Clinical outcomes of coronavirus disease 2019 (COVID-19) in cancer patients with prior exposure to immune checkpoint inhibitors
Abbreviations
-
- COVID-19
-
- coronavirus disease 2019
-
- CTLA-4
-
- cytotoxic T-lymphocyte-associated protein 4
-
- ICI
-
- immune checkpoint inhibitor
-
- IL-6
-
- interleukin-6
-
- PD-1
-
- programmed cell death protein 1
-
- PD-L1
-
- programmed cell death-ligand 1
-
- SARS-CoV-2
-
- severe acute respiratory syndrome coronavirus 2
Dear Editor,
The coronavirus disease 2019 (COVID-19) pandemic has affected over 6,000,000 people globally [1]. Patients with COVID-19 manifest with symptoms of fever, dry cough, dyspnea, and present with radiological changes that are consistent with atypical pneumonia [2]. Pathogenetic mechanisms for these abnormalities involve the systemic immune response that is associated with the hyperactivation of peripheral CD8+ and CD4+ T cells, and a cytokine storm [3]. Globally, the reported prevalence of patients with COVID-19 and cancer ranges from 0.5% to 6.0% in the different case series [4]. Consistent with these studies, we had previously reported that patients with cancer harbored an approximately 2-fold higher risk of COVID-19 than non-cancer patients, thereby indicating that this group of patients represents a susceptible population [5].
Immune checkpoint inhibitors (ICI) targeting the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), or programmed death-ligand 1 (PD-L1) immuno-inhibitory axis have demonstrated single-agent activity in treatment-refractory cancers, and have also demonstrated synergism with chemotherapy and radiotherapy in the first-line setting [6]. Anti-CTLA-4 and anti-PD-1/-PD-L1 antibodies work by targeting the T-cell exhaustion pathways, thereby resulting in reactivation of cytotoxic CD8+ T cells for anti-tumor activity [6]. Given the convergence of the downstream effects on the innate immunity by both ICI and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections, we therefore queried whether patients with cancer and prior exposure to ICI would present with a different trajectory of COVID-19 illness. We hypothesized that patients who received a longer course of ICI would more likely develop severe COVID-19 than those with brief exposure to this class of anti-cancer therapies.
In the present study, we reviewed the medical records of two tertiary cancer institutions, namely the Zhongnan Hospital of Wuhan University and the Tongji Hospital of Huazhong University of Science and Technology, both in Hubei, China, from the period of January 9, 2020, to March 20, 2020. This retrospective study was approved by the Medical Ethics Committee of Zhongnan Hospital of Wuhan University (ZN-IRB20200039) and the Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology (TJ-IRB20200349). Waiver of informed consent was approved for the aggregated data. Verbal informed consent for publication was obtained from living patients or the relatives of deceased patients.
Data of eleven cancer patients who had prior exposure to ICIs and subsequently diagnosed with COVID-19 (5th edition of COVID-19 diagnosis by the National Health and Health Commission of China) were obtained. Their median age was 66 (range: 29-73) years. Seven (63.64%) cases were lung cancers, while the remaining 4 (36.36%) patients had cervical, endometrial, hepatocellular, and colorectal cancers. The median number of ICI cycles underwent was 3 (range: 1-14). Ten (90.91%) patients received anti-PD-1 antibody, while 1 (9.09%) patient received doublet anti-PD-1 and anti-CTLA-4 antibody treatment (Table 1). The median interval between the date of last ICI treatment and the onset of COVID-19 symptoms was 25 (range: 3-47) days. Common symptoms of the eleven patients included fever (9 of 11, 81.82%), cough (9 of 11, 81.82%), and dyspnea (7 of 11, 63.64%). We observed lymphopenia (< 1.0 × 109/L) in 9 of 11 (81.82%) patients (median: 0.50 × 109 [range: 0.10-2.04 × 109]/L).
| COVID-19 infection severity | |||
|---|---|---|---|
| Total cases | Mild | Severe | |
| Variables | n (%) | n (%) | n (%) |
| Number of cases | 11 (100.00) | 4 (100.00) | 7 (100.00) |
| Clinical characteristics | |||
| Median age, years (range) | 66 (29-73) | 66 (61-69) | 66 (29-73) |
| Sex | |||
| Male | 8 (72.73) | 3 (75.00) | 5 (71.43) |
| Female | 3 (27.27) | 1 (25.00) | 2 (28.57) |
| Cancer types | |||
| Lung cancers | 7 (63.63) | 3 (75.00) | 4 (57.14) |
| Cervical squamous cancer | 1 (9.09) | 1 (25.00) | 0 |
| Endometrial cancer | 1 (9.09) | 0 | 1 (14.29) |
| Hepatocellular carcinoma | 1 (9.09) | 0 | 1 (14.29) |
| Colorectal adenocarcinoma | 1 (9.09) | 0 | 1 (14.29) |
| Smoking history | |||
| Absent | 5 (45.45) | 3 (75.00) | 2 (28.57) |
| Present | 6 (54.55) | 1 (25.00) | 5 (71.43) |
| Comorbidities | |||
| Absent | 6 (54.55) | 2 (50.00) | 4 (57.14) |
| Present | 5 (45.45) | 2 (50.00) | 3 (42.86) |
| ICI | |||
| Pembrolizumab | 3 (27.27) | 1 (25.00) | 2 (28.57) |
| Nivolumab | 1 (9.09) | 0 | 1 (14.29) |
| Sintilimab | 4 (36.36) | 3 (75.00) | 1 (14.29) |
| Camrelizumab | 2 (18.18) | 0 | 2 (28.57) |
| Ipilimumab + Nivolumab | 1 (9.09) | 0 | 1 (14.29) |
| Median cycles of ICI (range) | 3 (1-14) | 2 (1-3) | 3 (1-14) |
| Tumour response to ICI | |||
| PR | 4 (36.36) | 1 (25.00) | 3 (42.86) |
| SD | 3 (27.27) | 2 (50.00) | 1 (14.29) |
| PD | 2 (18.18) | 0 | 2 (28.57) |
| Non applicable | 2 (18.18) | 1 (25.00) | 1 (14.29) |
| Median time interval between ICI and COVID19, days (range) | 25 (3-47) | 28 (3-38) | 25 (3-47) |
| CT evidence of pneumonia | |||
| Absent | 0 | 0 | 0 |
| Present | 11 (100.00) | 4 (100.00) | 7 (100.00) |
| Laboratory characteristics median (range) | |||
| White blood cell (× 109/L) | 5.07 (1.50-12.37) | 4.51 (3.38-7.63) | 7.46 (1.50-12.37) |
| Lymphocyte (× 109/L) | 0.50 (0.10-2.04) | 0.55 (0.28-1.32) | 0.50 (0.10-2.04) |
| Lymphopenia (< 1.0 × 109/L) | |||
| Absent | 2 (18.18) | 1 (25.00) | 1 (14.29) |
| Present | 9 (81.82) | 3 (75.00) | 6 (85.71) |
| C-reactive protein (mg/L) | 82.10 (16.50-127.03) | 80.45 (31.4-112.0) | 82.10 (16.50-127.03) |
| Procalcitonin (ng/mL) | 0.10 (0.02-19.44) | 0.13 (0.05-19.44) | 0.10 (0.02-0.72) |
| IL-6 (pg/mL) | 12.87 (6.57-386.00) | 33.10 (6.57-59.63) | 12.87 (8.83-386.00) |
| D-dimers (ng/mL) | 2105.0 (439.0-36370.0) | 1795.5 (1021.0-2570.0) | 2105.0 (439.0-36370.0) |
| Treatments | |||
| Oxygen support | 11 (100.00) | 4 (100.00) | 7 (100.00) |
| Antiviral therapy | 8 (72.73) | 3 (75.00) | 5 (71.43) |
| Antibiotic therapy | 9 (81.82) | 3 (75.00) | 6 (85.71) |
| Use of corticosteroid | 5 (45.45) | 1 (25.00) | 4 (57.14) |
| Clinical outcome | |||
| Discharge | 6 (54.55) | 4 (100.00) | 2 (28.57) |
| Under treatment | 1 (9.09) | 0 | 1 (14.29) |
| Death | 4 (36.36) | 0 | 4 (57.14) |
- Abbreviations: COVID-19 = coronavirus disease 2019; ICI = immune checkpoint inhibitor; IL-6 = interleukin-6; PD = progressive disease; PR = partial response; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SD = stable disease.
Seven (63.64%) patients developed severe COVID-19 disease, which was defined by the presence of: 1) respiratory rate of ≥ 30 breaths per minute; 2) oxygen saturation of ≤ 93% at room air; 3) arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) of ≤ 300 mmHg; 4) respiratory failure that required mechanical ventilation; 5) presence of septic shock, and; 6) multi-organ failure that required intensive care unit treatment. We observed that 6 of 7 (85.7%) patients who received 3 or more cycles of ICI developed severe COVID-19 as compared to 1 of 4 (25%) patients receiving less than 3 cycles of ICI developed severe illness, albeit this difference was not statistically significant. (P = 0.09 by Fisher's exact test, Figure 1A and 1B). We also did not observe an association between the time interval from the last ICI administration and severity of COVID-19 (4 of 6 [66.67%] received ICI within < 28 days vs. 3 of 5 [60.00%] at ≥ 28 days, P = 1.00 by Fisher's exact test; Figure 1A). Interestingly, D-dimer levels were elevated in the severely affected cases than in the mildly affected cases (median: 2105.0 vs 1795.5 ng/mL, P = 0.64 by Mann Whitney test). Four (36.36%) of the eleven patients died from COVID-19. The clinical timelines of all the patients are outlined in the Supplementary file.
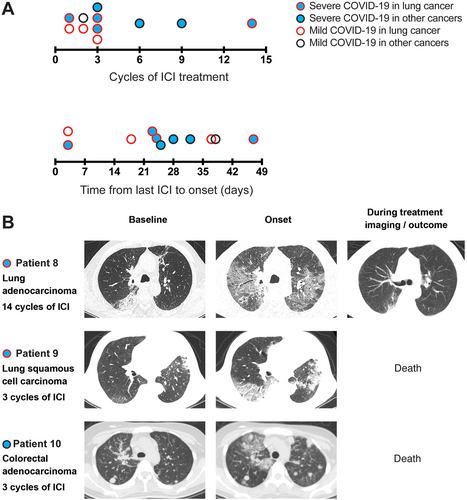
A recent study in lung cancer patients by Luo et al. [7] suggested that individuals with prior exposure to ICI were not more likely to develop severe COVID-19 than those who never received ICI, regardless of the interval from the last dose received. However, the authors did not test the association between duration and number of cycles of ICI and severity of COVID-19 in their cohort. Additionally, the effect of ICI treatment on COVID-19 severity in patients with other malignancies is uncertain. In this study, we added the outcomes of 11 patients with lung cancers (7 cases), cervical cancer (1 case), endometrial cancer (1 case), hepatocellular cancer (1 case), and colorectal cancer (1 case), who were diagnosed with COVID-19 and had previously received ICI. We observed a higher proportion of severe COVID-19 in cancer patients who received ≥ 3 cycles of ICI, although this finding was not statistically significant. Similar to the study by Luo et al. [7], we did not observe an association between the interval of last ICI administration and COVID-19 severity.
A plausible explanation underpinning the notion that ICI exposure may exacerbate COVID-19 illness is linked to the mechanism of T-cell hyperactivation causing further damage to the respiratory epithelium [8, 9]. Besides, severe cytokine release syndrome is also a known toxicity of ICI [10], despite interleukin-6 (IL-6) was not elevated among the severely affected patients in the present study. The cross-talk between the respective immune activation pathways that are secondary to ICI treatment and COVID-19-induced cytokine release syndrome remains unclear; admittedly, the former is linked to the innate immunity, while the latter is mostly triggered by the humoral immune response. Finally, we observed higher D-dimer levels in severe than mild cases in our study, which was consistent with previous findings showing its probable association with a higher case-fatality rate [11]. Elevated D-dimer levels are typically associated with disseminated coagulopathy and subsequent multiple organ dysfunction, which have been observed in patients who died from COVID-19 [12].
In the present case series, we report that a high proportion (63.63%) of patients with cancer, who received prior ICI, presented with severe COVID-19 complications. Here, we further observed that the onset of severe COVID-19 and mortality due to the illness may be linked to the duration of exposure based on the number of ICI cycles received; however, this association was not statistically significant, possibly owing to the limited size of our study cohort. Larger cohort studies are thus needed to better characterize the effects of ICI on cancer patients who develop COVID-19. Regardless, strict infection control measures to prevent the spread of SARS-Cov-2 such as social distancing, wearing of protective mask, hand hygiene, and temperature monitoring, are mandated, especially in patients with cancer, given the potential exacerbation of COVID-19 by anti-cancer therapies [13].
DECLARATIONS
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of Zhongnan Hospital of Wuhan University (ZN-IRB20200039) and the Ethics Committee of the Tongji Medical College of Huazhong University of Science and Technology (TJ-IRB20200349). Waiver of informed consent was approved for the aggregated data. Verbal informed consent was obtained from the living patients.
CONSENT FOR PUBLICATION
Verbal informed consent for publication was obtained from the living patients or the relatives of deceased patients.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
This study was supported by the National Medical Research Council Clinician-scientist award (NMRC/CSA/0027/2018), the Health Commission of Hubei Province Scientific Research Project (WJ2019H002), Health Commission of Hubei Province Medical Leading Talent Project, Fundamental Research Funds for the Central Universities (2042018kf1037, 2042019kf0329), Medical Science Advancement Program (Basic Medical Sciences) of Wuhan University (TFJC2018005), Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund (znpy2017049, znpy2018070).
AUTHORS' CONTRIBUTIONS
QJW, QC, HYZ, BY, XDH collected and analyzed the patient data. QJW, QC, HYZ, YHZ, XLY, MLKC, and CHX interpreted the results and discussed in the study. QJW, MLKC were major contributors in writing the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
Not applicable




