and metastasis. Shear stress activated Cav-1 could induced breast cancer cell motility, adhesion [135], invadopodia formation, anoikis resistance [142] and tumor metastasis [132].
4.1.4 Others
A number of other membrane-associated complexes have also been termed mechanosensors, including G-protein-coupled receptors, glycocalyx adhesion proteins, tyrosine kinase receptors, and membrane curvature sensors such as the BAR domain proteins guanosine triphosphatase (GTPase) regulator associated with FAK-1 (GRAF1) and protein interacting with protein kinase c alpha type-I (PICK1). Gated channels such as transient receptor potential (TRP) can be activated via the plasma membrane and curvature [143]. G protein rotational mobility and activation are regulated by a shear-induced increase in plasma membrane fluidity. As a transmembrane macromolecule, the glycocalyx undergoes a conformational change when subjected to shear stress, triggering signal transduction [144].
4.2 Mechanotransduction of tumor cells
Subjected to multitudinous mechanical forces, many signaling pathways in tumor cells are activated in response to surrounding mechanical cues and influence the growth, invasion and migration of tumor cells. The way in which mechanical cues are translated into biochemical signals is termed mechanotransduction (Figure 3).
4.2.1 Integrin- FAK signaling
In recent years, the bidirectional signaling of integrins has been demonstrated to serve key roles in cell adhesion, proliferation, differentiation and migration [145]. Among them, the outside-in signaling mediated by FAK involves a variety of signaling molecules to regulate cell behaviors. When stimulated by mechanical cues, the outside binding of ECM ligands to integrins stimulates a degree of conformational changes that activate FAK. FAK then binds and activates Src, and activated Src interacts with p130 CRK-associated substrate (p130CAS) to positively mediate the interactions between Src-FAK and Rac. On the other hand, (PKL/Git2)-β-Pix and β-pix are successively activated by FAK and transmit signaling by Rac and p21-activated protein kinases (PAKs) [146, 147]. In addition, the integrin-FAK signaling also affects a variety of other pathways, including the PI3K/AKT, Ras-ERK and YAP/TAZ pathways [148], transferring integrin-mediated external mechanical cues to the cell interior. Two major effectors of inside-out integrin signaling are talin and kindlin. Talin binding is important for the first step of integrin activation. Ras-related protein1 (RAP1) recruits talin directly or indirectly by Rap1-GTP-interacting adaptor molecule (RIAM), which then binds talin to cell surface integrins [149, 150]. The other effector kindlin binds the integrin β-subunit cytoplasmic domain to activate integrin at the first step [151]. Kindlin then recruits paxillin to nascent adhesions to activate the Rho GTPase Rac1, and it binds the actin-polymerizing Arp2/3 complex to mediate Rac1-mediated membrane protrusions [152].
4.2.2 Cytoskeletal mechanics
Recently, multiple studies have shown that many mechanosensor proteins, such as integrins and FAs, make interconnections with the cytoskeleton [10, 153, 154], and as reported, actin filaments act as mechanosensors for tension exerted on cells, activating downstream signaling pathways [155]. The cytoskeleton consists of actins, microtubules and intermediate filaments with different areas of responsibility. The actin cytoskeleton is made up of filamentous actin (F-actin) and globular actin molecules (G-actin), and the actins form F-actin and G-actin constantly via dynamic assembly and disassembly [156]. When adhered to stiff substrates or large nanopatterned substrates, the ratio of F-actin to G-actin increases, and stress fibrils form, causing the increased nuclear transcription of the transcription coactivator YAP, a key downstream signaling factor of the Hippo signaling pathway, thus promoting the expression of target genes by combining with the TEA/ATTS domain (TEAD) [157, 158]. This mechanical force and actin dynamics mediated YAP regulation is involved in the progression of malignant tumors and the control of organ size [88, 159]. In addition, the relaxed actin filaments facilitate cofilin binding that promotes disassembly of actin filaments. As mechanosensors, actins combine with the motor protein myosin II to generate contractile forces, pushing the plasma membrane forward [156], and tumor cells interact with ECM tension by this mechanism [160].
Microtubules are highly dynamic structures consisting of α and β tubulin heterodimers and play crucial roles in cellular growth, vesicle transport and especially mitosis [161]. As major components of the axonema of cilia, microtubules can help cilia sense and transduce many mechanical and chemical signals from the extracellular milieu [162]. Microtubules are important for mediating mechanical stress-directed spindle organization, chromosome alignment and segregation in mitosis [161]. Intermediate filaments, owing to their stable and resilient properties, serve as critical components in sensing the direction of mechanical stress endured by tumor cells [163].
4.2.3 Rho signaling
Rho-family small GTPases are a family of approximately 22 members that always cycle between an active GTP-bound form and an inactive GDP-bound form. The three most frequently studied Rho GTPases are RhoA, Cdc42 and Rac1, which regulate the remodeling of the cytoskeleton by modulating the activities of downstream proteins [164]. RhoA and RhoC act upon ROCK, ROCK1 and ROCK2 to regulate cytoskeletal properties. As Ser-Thr kinases, ROCK proteins target LIM kinases (LIMK), by which they can control actin depolymerization and regulate myosin contractility [165]. More specifically, ROCK activity causes the phosphorylation of its targets, myosin regulatory light chain 2 (MLC2) and LIMK, to enhance actin polymerization and myosin contractility. In turn, this situation initiates a paracrine signaling mechanism, resulting in increased ECM components. Furthermore, the increased ECM components boost ECM tension that feeds back to activate ROCK.
Several studies have shown that the activity of Rho-specific guanine nucleotide exchange factors (Rho GEFs) and Rho GTPase-activating proteins (Rho GAPs) is regulated by mechanical cues in the TME, and these Rho-GEFs and Rho-GAPs respond to surrounding mechanical signals by regulating actin cytoskeletal remodeling [166, 167]. Yang et al. [168] demonstrated that a RhoA-targeting GAP termed p190RhoGAP participates in the transient inhibition of RhoA and remodeling of actin induced by shear stress through Src-mediated phosphorylation of p190RhoGAP downstream of mechanosensor Cav-1 and integrin β1.
4.2.4 Hippo signaling
The Hippo signaling pathway is a well-conserved signaling pathway that comprises a sequence of transcription factors and protein kinases and plays important roles in tissue regeneration, organ development, wound healing, cell proliferation and apoptosis [169, 170]. Several recent studies have suggested that the Hippo pathway is also involved in tumorigenesis and metastasis in many cancers. Various upstream factors of the TME are able to activate the Hippo pathway, including many mechanical signals (such as stiffness and shear stress), cellular stress and cell polarity [171, 172].
After receiving upstream signals, such as activated moesin-ezrin-radixin-like protein (Merlin) and FERM domain-containing protein (FRMD) [173], the pathway is triggered by the activation of mammalian STE20-like kinase (MST1/2), which is associated with Salvador homolog-1 (SAV1) [174]. This complex is capable of phosphorylating and activating large tumor suppressor homologs 1 and 2 (LATS1/2) and its cofactor MOB1, which in turn phosphorylates the key transcription cofactors YAP/TAZ. The phosphorylated YAP/TAZ is detained in the cytoplasm by 14-3-3 protein binding or is ubiquitinated and degraded [172], causing the inhibition of its nuclear translocation and the loss of its ability as a transcription cofactor. In contrast, unphosphorylated YAP/TAZ can successfully enter the nucleus and combine with some transcription factors that have DNA binding domains, such as the TEADs family [175] and RUNX family, to regulate the expression of downstream related target genes, enhancing the promoter activity of target genes associated with cell migration and invasion [172, 176]. Other upstream Hippo pathway modulators, such as the actin cytoskeleton, neurofibromine 2 (NF2), G-coupled receptors (GPCRs), Sonic hedgehog (Shh) signaling pathways or contact inhibition, and even DNA damage, could also trigger the activation of the Hippo pathway [177-179].
4.3 Cell nuclear mechanics
Physically connected with the plasma membrane via the internal cytoskeleton, the nucleus responds to mechanical factors in the TME through this physical connection. However, recent studies have expounded that the nucleus also acts as a source of information, which means it plays a crucial role in bidirectional signaling transmission [180, 181].
4.3.1 Linker of nucleoskeleton and cytoskeleton (LINC)
LINC complexes are well- known central components in the mechanotransduction process, which transmit mechanical cues from the plasma membrane to the nucleus. Through the functionality of LINC complexes, the nucleus is capable of responding to mechanical forces by changing nuclear structure, chromosomal reorganization and gene location and expression [182]. Klarischt/ANC-1/SYNE homology (KASH) domain-containing proteins on the outer nuclear membrane and Sad1/UNC-84 (SUN) domain-containing proteins on the inner nuclear membrane mainly form LINC complexes. Six KASH proteins (including nesprins-1-4, KASH5, and lymphocyte-restricted membrane protein) and five SUN proteins (SUNs 1-5) are encoded in mammals, combine with cytoskeletal elements in the cytoplasm and associate with lamin proteins and chromatin in the nucleus to exert their effects.
According to published data, an entire mechanotransduction pathway is able to realize the transmission of mechanical cues from the extracellular environment to the nuclear genome. In this pathway, the actin cap could connect to the nuclear envelope by nesprin-2 and nesprin-3, which in turn anchor to the lamina and chromatin genome by the interactions of KASH-SUN proteins and lamin A/C [183]. On the other hand, LINC complexes make the transition of inside-out signaling possible via many approaches. Sun2 promotes the activation of RhoA and induces an increase in FAs in cells; Sun1 inhibits the assembly of stress fibers by limiting Sun2 activity [184]. The inhibition of ROCK or the ROCK-dependent actin remodeling regulator formin homology 2 domain containing 1 (FHOD1) could rescue the mutant cell morphology, which means that the integrity of nesprin-1 and lamin A/C is necessary for the activity of FHOD1 [185]. In addition, LINC complexes transmit mechanical stimulation via a chromatin regulator, barrier-to-autointegration factor, to modulate the progression of synchronized cell-cycle progression [186].
4.3.2 Nuclear lamins
Under the nuclear membrane, the nuclear lamina is a dense meshwork consisting of karyoskeletal intermediate filament proteins, termed lamins, which provide physical stability for the nucleus and are involved in cell migration, nuclear localization, chromatin organization and DNA replication [187-189]. There are two main categories of lamins: A-type lamins and B-type lamins. The former includes lamin A, C, AΔ10, and C2, which are encoded by the LMNA gene; the latter contains lamin B1 and B2/B3, which are encoded by the LMNB1 and LMNB2 genes and can be continuously and steadily expressed in all cells [190-192]. Localized close to the nuclear envelope, lamins interact with the majority of nuclear envelope-associated proteins, building a mechanical support through this connection [193].
A-type lamins anchor proteins play a role in the mechanotransduction pathway and chromatin remodeling. Poh et al. [194] noticed that cells expressing lamin A mutants or lacking A-type lamins cannot transmit mechanical forces directly to the nucleus. Swift et al. found that the protein levels of lamin A are directly influenced by matrix stiffness. Low lamin A levels promote stem cells to differentiate into fat on soft matrix, while on stiff matrix, high lamin A levels enhance the differentiation of those stem cells into bone [195]. Other studies have demonstrated that lamins act as transcription factors and signaling molecules in the mechanotransduction pathway. As transcriptional regulators, lamins attenuate signaling pathways by interacting with c-fos [196] or participating in the Notch pathway [197]. As signaling molecules, both ERK and phosphorylated c-fos bind to lamins A/C, which activates c-fos/AP-1-driven transcription [198].
4.3.3 Chromosomal reorganization
Repeating to form nucleosomes, chromatin is composed of DNA and core histone proteins, and changes in chromatin structure are considered to turn gene expression on or off. The force-induced nuclear deformations could alter the chromatin organization and the interactions between the nuclear lamina and chromatin, activating or repressing nuclear transcription factors [199]. A recent study suggests that Cajal bodies interacting with chromatin dissociate rapidly when subjected to force on the cell membrane [194]. Force is transmitted from the cell membrane to the nucleus via actin cytoskeleton remodeling, leading to chromatin depolymerization within 5 s. Compared with microtubules, the actin cytoskeleton plays a more important role in force transmission [194, 200]. Further results demonstrated that the fluorescence anisotropy values of the core histone H2B fusion plasmid (H2B-EGFP) were changed in euchromatin regions upon force application, which means that these areas were more sensitive to changes in mechanical cues. In addition, chromatin structure is also regulated by some posttranslational modifications, such as methylation, acetylation/deacetylation, and phosphorylation. Li et al. [201] revealed a new mechanism by which histone acetylation and histone deacetylase (HDAC) activity could be modulated by biophysical cues (microtopographic pattern or anisotropic mechanical strain), thus regulating the structure of chromatin and the pattern of gene expression. The related chromatin remodeling proteins work cooperatively with β-catenin, orchestrating the modification of epigenetics and the activation of transcription of Wnt-responsive genes [202]. Moreover, nuclear actin as well as its binding proteins can also promote the recruitment of histone remodelers to appropriate transcription sites and further induce changes in gene expression [203].
4.4 Mechanical cues and Tumor metastasis
The majority of cancer-related deaths are not caused by primary tumors but by distant metastases. Distant tumor metastasis occurs by way of several steps: primary tumor formation, local invasion, intravasation, survival in the circulation, arrest at a distant organ, extravasation, and proliferation in the secondary sites [204]. For decades, the influence of mechanical cues in TME on tumor metastasis has been increasingly recognized. In particular, tissue rigidity is functionally important in metastasis. Fattet et al. [205] showed that high ECM stiffness could promote breast cancer cell EMT, invasion and metastasis through an EPH receptor A2 (EPHA2) /LYN/TWIST1 mechanotransduction signaling. EMT, as a key process in metastasis, could be stimulated and enforced via a positive feedback loop among EMT, lysyl oxidase like 2 (LOXL2), and ECM stiffness [206, 207]. In addition, ECM stiffness can achieve the regulation of tumor metastasis-related cell behaviors through activating of mechanosensitive transcription factors, such as YAP/TAZ signaling. The decrease in the capping protein inhibiting regulator of actin dynamics (CRAD) in soft substrates induces YAP retention in the cytoplasm to inhibit the expression of the stemness markers Nanog and Oct4, thereby promoting the metastasis of colorectal cancer stem cells [208]. However, another study indicated that cells can adopt alternative YAP-independent pathways when sensing substrate stiffness. Cells cultured on a stiff matrix retain more nuclear YAP to form larger FAs and yield higher actomyosin expression; therefore, these cells migrate faster [209]. Different substrate stiffnesses affect cell motility by regulating cell migration models. High stiffness promotes individual prostate cancer cell migration by inducing the nuclear localization of YAP/TAZ in bone metastasis-derived cells, whereas low stiffness promotes prostate cancer cell migration by inducing lymphatic metastasis-derived cells to form clusters characterized by highly expressed CD44 [210].
In addition to stiffness, ECM architecture is able to direct migratory behavior. Cells adhere to the ECM matrix, and generate traction forces to drive the cell body to move forward. The aligned ECM can polarize FAs and facilitate the assembly of actin stress fibers, which also localize Rac activity to stabilize cell protrusions to direct uniaxial cell migration with enhanced speed and persistence [207]. Moderate cell adhesion is conducive to rapid cell migration. For instance, cells cultured on 91 nm-spacing substrates were found to migrate fastest, and the expression of FA genes (such as paxillin, vinculin and α-actinin) was moderately downregulated [208]. In the future, when and how the mechanical cues in the TME trigger the collective migration of cells and the impact of single mechanical factors on tumor metastasis in the comprehensive TME still need to be investigated. Moreover, to a large extent, flow shear stress may act as a positive factor of tumor metastasis by promoting the survival and metastatic potential of CTCs. In addition to regulating tumor growth, the increased solid stress also leads to a lack of lymphocytic infiltration into the metastatic lesions, and inhibits T-cell-mediated anti-tumor response [209].
5 APPLICATIONS AND CLINICAL SIGNIFICANCE OF TUMOR MECHANICAL MICROENVIRONMENT
The response to tumor treatments is largely determined by the generation of mechanical cues in the TME. Following the outcome, several strategies have been provided for drug delivery and therapy to eliminate or alleviate tumor progression, such as cellular, nano-, molecular and immuno-medicines [210].
5.1 Stress-alleviation strategy
Cell proliferation-induced solid stress or residual solid stress compresses the surroundings, leading to an increase in fluid pressure, which further complicates targeted drug delivery and therapy [211]. Therefore, the stress-alleviation strategy yields positive effects on decompressing tumor vasculature and restoring tumor perfusion and oxygenation, thus enhancing drug efficacy [212]. Proliferating tumor cells, CAFs, hyaluronan and collagen are important components that cause solid stress accumulation in tumors. Hence, targeting the therapeutic depletion of these TME components is able to alleviate solid stresses and reduce interstitial flow pressure to some degree. Stylianopoulos et al. [212] proved that an inhibitor termed saridegib, which is used to target CAFs in some kinds of solid tumors (such as highly desmoplastic or hypovascular pancreatic tumors), could alleviate solid stress, further decompress the tumor vasculature and strengthen the effects of the fraction of perfused tumor vessels by 47% [34, 210]. In addition, as an angiotensin receptor blocker and an inhibitor of TGF-β, losartan is widely used to reduce the contents of collagen and hyaluronan to alleviate solid stress and improve drug delivery in breast and pancreatic tumors as well as lung or renal cancers, consequently promoting treatment [213-215]. Locally advanced pancreatic cancer patients have historically poor outcomes, and single radiotherapy or drug therapy cannot improve overall survival (OS). Recently, the multidrug regimen FOLFIRINOX composed of fluorouracil, leucovorin, oxaliplatin, and irinotecan has significantly prolonged OS [216], which has attracted extensive attention from relevant clinical studies [216]. Murphy et al. [217] assessed the margin-negative (R0) resection rates of losartan and total neoadjuvant FOLFIRINOX followed by chemoradiotherapy for locally advanced pancreatic adenocarcinoma. It is gratifying that total neoadjuvant therapy with chemoradiotherapy, losartan and FOLFIRINOX reduces the downstaging of locally advanced pancreatic adenocarcinoma and increases the R0 resection rate to 61%, which is much higher than expected.
5.2 Vascular normalization strategy
With leaky, tortuous, saccular, and dilated abnormal vascular network characteristics, the tumor vasculature causes increased interstitial fluid pressure and poorly perfused tumors. Impaired tumor perfusion leads to a hypoxic microenvironment and reductions in immune cell tumor-killing action and chemotherapeutic radiotherapy efficiency [18]. In contrast to stress-alleviation, vascular normalization strategies aim to directly control tumor neovascularization or promote blood vessel maturation to decrease interstitial fluid pressure and increase tumor perfusion. Since it was discovered to be a key regulator of blood vessel growth, VEGF or its receptor is considered to be an ideal target to control tumor neovascularization. Several drugs have been placed into use, including bevacizumab (an anti-VEGF-A monoclonal antibody) and some tyrosine kinase inhibitors (cediranib, sunitinib, semaxanib) [43, 218, 219]. Other drugs combined with bevacizumab have been evaluated in tumor models, with bevacizumab combined with cisplatin/gemcitabine in non-small-cell lung cancer [220] or with the combination of paclitaxel and bevacizumab in breast cancer [221]. Another part of the vascular normalization strategy aims to increase pericyte coverage to maintain vascular quiescence and integrity [222] by some signaling pathways (such as angiopoietins, the PDGF-B/PDGFR axis [223] and BMP signaling [224]) or to enhance cell adherent junctions to increase vascular permeability by inhibiting VE-cadherin [225]. In addition, Liu et al. [226] also noted the positive therapeutic effects of vascular normalization in immunotherapy in a variety of cancers, such as the use of anti-CTLA-4/anti-PD-1 in breast cancer [227] and hepatocellular carcinoma [228], interferon-γ (IFNγ)/IFNγ-RGR in pancreatic neuroendocrine tumors [229], 3'3'- cyclic GMP-AMP (cGAMP) in colorectal cancer [230], and IFN-β in glioma [231] as well as alveolar rhabdomyosarcoma [232].
5.3 Targeting ECM stiffness
The ECM stiffens during the pathological progression of fibrotic diseases, cardiovascular disease and in different kinds of tumors [233, 234]. An emerging field termed mechanomedicine is aimed at therapeutically targeting tissue mechanics because extensive data have shown that mechanical cues are able to drive tumor progression, in turn, these cues can be targeted for drug delivery and therapy [235]. Hence, there are two main approaches to target ECM stiffness to attenuate disease, including limiting the cross-linking and stiffening of ECM fibrils and disrupting cell responses to increased ECM stiffness. For the former, directly targeting CAFs or their differentiation may alleviate fibrosis, related drugs and approaches include the monoclonal antibody C1-3, targeting growth factor TGF-β to suspend CAFs differentiation and matrix production, such as with the small molecule pirfenidone, and the monoclonal antibody GC1008 [236], LOXs could be inhibited by using the competitive inhibitor β-aminopropionitrile to mitigate ECM stiffness [237]. In contrast to the former, inhibiting the cellular response to surrounding stiff ECM is another way to reduce the adverse effects of ECM stiffening. Targeting integrins, especially the αv family, is a chief choice to obstruct ECM mechanosensing, such as with the monoclonal antibody against αvβ6 named BG00011/STX-100 and the integrin inhibitor cilengitide [238]. The activation of Rho GTPase signaling downstream of integrin also contributes to increase ECM stiffness, and guanine nucleotide-exchange factors are a target to control Rho activity, for instance, Rhosin suspends a nucleotide-exchange factor docking site on RhoA [239]. Additionally, FAK, ROCK, myocardin-related transcription factor-A/B, nuclear factor κB and JMJD1, Brd4, G9a and miR-21 can also serve as targets to modulate ECM stiffness [3, 4, 234, 240].
Although these drugs reduce the stiffness of the ECM to some extent, there are still some limitations in clinical application. Pirfenidone has off-target effects, and its adverse drug reactions (ADRs) are relatively common; thus drug safety tests and patient tolerance should be improved [241]. Smith et al. [242] found that TGF-β inhibitors (such as Fresolimumab) may impose harmful effects on heart function in breast cancer and glioblastoma. To address this problem, some strategies for inhibiting pathological TGF-β activation have been developed, including the inhibition of αv-integrin heterodimers with therapeutics such as c8 [243] and STX-100 [244]. Although β-aminopropionitrile was found to be nontoxic in mice and rats [245], the high toxicity in clinical trials for the treatment of patients with scleroderma may [246] disallow its clinical application. Furthermore, cilengitide failed to significantly improve the median OS times in the context of pancreatic cancer [247].
6 CONCLUDING REMARKS AND PERSPECTIVES
Recognizing that the mechanical TME is a trigger of multiple pathologies, researchers have put forward new hopes and treatment strategies for patients. Understanding the pivotal principles of the origins and consequences of tumor physiology and pathology is necessary to improve therapy efficiency. Here, we have discussed the major mechanical factors in the TME and molecular mechanotransduction pathways to help increase the understanding of solid tumor pathological deformation, expansion and extrusion, and stiffening in cancer growth and metastasis.
Although several in vitro tumor mechanical microenvironment models have been summarized in this review, it is still difficult to simulate the complicated vasculature and the simultaneous effects of various external and internal mechanical factors. More specifically, for instance, simultaneous simulation of interstitial flow and solid stress in a 3D matrix is difficult. Fortunately, some studies have focused on improving the adverse factors in the mechanical TME rather than being limited to the treatment of the tumor itself. Targeting the ECM or vascular system mechanics by preventing tissue stiffening, arresting the cellular response, decompressing tumor vasculature or normalizing the vascular network are representative therapeutic approaches with clinical potential.
Although the influences of mechanical factors in the TME on the occurrence and progression of tumors have been detailed described, the recurrence and drug resistance related to the physical TME have not been clearly elucidated. Moreover, how the physical characteristics of the TME play roles in different stages of tumorigenesis, how to achieve the precise localization and time of drug release in vivo, and how to use the TME for high-throughput drug discovery and screening are worthy of further attention. Studies on the molecular basis and mechanomedicine in the past have established a solid foundation for understanding the importance of TME mechanical factors and mechanotransduction. Future studies must be sure to focus on ameliorating the increased ECM stiffness, altered topology, increased solid stress and fluid pressure in the TME, minimizing the physiological side effects induced by immunity, exploring the possibility of blocking the secondary recurrence of tumors.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGEMENTS
This research was supported, in part or in whole, by the National Natural Science Foundation of China (U19A2006, 12132004, 11972111, 31900940, 32071304, 32171309, and 32171395), the Sichuan Science and Technology Program (21YJ0130), and the Joint Funds of Center for Engineering Medicine (ZYGX2021YGLH017, ZYGX2021YGLH010, and ZYGX2021YGLH023).
AUTHORS' CONTRIBUTIONS
Hanying Zhou: conceptualization, visualization, writing-original draft, resources, and investigation. Meng Wang: visualization. Yixi Zhang: visualization. Qingqing Su: resources. Zhengxin Xie: investigation. Xiangyan Chen: resources. Ran Yan: resources. Ping Li: resources. Tingting Li: resources. Xiang Qin: resources. Hong Yang: investigation. Chunhui Wu: resources. Fengming You: resources. Shun Li and Yiyao Liu: conceptualization, supervision, funding acquisition, writing-review, and editing.