Abscopal effect of stereotactic radiotherapy combined with anti-PD-1/PD-L1 immunotherapy: Mechanisms, clinical efficacy, and issues
Abbreviations
-
- BED
-
- biological effective dose
-
- GM-CSF
-
- granulocyte-macrophage colony-stimulating factor
-
- IL
-
- interleukins
-
- MHC
-
- major histocompatibility complex
-
- PD-1
-
- programmed cell death-1
-
- PD-L1
-
- programmed death ligand 1
-
- SABR
-
- Stereotactic radiotherapy
-
- TAA
-
- tumor-associated antigen
-
- TNFs
-
- tumor necrosis factors
Immunotherapy, as one of the major strategies for cancer treatment, has shown promising efficacy in cancer treatment by the clinical application of programmed cell death protein-1/ programmed cell death-ligand 1 (PD-1/PD-L1) antibodies which have demonstrated a significant prolongation in the survival time of melanoma, lung, and liver cancer patients. [1-3]. Meanwhile, the clinical observation of partial responses in unirradiated lesions after radiotherapy, termed as abscopal effect, has gradually attracted a lot of attention and has inspired oncologists to combine stereotactic radiotherapy (SABR) with immunotherapy for improving clinical outcomes. It is the abscopal effect phenomenon that crucially determines the anti-tumor efficiency of SABR and immune checkpoint inhibitors combination strategy [4]. However, due to the short application time and limited experience of the clinical utilization of SABR and anti-PD-1/PD-L1 combination therapy, there are plenty of theoretical and practical issues that need to be systematically discussed, including the mechanisms underlying an abscopal effect, the efficacy and toxicity of combination therapy, and the validity or reliability of corresponding clinical trials. Here, we reviewed previous landmark reports and summarized the research progress of SABR combined with anti-PD-1/PLD-1 therapy to improve the understanding on abscopal effect.
1 THE POSTULATED MECHANISM OF THE ABSCOPAL EFFECT IN SABR AND ANTI-PD1/PD-L1 COMBINATION THERAPY
The abscopal effect phenomenon in SABR and anti-PD1/PD-L1 combination therapy involves a series of chain reactions. First, high-dose of ablative radiation induces DNA double-strand breaks and damages the tumor cells. Second, the damaged cells release large amounts of tumor-associated antigen (TAA) fragments into the blood within a short time for antigen-presenting cells, called antigen outbreak. During this process, cascades of inflammatory cytokines, such as interleukins (IL) and tumor necrosis factors (TNFs), are secreted simultaneously, Subsequently, these TAA fragments and inflammatory cytokines are recognized and engulfed by dendritic cells, which thereby promotes the dendritic cells’ cross-presenting tumor antigens on class I major histocompatibility complex (MHC) for activating cytotoxic T cells. Finally, the activated polyclonal antigen-specific T cells are recruited to attack the tumor cells outside the irradiated field deliberately. In particular, those cascade immune reactions originated by TAA fragments approve the participation of other immune checkpoint inhibitors [5-9]. Therefore, anti-PD1/PLD-1 antibodies could theoretically enhance the anti-tumor immune modulation of SABR and achieve better outcomes synergistically [9-11]. The process of the abscopal effect described above elucidated that based on the antigen outbreak microenvironment triggered by SABR, the immune modulation, which acts as a bridge, could be dramatically elevated via the co-stimulation of anti-PD1/PD-L1 antibodies, and thus exert an obvious killing effect on tumors (Figure 1).
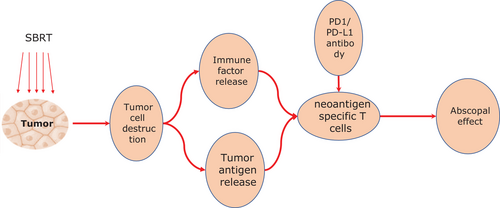
2 CLINICAL EFFICACY OF THE ABSCOPAL EFFECT IN SABR AND ANTI-PD1/PD-L1 COMBINATION THERAPY
Early observations on the abscopal effect were reported by some independent clinical cases, which subsequently encourages the progress of systematic investigations in this field. With the advancement in radiobiology at different periods, various new biological theories have been proposed by oncologists to reasonably explain and support the abscopal effect. However, the effectiveness and efficiency of the abscopal effect, as well as the radiobiological mechanism, remain unclear until now. In 2012, Postow et al. [11] published a landmark case on the abscopal effect in the New England Journal of Medicine and was the first report about those specific manifestations after immunotherapy. This case, together with the related phenomenon, caused wide public concern and a growing number of similar reports have been since published in various journals. Several studies [12-19] have detailed the characteristics and results of clinical cases after SABR and anti-PD-1/PD-L1 combination treatment over the recent years (Table 1). However, public opinions are still polarized on those insufficient statistical results regarding the abscopal effect. On one hand, the incidence of the abscopal effect is relatively low. On the other hand, there is no uniform standard for evaluating the occurrence and level of the abscopal effect to date. Some studies, therefore, raised the controversial question of whether the regression of unirradiated abscopal tumors should attribute to the context of SABR and immune checkpoint combination therapy or the latter alone. Based on the thorough review of the previous findings and reports, we summarize the clinical key points of the abscopal effect as follows: 1) the phenomenon of the abscopal effect does exist. 2) the exact identification and evaluated-criteria for the abscopal effect are urgently needed via high-level evidence-based medical researches. 3) the overall incidence of the abscopal effect in the clinic is relatively low; 4) no prospective studies with large sample sizes and long follow-up periods in this field have been conducted till now, and 5) classifying the targeted beneficiary group will be the further step in the abscopal effect exploration [20]. These suggest that the clinical efficacy of the abscopal effect in the context of radiation and immune checkpoint combination therapy needs to be firmly confirmed in future investigations (Table 1). More importantly, extensive investigations are required before taking the advantage of the abscopal effect in conducting the clinical treatment (Table 2).
| Authors | Year of publication | Journal | Tumor | Cases | Results on abscopal effect |
|---|---|---|---|---|---|
| Postow et al.[11] | 2012 | N Engl J Med | Melanoma | 1 | Exact abscopal effect |
| Hiniker et al. [12] | 2012 | Transl Oncol | Melanoma | 1 | Some unirradiated liver lesions and an unirradiated axillary lesion had completely resolved |
| Grimaldi et al. [13] | 2014 | Oncoimmunology | Melanoma | 21 | The abscopal response was observed in 11 patients (52%) |
| Levy et al. [14] | 2016 | Eur J Cancer | Multiple cancer species | 15 isolated lesions | Outfields disease evaluation retrieved 10/14 SD and 4/14 PD. But no outfield OR or outfield difference between those two periods were obtained according to TGR (p = 0.09). |
| Brenneman et al. [15] | 2019 | Front Oncol | Retroperitoneal Sarcoma | 1 | Non-irradiated metastases influenced by the abscopal effect |
| Choi et al. [16] | 2019 | Ann Otol Rhinol Laryngol | head and neck squamous cell carcinoma | 2 | Two patients with metastatic HNSCC developed complete responses |
| Garelli et al. [17] | 2019 | Future medicine | Lung cancer | The abscopal effect was observed in all 3 patients | |
| Kim et al. [18] | 2019 | Front Oncol | mucosal melanoma | 23 patients with 31 tumors | No abscopal effect was observed |
| Trommer et al. [19] | 2019 | Front Pharmacol | 2019 | 24 | The abscopal effect was observed in 29% (7/24) of the cases |
| NCT number | Hospital/ Institute | Status | Study Title | Conditions | Interventions | Phase | Outcome Measures |
|---|---|---|---|---|---|---|---|
| NCT03480334 | Cologne University Hospital | Recruiting | Abscopal Effect of Radiotherapy and Nivolumab in Relapsed Hodgkin Lymphoma After Anti-PD1 Therapy | Classical Hodgkin Lymphoma | Nivolumab plus radiotherapy | 2 | Abscopal response rate |
| NCT04238169 | Xinqiao Hospital of Chongqing | Not yet recruiting | Clinical Trial Assessing the Efficacy of Abscopal Effect Induced by SBRT and Immunotherapy in Advanced NSCLC | Non-Small-Cell Lung Cancer Stage IV |
Drug: Bevacizumab Drug: Toripalimab |
2 |
Objective response rate Objective response of non-target lesion Progression-free survival (PFS) |
| NCT04168320 | KABEG Klinikum Klagenfurt, Institute for Radiation Oncology | Recruiting | SBRT-based PArtial Tumor Irradiation of HYpoxic Segment | Unresectable Malignant Solid Neoplasm | Radiation: SBRT-PATHY (SBRT-based PArtial Tumor irradiation targeting HYpoxic segment | 1 |
Bystander and abscopal effects Overall survival Progression-free survival |
| NCT03396471 | Mayo Clinic; MD Anderson Cancer Center | Recruiting | Study of Pembrolizumab and Concurrent Radiation in Patients With Previously Treated Carcinoma of Unknown Primary | Carcinoma, Unspecified Site |
Drug: Pembrolizumab Radiation: External Beam Radiation Therapy |
2 |
Abscopal Response Rate Response Rate Assess Adverse Events |
| NCT04245514 |
Kantonsspital Aarau Aarau, Switzerland |
Recruiting | Multimodality Treatment in Stage III Non-small Cell Lung Cancer (NSCLC) | Non-small Cell Lung Cancer |
Drug: Durvalumab Radiation: Radiotherapy |
2 |
Event-free survival (EFS) at 12 months Event-free survival (EFS) Recurrence-free survival (RFS) after R0 resection |
| NCT03993678 |
Kantonsspital Graubünden Chur, Switzerland |
Recruiting | Intratumoral Injection of IP-001 Following Thermal Ablation in Patients With Advanced Solid Tumors. | Advanced Solid Tumors |
Drug: IP-001 Device: Thermal Ablation |
1-2 | Toxicity and tumor control |
3 ISSUES TO BE SOLVED FOR THE ABSCOPAL EFFECT OF SABR AND ANTI-PD-1/PLD-1 COMBINATION THERAPY
3.1 What are the underlying molecular mechanisms of the abscopal effect?
The phenomenon of spontaneous regression in unirradiated tumors is defined as the abscopal effect, which is commonly found after SABR, and hypothetically attributed to an increase in immune response evoked by tumor antigens cascade. However, it is very difficult to identify the presence and strength of the abscopal effect in clinical reality due to the unmeasurable activation of the immune response. Moreover, some concerns are raised that the shrink of abscopal tumors may entirely be induced by immunotherapy but not SABR, especially in patients who received SABR and immune-checkpoint inhibitors at the same time. However, we speculate that the partial or complete regression of unirradiated tumors after SABR combined with immunotherapy, particularly in the patients with stable or progressive conditions after immunotherapy alone, should be ascribed to the occurrence of the abscopal effect (Figure 2). Accordingly, comparing the response rate of abscopal tumors in the homogeneous metastasis group who received immunotherapy alone or SABR combined immunotherapy separately, could be an appropriate strategy in conducting related clinical trials [21]. Indeed, owing to the various efficacy of each independent case, a clear distinction between the abscopal effect and immune-checkpoint inhibiting effect is also practically needed.
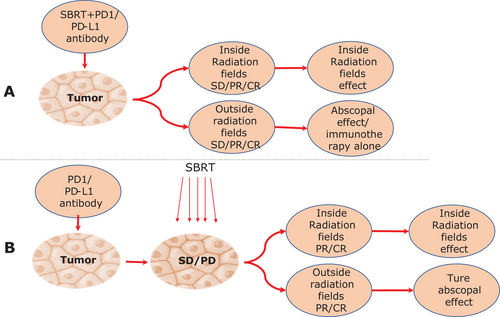
Distinguishing the independent and suspicious abscopal effect. (A) The abscopal effect should be distinguished from the immunotherapy effect alone. (B) The definition of the abscopal effect.
The abscopal effect should be distinguished from the immunotherapy effect alone during combination therapy, especially when immunotherapy and SABR were used sequentially or at the same time. If the unirradiated-tumor was relieved before SABR, we could surely not attribute this phenomenon to the abscopal effect, as it is probably induced by immunotherapy alone. If the patients have stable or progressive conditions during immunotherapy but obtain relief in local as well as abscopal lesions after SABR, we should consider this phenomenon as “real” abscopal effect
Besides, not only imaging examinations but also advanced technology should be used to qualify and quantify the abscopal effect such as AI, radiomics, and radio-genomics techniques. Meanwhile, biomarkers of abscopal effect such as antigen outbreak fragments, tumor-related molecules, and circulating immune factors, also need to be further researched and validated.
3.2 Can the abscopal effect confer survival benefit?
Undoubtedly, the abscopal effect has been a hot point of discussion in the current research field. Some radiologists have pointed out that the abscopal effect confers SABR on systemic curative effects, while more precious evidence and studies are urgently required. First of all, the occurrence of the abscopal effect is rare and slight in patients with SABR alone. Our ten years of SABR experience with more than 7000 patients suggest that the abscopal effect could hardly be aroused in patients with SABR alone. Nevertheless, more detailed observations and more strictly randomized controlled trials are demanded to differentiate the abscopal effect and the immune-checkpoint inhibiting effect in patients who received SABR combined with anti-PD-1/PLD-1 therapy. The abscopal effect may have organ- and host-dependent manners as it has only been observed in some patients with oligometastatic or some specific patients with multiple lesions; possibly owing to the different immune situations in patients. In the author's opinion, utilizing the abscopal effect of SABR to conduct the overall therapeutic effect for patients is too advanced in the current clinic. Besides, along with the progress of immunotherapy, more efforts could be made to seek biomarkers of the abscopal effect and classify the beneficial groups during clinical practice [22], which may elevate the better application of the abscopal effect.
3.3 In which way could the abscopal effect be enhanced?
Although the incidence of the abscopal effect is relatively low and the validation of the curative effect evoked by the abscopal effect is unclear, we cannot deny the existence of the abscopal effect phenomenon. If we want to take the advantage of the abscopal effect as an efficient strategy in tumor control and make SABR a part of “systemic treatment” in the context of immunotherapy, more efforts are urgently and additionally needed to support and enhance the curative impact of the abscopal effect. Initially, from the view of clinical practice [20], SABR should be conducted in the guideline-recommended patients with high tumor burden and relatively high irradiated-volume, which could trigger the release of anti-tumor antigens as much as possible. Simultaneously, lesions in different sites should be separately treated with discrepant SABR strategy due to the organ-dependent characteristics (for example, bone metastases) of the abscopal effect and the tumor heterogeneity, which may release a diversity of antigens. Moreover, the sequence of SABR and immunotherapy must be appropriately arranged to stimulate the maximum curative effect of those two methods. Eventually, the dose and fraction of SABR as an assistant of immunotherapy also require further study to better exploit the potency of anti-tumor antigens evoked by SABR [22]. Notably, applying some immunopotentiators during different stages of the combination therapy may enhance immune response based on the theoretical radiobiological mechanisms of the abscopal effect such as granulocyte-macrophage colony-stimulating factor (GM-CSF) [23].
4 CONCLUSION
The abscopal effect is an important mechanism of stereotactic ablative radiotherapy (SABR) combined with immunotherapy on killing tumor cells. SABR kills tumor cells to release tumor-associated antigens and activates immune responses in the human body. However, SBAR combined with PD-1/PD-L1 inhibitor has only been applied in clinical practice for a short time, and most findings are from clinical trials. The abscopal effects are currently far from clear, and the related mechanisms, efficacies, and issues need to be solved in more elaborated studies.
DECLARATIONS
AUTHORS' CONTRIBUTIONS
HQZ contributed substantially to the conception and design of this study. HQZ collected material and wrote the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
COMPETING INTERESTS
The author declares no competing interests.
FUNDING
This research was funded by key clinical projects of Peking University Third Hospital (Peking University talent introduction fund, BYSY2017030).
ACKNOWLEDGEMENTS
Not applicable.




