Mutational characteristics determined using circulating tumor DNA analysis in triple-negative breast cancer patients with distant metastasis
Abbreviations
-
- TNBC
-
- Triple-negative breast cancers
-
- ER
-
- estrogen receptor
-
- PR
-
- progesterone receptor
-
- HR
-
- hormonal receptor
-
- HER2
-
- human epidermal growth factor receptor 2
-
- NGS
-
- next-generation sequencing
-
- ctDNA
-
- circulating tumor DNA
-
- AMPK
-
- 5-AMP-activated protein kinase
-
- PI3KCA
-
- phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha
-
- TMB
-
- tumor mutation burden
-
- MSAF
-
- maximum mutant allele frequency
-
- TCGA
-
- The Cancer Genome Atlas
-
- MSK
-
- Memorial Sloan Kettering Cancer Center
-
- METABRIC
-
- Molecular Taxonomy of Breast Cancer International Consortium
-
- PIK3CB
-
- Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta; FANCM: FA complementation group M
-
- PRKAA1
-
- protein kinase AMP-activated catalytic subunit alpha 1
-
- MAP2K4
-
- mitogen-activated protein kinase kinase 4
-
- NCOR1
-
- nuclear receptor corepressor 1
Dear editor,
Breast cancer has been considered as the most common malignancy and the leading cause of death in women worldwide [1]. Triple-negative breast cancer (TNBC) accounts for 10%-20% of breast cancers, which are characterized by the absence of estrogen receptor (ER), progesterone receptor (PR), and amplification of human epidermal growth factor receptor 2 (HER2) [2]. TNBC is well known for its rapid progressiveness, high rate of distant organ recurrence, and poor prognosis. Metastasis remains the major cause of death for patients with TNBCs. Up to date, the only direct treatment for metastatic TNBCs is chemotherapy, which has been challenged by tumor heterogeneity and drug resistance. Lack of effective targeted therapy for metastatic TNBCs impels researchers to focus on discovering potentially actionable targets through mutational profiling.
Although next-generation sequencing (NGS) has shown valuable benefits in oncology, its use in metastatic TNBC demands attentions. On one hand, in view of cellular heterogeneity, the genomic profiles of tissue biopsies might not be representative for lethal tumors. There exists trauma, pain, and other safety issues when obtaining metastatic tissues in clinical practices, since metastatic TNBCs frequently involve distant organs including the liver, lungs, bones, or brain. Recently, circulating tumor DNA (ctDNA) sequencing has been considered a promising tool for its convenience, accuracy, and minimal invasiveness in investigating the tumor mutational landscape, assess tumor burden, and dynamically monitor drug response [3, 4]. However, available data of metastatic TNBCs are insufficient. In the present work, we enrolled 128 TNBC patients with lung, liver, bone, or brain metastasis, collected their peripheral blood samples, and performed ctDNA profiling using targeted sequencing (Supplementary Materials and Methods). The median age of patients was 52 years (range, 31-78 years).
Based on our ctDNA analysis, 236 mutated genes were identified in 110 of the 128 included patients with metastatic TNBC. The characteristics of patients are summarized in Supplementary Table S1. The most frequently mutated genes were TP53 (56%) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) (19%) (Figure 1A), which were concordant with the tissue-based sequencing results for the public Memorial Sloan Kettering Cancer Center (MSK) metastatic TNBC cohort (r = 0.88, P < 0.001) (Figure 1B). Totally 527 nonsynonymous somatic mutations were identified, comprising of 465 single nucleotide variants and 62 insertions and deletions (indels). Specifically, 75 somatic mutations of TP53 (43 missense mutations, 13 nonsense mutations, 7 splice site mutations, and 12 indels) were identified in 72 patients, while 26 mutations of PIK3CA (1 deletion and 25 missense mutations) were identified in 24 patients.
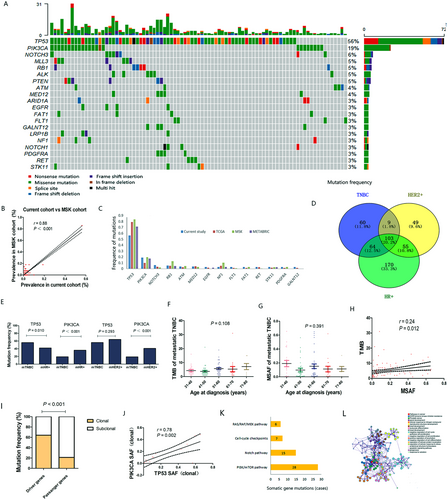
To evaluate the mutated genes in TNBC patients with distant metastases, we compared the frequency of mutations observed in the current cohort (n = 128) to those in early-stage TNBC patients from The Cancer Genome Atlas (TCGA) (n = 207), MSK (n = 198), and Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) cohorts (n = 309). Fourteen genes were observed as mutated with different frequencies in metastatic and early-stage TNBC patients (P < 0.05) (Figure 1C). We then compared the frequencies of these mutations in the 128 metastatic TNBC patients with those in 261 metastatic hormonal receptor (HR)-positive patients, and 70 HER2-positive patients from our NGS datasets (Supplementary Materials and Methods). Of the 236 mutated genes, 60 were only observed in metastatic TNBCs, while the remaining 176 were also found in metastatic HR-positive or HER2-positive breast cancers (Figure 1D). PIK3CA was less frequently mutated in metastatic TNBCs than in metastatic HR-positive and HER2-positive breast cancers (both P < 0.001), while TP53 was more frequently mutated in metastatic TNBCs than in HR-positive breast cancers (P = 0.010) (Figure 1E).
Considered as potential prognostic factors, tumor mutation burden (TMB) and maximum mutant allele frequency (MSAF) were calculated based on an NGS panel of 1021 genes. Among the 110 patients with gene mutations, TMB varied remarkably, ranging from 1 to 31 (median, 3); MSAF ranged from 0.3% to 65.4% (median, 5.4%). Then, we assessed the relationships of TMB and MSAF with age at diagnosis, but no significant relationship were observed (Figure 1F, G). Also, there was weak correlation between TMB and MSAF in metastatic TNBCs (r = 0.24, P = 0.012) (Figure 1H).
Moreover, clonal analysis of metastatic TNBCs was performed to evaluate the somatic evolutionary relationship. Based on our data, driver genes had a higher proportion of clonal mutations than potential passenger genes (64.3% vs. 21.3%, P < 0.001) (Figure 1I). TP53, with a clonal mutation proportion of 70.7% (53/75), and PIK3CA, with a clonal mutation proportion of 61.5% (16/26), were the most likely to participate in the carcinogenesis and development of TNBCs. Thirteen metastatic TNBC patients harbored clonal mutations of both TP53 and PIK3CA. Somatic allele frequency of TP53 clonal mutations were markedly correlated with that of PIK3CA clonal mutations (r = 0.78, P = 0.002) (Figure 1J), indicating their coexistence pattern in tumor clonal structure of TNBCs.
Additionally, 70 somatic mutations involved in potentially actionable pathways in TNBC were observed in 46 patients of the current cohort, mainly including the phosphatidylinositol 3-kinase (PI3K)/mechanistic target of rapamycin kinase (mTOR) pathway (28 cases), NOTCH pathway (15 cases), cell cycle checkpoints (7 cases), and RAS/RAF/mitogen-activated protein kinase (MEK) pathway (6 cases) (Figure 1K). Functional enrichment analysis showed that the mutated genes in metastatic TNBCs were tightly related to pathways in cancer (hsa05200), EGFR tyrosine kinase inhibitor resistance (hsa01521), gland development (GO: 0048732), diseases of signal transduction (R-HSA-5663202), and PI3K/AKT signaling in cancer (R-HSA-2219528) (Figure 1L, Supplementary Figure S1).
Of the 128 metastatic TNBC patients, 45 had lung metastasis, 29 had liver metastasis, 41 had bone metastasis, and 18 had brain metastasis; 62 (56.4%) had multiple distant organs being involved. The number of involved distant organs was not correlated with TMB (r = 0.134, P = 0.162) and MSAF (r = 0.256, P = 0.007). Of the 236 mutated genes, 31 were only observed in patients with lung metastasis, 23 in patients with liver metastasis, 22 in patients with bone metastasis, and 4 in patients with brain metastasis (Supplementary Figure S2). TMB of metastatic TNBCs was not closely related with any involved distant organ, while MSAF was higher in patients with bone metastasis than in those without bone metastasis (P = 0.004) (Supplementary Figure S3A), indicating that tumor-related bone destruction might increase the amount of metabolically active tumor cells shedding into the blood.
Among the unique genes identified in patients with different metastatic sites, NOTCH1 (9%) was most frequently mutated and significantly associated with lung involvement; phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta (PIK3CB) (10%) and FA complementation group M (FANCM) (10%) were most frequently mutated and significantly related with liver involvement; NOTCH4 (7%) and protein kinase AMP-activated catalytic subunit alpha 1 (PRKAA1) (7%) were most frequently mutated and significantly associated with bone involvement; mitogen-activated protein kinase kinase 4 (MAP2K4) (11%) and nuclear receptor corepressor 1 (NCOR1) (11%) were most frequently mutated and significantly related with brain involvement (Supplementary Figure S3B). Functionally enriched genes varied in patients with different sites of metastases using Metascape (Supplementary Figure S2).
The above mentioned 7 genes were linked to specific organ involvement in metastatic TNBCs. A total of 20 variants of these genes were identified: 1 nonsense and 3 missense mutations of NOTCH1, 3 missense mutations of PIK3CB, 3 missense mutations of FANCM, 3 missense mutations of NOTCH4, 3 missense mutations of PRKAA1, 2 missense mutations of MAP2K4, and 1 nonsense and 1 missense mutations of NCOR1. The consequence of these mutations on the activity of the encoded proteins was assessed using cBioPortal (Supplementary Figure S3C). Among them, 2 nonsense and 3 missense mutations were classified as pathogenic, which have been reported in the COSMIC database, and 8 missense mutations were interpreted to be likely pathogenic via in silico analysis.
PIK3CB c.2642T>A, NOTCH1 c.2983G>A, NOTCH4 c.3721G>C, and FANCM c.437T>C were evaluated as novel variants for further study of metastatic TNBCs (Supplementary Table S2). These candidate genes might be closely associated with carcinogenesis and tumor progression. NOTCH1, a key receptor in the Notch signaling pathway, has been shown to promote proliferation, invasion, and metastasis of cancer cells [5]. PIK3CB, known as PI3K p110β subunit, has been recognized as an important biomarker of cancer recurrence and prognosis [6]. FANCM, a breast cancer susceptibility gene, confers a particularly strong predisposition for TNBC [7]. NOTCH4 was found to be associated with metastasis in various cancers [8]. PRKAA1 has been reported to encode the α-subunit of 5-AMP-activated protein kinase (AMPK) and participate in cancer pathogenesis and progression [9]. MAP2K4 was found to play an important role in cancer metastasis [10]. NCOR1 was down-regulated in several types of cancer and associated with poor prognosis [11].
Overall, using ctDNA target-captured sequencing of metastatic TNBCs, we found that the mutational profile of metastatic TNBC was different from those of early-stage or HR-/HER2-positive breast cancers), and identified PIK3CB c.2642T>A, NOTCH1 c.2983G>A, NOTCH4 c.3721G>C, and FANCM c.437T>C as potentially actionable targets for further study of metastatic TNBCs.
ACKNOWLEDGMENTS
This work was supported by the National Key R&D Program of China (2018YFC1312101), Chinese Academy of Medical Sciences (CAMS) Initiative for Innovative Medicine (CAMS-12M-1-010, 2017-12M-3-004), Major Project of Beijing Municipal Science and Technology Commission (D161100000816004), National Science and Technology Major Project of the Ministry of Science and Technology, China (2015ZX09101007), and Beijing Municipal Science and Technology Project (D161100000816004).
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research including animal study was approved by the Institutional Review Board and Human Ethics Committee of at National Cancer Center/ National Clinical Research Center for Cancer/ Cancer Hospital, Chinese Academy of Medical Science, and Peking Union Medical College. Written informed consent for using the samples for research purposes was obtained from all patients.
CONSENT FOR PUBLICATION
The authors declare that written informed consent has been provided for the publication of any associated data and accompanying images.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
RG interpretation of data and drafting the manuscript
YZ sample collection and interpretation of data
MF sample collection and draft revision
GY, DNA sequencing analysis
YX, DNA sequencing analysis
LL, DNA sequencing analysis
XB, design of the work and draft revision
All authors read and approved the final manuscript.
AVAILABILITY OF DATA AND MATERIAL
All data generated or analyzed during this study are included in this published article.




