A meta-analysis of the accessory left atrial appendage and the left atrial diverticulum
Abstract
Left atrial (LA) structures, including the accessory left atrial appendage (aLAA) and left atrial diverticulum, have been studied based on their prevalence, shape, and association with arrhythmia and thrombi formation. A pooled prevalence with morphometric data has not been determined in previous research. Our goal is to provide structured, clinically relevant information on said structures for clinical practitioners to use in their daily work. We propose that morphometric data of additional LA structures is necessary when considering the possible complications during cardiac interventions. We conducted a meta-analysis of all relevant studies which used electrocardiogram (ECG)-gated computed tomography (CT) imaging to determine the prevalence of LA structures and record their morphometric characteristics as well as the presence of thrombi. Data were extracted from 19 studies (n = 6643 hearts). The pooled prevalence estimate of left atrial diverticulum and/or aLAAs were reported from 14 studies and was 28.8%. The most common location noted was anterosuperior in the LA with 70.2% of structures found there. Data regarding thrombi presence in left atrial diverticulums or aLAAs were extracted from 11 studies and a thrombus was present in 0.2%. The prevalence rates of aLAAs and left atrial diverticulums are essential in performing uncomplicated cardiac interventions and reducing risk of electrophysiological procedures. Our findings show a considerable prevalence of LA structures in varying populations, provides information regarding the general characteristics of said structures, and does not support the previously theorized associated risk of thrombus formation in relation to LA structure presence.
1 INTRODUCTION
The left atrium has four components consisting of left atrium vestibule, pulmonary veins, interatrial septum, and left atrial appendage. The left atrium, an integral component of the cardiovascular system, stands as one of the four chambers composing the heart's architecture. When observed from the anterior perspective of the thorax, the left atrium occupies the most posterior position among the cardiac chambers. The left atrium is more posteriorly and superiorly situated relative to the right atrial chamber, anterior to the esophagus and thoracic spine. It is bordered superiorly by the left pulmonary artery and inferiorly by the left ventricle. The four pulmonary veins, originating from the lungs, enter the posterior wall of the left atrium. Their location typically coincides with the junction of the left atrium and the left atrial appendage. The interatrial septum separates the left atrium from the right atrium and serves as a partition between them. The left atrial appendage (LAA) arises from the anterior aspect of the left atrium, commonly in the superior and anterior regions. Its location can exhibit variations contributing to the complexity of the left atrial anatomy (Bochenek & Reicher, 2015; Whiteman et al., 2019; Yen Ho et al., 2012) (Figures 1-4).
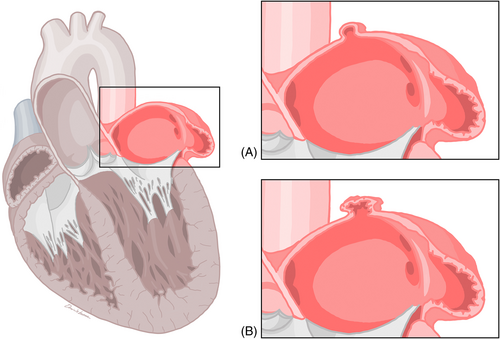
The accessory left atrial appendage (aLAA) and left atrial diverticulum are two morphologically and structurally variable focal outpouchings identified in the left atrium (LA). Even though a consistent definition has not been established, the aLAA and left atrial diverticulum can be differentiated based on their shape, ostium characteristics, and body contour. The left atrial diverticulum typically has a sac-like, smooth body with a broad-based ostium. The aLAA can be identified by its ostium associated with a visible neck, and body with irregular contours that are continuous with the pectinate muscles (Abbara et al., 2009; Balli et al., 2012; Duerinckx & Vanovermeire, 2008; Hoey et al., 2011; Incedayi et al., 2012; Ko et al., 2013; Lazoura et al., 2012; Patel et al., 2013). These differences can be visualized when using multidetector computed tomography (MDCT), the main method of their study. Controversy still exists with regards to the etiology of left atrial diverticulums and aLAAs. Some evidence supports a congenital etiology. aLAAs are most likely embryological remnants of a primitive LA. On the other hand, left atrial diverticulums may arise in areas of lower tissue resistance where the LA wall is weaker and wall pressure forms an outpouching (Nagai et al., 2011; Nomura et al., 2008; Srinivasan et al., 1980; Terada et al., 2000). An acquired etiology also exists whereby a weakening in the LA wall forms due to pathophysiological complications such as arrhythmia, systemic thrombosis, or myocardial infarction (Wan et al., 2009).
The function of both the aLAA and left atrial diverticulum has not been elucidated, as they tend to be incidentally discovered. There have been isolated associations with arrhythmias, thromboemboli, mitral regurgitation, and pericarditis (Poh et al., 2008; Srinivasan et al., 1980; Terada et al., 2000). However, like the left atrial appendage, the left atrial diverticulum and aLAA are theoretical sites of thrombus formation, especially in patients with atrial fibrillation or coagulation disorders, thus they may be a source of embolic stroke (Abbara et al., 2009; Duerinckx & Vanovermeire, 2008; Hołda et al., 2017; Incedayi et al., 2012; Killeen et al., 2010; Ko et al., 2013; Lazoura et al., 2012; Peng et al., 2012; Poh et al., 2008; Troupis et al., 2012; Üçerler et al., 2013). In addition, procedures involving catheter placement in the LA could be associated with potential complications, such as catheter entrapment and left atrial wall perforation if the presence of these structures is unknown (Hołda et al., 2017; Peng et al., 2012). It is, therefore, essential that possible aLAAs or left atrial diverticulums are identified before these procedures.
In this study, we completed a meta-analysis to investigate various parameters of the aLAA and left atrial diverticulum including their length, width, ostium diameter, shape, location, and number. We also aimed to determine the anatomical prevalence of the aLAA and left atrial diverticulum as well as the presence of associated thrombi. Knowledge of these characteristics are important for the successful completion of cardiac procedures. The anatomical studies were deemed reliable by following the criteria of the Anatomical Quality Assessment (AQUA) tool.
2 METHODS
2.1 Search strategy
We performed a systematic literature review of articles involving the aLAA. Using the search terms ‘accessory left atrial appendage’ or ‘left atrial appendage anatomy’, we searched for articles through January 10, 2019 in the main electronic databases (PubMed, Embase, Science Direct, Web of Science, and Google Scholar). No language or lower date restrictions were applied. We then also included relevant references from the initial searched articles. This search and the performance of the meta-analysis was done in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
2.2 Criteria for study extraction
Two independent reviewers (F.R. & J.R.) evaluated all studies to determine their eligibility for data extraction. Studies were eligible if they (1) included complete prevalence data for aLAAs and/or left atrial diverticulums in human hearts, (2) included aLAA and/or left atrial diverticulum morphometric data, and (3) were radiological assessments or cadaveric dissections. The eligibility assessment for the acquired articles was performed by two independent reviewers (F.R. & J.R.).
The exclusion criteria used for data extraction were (1) case studies, reviews, letters to the editor, essays, and conference abstracts, (2) studies containing irrelevant, partial, missing, or incomplete data. Disagreements regarding the eligibility of studies for data extraction were resolved via consensus among the reviewers and by contacting the original study authors.
2.3 Data extraction
Two independent reviewers (F.R. & J.R.) reviewed the data extraction. Data from the extraction included the total pooled prevalence, locations, length, width, and ostium diameter of the left atrial diverticulum and aLAAs. The shape of left atrial diverticulum or aLAA, the number (single or multiple) found in each heart, and the presence of thrombi were also assessed.
2.4 Quality assessment
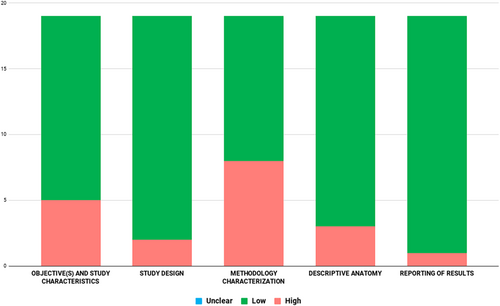
Two co-authors (F.R. & J.R.) independently analyzed the studies for possible biases using the AQUA tool. Studies were examined for risk of bias in the following five domains: objective(s) and study characteristics, study design, methodology characterization, descriptive anatomy, and reporting the results. Bias was categorized according to ‘high’, ‘low’, or ‘unclear’ risk. A ‘high’ risk of bias was associated with multiple domains being unsatisfactory after analysis. A ‘low’ risk of bias was associated with few or no domains being unsatisfactory after analysis. If bias was determined to be ‘unclear’ there was insufficient or unclear data to determine the bias associated with the study.
2.5 Statistical analysis
The prevalence data for the included studies was pooled via MetaXL version 5.3 (EpiGear International). Comprehensive Meta-Analysis (Version 3.3, Biostat) was used to pool morphometric data. Single and multi-categorical pooled prevalence rates were calculated via a random-effects model. The chi-square test and I2 statistic were used to determine the heterogeneity for each study in accordance with the guidelines from the Cochrane Handbook for Systematic Reviews of Interventions. p values of <0.10 were considered statistically significant for heterogeneity in the chi-square test. For the I2 statistic, values were interpreted as follows: 0%–40% may be unimportant, 30%–60% may indicate moderate heterogeneity, 50%–90% may indicate substantial heterogeneity, and 75%–100% may indicate considerable heterogeneity.
Subgroup analysis was used in assessing sources of heterogeneity; studies were categorized according to study type (radiological or cadaveric dissection) and geography region. Significant differences between two subgroups were determined via 95% CIs, and differences were considered statistically insignificant if 95% CIs between two groups or subgroups overlapped.
3 RESULTS
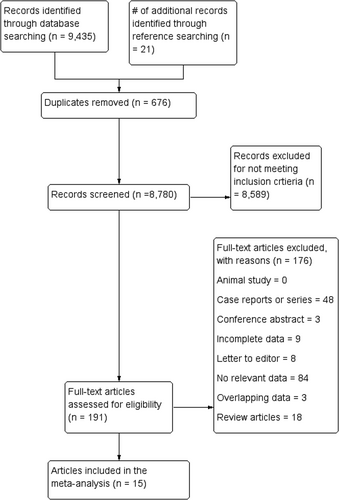
3.1 Study identification and characteristics of included studies
After the initial search, 150 studies were identified. Through analysis of the data, 135 studies were excluded. Data were extracted from 15 articles, where four of the included articles had two separate populations, making it a total of 19 studies. Characteristics of these studies are shown in Table 1. These 19 studies were published from 2008 (Duerinckx & Vanovermeire, 2008) to 2018 (Hołda et al., 2017) for a total of 6643 hearts. With the exception of one cadaveric study, all were radiological. All studies originated from eight different countries in North America, Europe, Asia, or Australia (Table 1). After assessment with the AQUA tool, most studies that were included in this meta-analysis showed ‘low’ risk of bias in most domains. The highest risk of bias was in domain three (methodology characterization) (Appendix A).
| Study ID | Country | Type of study | n = (patients) |
|---|---|---|---|
| Abbara et al., 2009 | USA | Radiological | 529 |
| Duerinckx 2008 | USA | Radiological | 166 |
| Genc 2014 | Turkey | Radiological | 1305 |
| Hołda 2017 | Poland | Radiological | 294 |
| Incedayi 2012 | Turkey | Radiological | 454 |
| Killeen 2009 | Ireland | Radiological | 102 |
| Ko 2013 | South Korea | Radiological | 270 |
| Ko 2013 (stroke) | South Korea | Radiological | 270 |
| Lazoura et al., 2012 (atrial fibrillation) | UK | Radiological | 200 |
| Lazoura et al., 2012 (sinus rhythm) | UK | Radiological | 200 |
| Patel 2013 | UK | Radiological | 46 |
| Peng 2012 (atrial fibrillation) | China | Radiological | 214 |
| Peng 2012 (sinus rhythm) | China | Radiological | 214 |
| Poh 2008 | USA | Radiological | 50 |
| Shin 2011 | South Korea | Radiological | 2059 |
| Troupis 2011 (atrial fibrillation) | Australia | Radiological | 47 |
| Troupis 2011 (sinus rhythm) | Australia | Radiological | 47 |
| Ucerler 2013 | Turkey | Cadaveric | 56 |
| Wan 2008 | China | Radiological | 120 |
3.2 Prevalence of left atrial diverticulum and/or aLAA
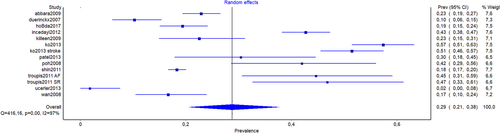
The pooled prevalence estimate (PPE) of left atrial diverticulum and/or aLAAs were reported from 14 studies (n = 4510). The overall PPE of left atrial diverticulum and/or aLAA was 28.8% (95% CI, 20.6%–37.7%) (Table 2). When PPE was divided based on continent of origin, the value for Europeans (n = 442) was 22.1% (95% CI, 17.0%–27.6%), for Asians (n = 3229) was 28.6% (95% CI, 13.5%–46.4%), and for North Americans (n = 745) was 22.4% (95% CI, 10.1%–37.6%). The total PPE of left atrial diverticulum only was extracted from 9 studies (n = 3290) and was 29.8% (95% CI, 20.9–39.6%). For Asians, data were extracted from three studies (n = 1973); the PPE of left atrial diverticulum was 40.6% (95% CI, 33.2%–48.1%). The total PPE of aLAAs only was extracted from nine studies (n = 3132). The overall PPE was 5.5% (95% CI, 4.2%–6.9%). For Asians, the total PPE of aLAA data were extracted from three studies (n = 1815) and the PPE was 4.0% (95% CI, 2.8%–5.4%).
| Category | # of studies (# of subjects) | Pooled prevalence: % (95% CI) | I2 (95% CI) |
|---|---|---|---|
| Overall | 14 (4510) | 28.8 (20.6–37.7) | 96.9 (95.8–97.7) |
| Female | 6 (446) | 19.8 (10.5–31.1) | 84.0 (66.7–92.3) |
| Male | 6 (757) | 25.5 (17.2–34.8) | 83.7 (66.1–92.2) |
| Europe | 3 (442) | 22.1 (17.0–27.6) | 32.1 (0.0–92.9) |
| Asia | 6 (3229) | 28.6 (13.5–46.4) | 98.6 (98.0–99.0) |
| North America | 3 (745) | 22.4 (10.1–37.6) | 92.4 (80.9–96.9) |
3.3 Most common location of left atrial diverticulum and/or aLAAs
Data regarding the location of left atrial diverticulum and/or aLAAs was extracted from nine studies (n = 1885). For 70.2% (95% CI, 56.0%–79.5%) of cases, the location was anterosuperior; for 15.7% (95% CI, 7.2%–25.5%) the location was lateral; for 3.5% (95% CI, 0.1%–9.9%) the location was medial; and in 10.6% (95% CI, 3.7%–19.4%) of patients, the location was described as ‘Other’. Data regarding the location of left atrial diverticulum were extracted from six studies (n = 1081). The most common location was anterosuperior (78.7%; 95% CI, 55.7%–88.3%). Data regarding the location of aLAAs were retrieved from three studies (n = 115). The most common location of the aLAAs was lateral (62.5%; 95% CI, 16.9%–100.0%) (Tables 3–7).
| Category | # of studies (# of subjects) | Pooled prevalence: % (95% CI) | I2 (95% CI) |
|---|---|---|---|
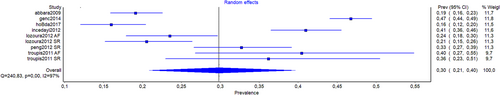
| Overall | 9 (3290) | 29.8 (20.9–39.6) | 96.7 (95.2–97.7) |
| Female | 4 (453) | 24.4 (12.3–38.7) | 90.2 (77.9–95.7) |
| Male | 4 (958) | 34.9 (24.7–45.9) | 91.3 (80.7–96.0) |
| Asia | 3 (1973) | 40.6 (33.2–48.1) | 88.3 (67.5–95.8) |
| Category | # of studies (# of subjects) | Pooled prevalence: % (95% CI) | I2 (95% CI) |
|---|---|---|---|
| Overall | 9 (3132) | 5.5 (4.2–6.9) | 54.3 (3.2–78.4) |
| Asia | 3 (1815) | 4.0 (2.8–5.4) | 28.8 (0.0–92.6) |
| Category | # of studies (# of subjects) | Anterosuperior % (95% CI) | Lateral % (95% CI) | Medial % (95% CI) | Other % (95% CI) | I2 (95% CI) |
|---|---|---|---|---|---|---|
| Overall | 9 (1885) | 70.2 (56.0–79.5) | 15.7 (7.2–25.5) | 3.5 (0.1–9.9) | 10.6 (3.7–19.4) | 95.6 (93.5–97.1) |
| Asia | 4 (1647) | 43.1 (30.3–54.3) | 29.3 (18.2–40.3) | 6.4 (1.4–13.7) | 21.2 (11.6–31.5) | 95.4 (91.0–97.6) |
| Category | # of studies (# of subjects) | Anterosuperior % (95% CI) | Lateral % (95% CI) | Medial % (95% CI) | Other % (95% CI) | I2 (95% CI) |
|---|---|---|---|---|---|---|
| Overall | 6 (1081) | 78.7 (55.7–88.3) | 7.0 (0.0–19.2) | 5.5 (0.0–14.8) | 8.8 (0.5–21.6) | 95.8 (93.1–97.5) |
| Category | # of studies (# of subjects) | Anterosuperior % (95% CI) | Lateral % (95% CI) | Medial % (95% CI) | Other % (95% CI) | I2 (95% CI) |
|---|---|---|---|---|---|---|
| Overall | 3 (115) | 14.3 (0.0–54.5) | 62.5 (16.9–100.0) | 17.1 (0.0–59.0) | 6.2(0.0–38.4) | 92.8 (82.4–97.1) |
3.4 Most common morphometric data for left atrial diverticulum and/or aLAAs
The average length of left atrial diverticulum and/or aLAAs was 5.0 mm (95% CI, 4.9–5.2 mm; eight studies, n = 882), further subdivided by geographic origin (Table 8). The average length of left atrial diverticulum was 5.7 mm (95% CI, 5.5–5.9 mm; nine studies, n = 952).
| Category | # of studies (# of subjects) | Length [mm] (95% CI) | I2 |
|---|---|---|---|
| Overall | 8 (882) | 5.0 (4.9–5.2) | 91.3 |
| Europe | 3 (85) | 6.5 (5.8–7.2) | 86.6 |
| Asia | 3 (649) | 4.8 (4.6–5.0) | 77.6 |
The average width of left atrial diverticulum and/or aLAAs was 4.7 mm (95% CI, 4.6–4.9 mm; three studies, n = 680). The ostium diameter of left atrial diverticulums and/or aLAAs was ostium diameter was 5.6 mm (95% CI, 5.1–6.0 mm, four studies, n = 179). Most left atrial diverticulum and/or aLAAs were cystic (72.2%; 95% CI, 51.8%–88.2%, three studies, n = 1267) (Tables 9–16).
| Category | # of studies (# of subjects) | Width [mm] (95% CI) | I2 |
|---|---|---|---|
| Overall | 3 (680) | 4.7 (4.6–4.9) | 90.1 |
| Category | # of studies (# of subjects) | Ostium diameter (95% CI) | I2 |
|---|---|---|---|
| Overall | 4 (179) | 5.6 (5.1–6.0) | 51.8 |
| Category | # of studies (# of subjects) | Length (95% CI) | I2 |
|---|---|---|---|
| Overall | 4 (952) | 5.7 (5.5–5.9) | 72.9 |
| Category | # of studies (# of subjects) | Cystic % (95% CI) | Tubular % (95% CI) | Other % (95% CI) | I2 (95% CI) |
|---|---|---|---|---|---|
| Overall | 3 (1267) | 72.2 (51.8–88.2) | 26.1 (10.0–45.5) | 1.7 (0.0–8.9) | 97.3 (94.7–98.6) |
| Category | # of studies (# of subjects) | Single % (95% CI) | Multiple % (95% CI) | I2 (95% CI) |
|---|---|---|---|---|
| Overall | 10 (532) | 87.0 (81.2–93.0) | 13.0 (7.6–19.7) | 69.9 (42.2–84.3) |
| Asia | 5 (392) | 81.5 (77.5–85.2) | 18.5 (14.8–22.5) | 0.0 (0.0–76.3) |
| Category | # of studies (# of subjects) | Single % (95% CI) | Multiple % (95% CI) | I2 (95% CI) |
|---|---|---|---|---|
| Overall | 7 (858) | 90.9 (88.0–93.5) | 9.1 (6.5–12.0) | 17.3 (0.0–61.1) |
| Category | # of studies (# of subjects) | Single % (95% CI) | Multiple % (95% CI) | I2 (95% CI) |
|---|---|---|---|---|
| Overall | 7 (110) | 94.2 (89.0–97.9) | 5.8 (2.1–11.0) | 0.0 (0.0–52.1) |
| Category | # of studies (# of subjects) | Present % (95% CI) | I2 (95% CI) |
|---|---|---|---|
| Overall | 11 (984) | 0.2 (0.0–0.6) | 0.0 (0.0) |
3.5 Prevalence of thrombi in left atrial diverticulum and/or aLAAs
Data regarding the presence of thrombi in left atrial diverticulum and/or aLAA was extracted from 11 studies (n = 984) and a thrombus was present in 0.2% (95% CI, 0.0%–0.6%) of cases.
4 DISCUSSION
Through this meta-analysis, we collected and merged published data which investigated the prevalence of both aLAAs and left atrial diverticulum in various populations, and specific characteristics such as their most common shape, size, and ostium diameter. Notably, we investigated the presence of thrombi in a greater population of aLAAs and left atrial diverticulum (n = 984). With this meta-analysis, we hoped to consolidate the most relevant information regarding the two important structures outpouching from the LA and, as a result, improve outcomes related to cardiac pathology, transcatheter interventions, and cardiac surgery. This was completed using AQUA guidelines for anatomical studies to minimize bias and ensure accurate results.
Variations in LA anatomy include the presence of sac-like structures or outpouchings, the left atrial diverticulum and aLAA. Although these structures have vastly different characteristics, the subtle variations in their morphology may confer a challenge when differentiating them using CT and their classification can be highly subjective to each individual observer (Igawa et al., 2008). For this reason, when LA structures were not differentiated in previous research, the data were combined and labeled as left atrial diverticulum and/or aLAA in our meta-analysis. In our study, the total PPE of both left atrial diverticulums and aLAAs was found to be 28.8% (95% CI, 20.6%–37.7%). This value was most similar to the total PPE of left atrial diverticulums which was 29.8% (95% CI, 20.9%–39.6%). The total PPE of only aLAAs was found to be 5.5% (95% CI, 4.2%–6.9%). This could be a result of the difficulty in differentiating left atrial diverticulums from aLAAs and their subjective identification or different etiology of analyzed structures.
A considerable difference was found in the PPE of left atrial diverticulums and/or aLAAs in Asian populations as compared with both European and North American populations. The total PPE was 28.6% (95% CI, 13.5%–46.4%) in Asians; meanwhile it was 22.1% (95% CI, 17.0%–27.6%) and 22.4% (95% CI, 10.1%–37.6%) in Europeans and North Americans, respectively. Furthermore, our data showed the PPE of left atrial diverticulum only was 40.6% (95% CI, 33.2%–48.1%) in Asian populations as compared to the overall prevalence of 29.8% (95% CI, 20.9%–39.6%). This correlated with other studies looking at only Asian populations that found a high left atrial diverticulum prevalence (Incedayi et al., 2012; Ko et al., 2013; Peng et al., 2012; Shin et al., 2011; Üçerler et al., 2013; Wan et al., 2009). This could support a potential genetic component for the presence of the left atrial diverticulum in different global populations.
The location of these structures is difficult to categorize as it is strongly dependent on the observer who classifies the positions. For this reason, we simplified the locations into the most common ones: anterosuperior, lateral, medial, and ‘other.’ The ‘other’ classification contains all data that does not fit into the main criteria. When both aLAAs and left atrial diverticulums were considered or not differentiated, the most common location was anterosuperior, where 70.2% (95% CI, 56.0%–79.5%) of LA structures were located in this position. The second most common location was lateral at 15.7% (95% CI, 7.2%–25.5%). When separated based on accurate definitions of left atrial diverticulums and aLAAs, the most common left atrial diverticulum location was anterosuperior (78.7%; 95% CI, 55.7%–88.3%). While 8.8% (95% CI, 0.5%–21.6%) were found in a different location. Similarly to other studies, the anterosuperior location was the most common location for left atrial diverticulum (Abbara et al., 2009; Genç et al., 2014; Hołda et al., 2017; Incedayi et al., 2012; Lazorua et al., 2012; Peng et al., 2012). On the other hand, 62.7% (95% CI, 16.9%–100.0%) of aLAAs were found laterally while 17.1% (95% CI, 0.0%–59.0%) were found medially. As in previous research, the location of aLAAs was most typically inferolateral (Abbara et al., 2009; Genç et al., 2014; Incedayi et al., 2012). The differences in pooled location may be due to the increased prevalence of left atrial diverticulums compared with aLAAs, which may falsely show the most common location of both structures as anterosuperior.
Generally, accessory LA structures are deemed to have a cystic shape if the ratio of its length to ostium diameter is less than 1.5; previous studies agree that the structure is tubular if the ratio is above 1.5 (Genç et al., 2014; Ko et al., 2013; Shin et al., 2011). Genç et al. (2014) found that left atrial diverticulums may have cystic, tubular, conical, hook-shaped, bilobular, or both tubular and cystic forms. Similarly to the definition of location, the classification of the shape of the LA structure depends on the experience of the observer describing the findings. The most identifiable shape descriptions were cystic and tubular; all other descriptors specific to certain studies were classified as ‘other’. In this meta-analysis, 72.2% (95% CI, 51.8%–88.2%) of the left atrial diverticulums and/or aLAAs (n = 1267) were found to have a cystic shape and 26.1% (95% CI, 10.0%–45.5%) of these structures were said to have a tubular shape. These findings correlated with previous research that found 440 cystic left atrial diverticulums out of the 708 left atrial diverticulums observed (Genç et al., 2014). Variations in shape of the LA structure may confer increased risk for complications due to lodging of catheters into the more tubular shape. This can result in steam pop or coagulum formation (Igawa et al., 2008; Peng et al., 2012). Furthermore, the left atrial diverticulum wall was found to be thinner than the proper LA wall, making it more vulnerable to perforation (Peng et al., 2012).
Accurate classification of aLAAs is achieved using histopathology. Igawa et al. (2008) found an LA structure located near the atrial septum. Upon examination, the endocardial and myocardial layers resembled the surrounding tissue. Trabeculated myocardium was visible continuously with the normal LA wall. This was previously visualized in a case report by Terada et al. (2000) who also found normal myocardial wall morphology in LA structures which contracted in synchrony with the rest of the myocardium. Killeen et al. (2010) studied the contractile properties of both LA structures; they found that when ostium diameters measured over 10 mm, significant coordinated contraction was evident. Because these LA structures contain myocardial contractile tissue which contract in synchrony with the rest of the cardiac myocardium and this activity is greater with larger diameters, there is a possible risk of ectopic foci of electrical activity causing arrhythmia. Our study compiled the average ostium diameter of both additional LA structures (n = 179) and it was determined to be 5.6 mm (95% CI, 5.1–6.0 mm). Because our results found the ostium diameter to be less than 10 mm on average, these LA structures are less likely to be the cause of ectopic foci of electrical activity. This is further supported by Peng et al. (2012) who looked at the prevalence of left atrial diverticulums in a group of patients with atrial fibrillation and a control group in sinus rhythm. They found no significant difference in prevalence of LA structures between the two groups. Troupis et al. (2012) and Lazoura et al. (2012) also found no difference in prevalence of LA structures in patients with atrial fibrillation and in sinus rhythm. Patel et al. found no difference in prevalence of LA structure in patients with recurrent atrial fibrillation after catheter ablation as compared with control patients. However, a recent study by Hołda et al. (2017) found a significant association between the presence of any additional LA structure and atrial fibrillation occurrence. This significant difference was between all LA structures, including the aLAA, left atrial diverticulum and left atrial septal pouch, which was shown to be significantly associated with atrial fibrillation. When the presence of left atrial diverticulums and aLAAs were compared with controls independently of other LA structures, there was no significant difference in atrial fibrillation occurrence.
A theorized complication of both left atrial diverticulums and aLAAs is the formation of thrombi, with a mechanism similar to that seen in LAAs. Previous studies speculated that cryptogenic strokes may be associated with aLAAs or left atrial diverticulums (Gonçalves et al., 2009; Lee et al., 2008). In our study of LA structures (n = 984), the presence of thrombi was 0.2% (95% CI, 0.0%–0.6%). This supports previous findings by Ko et al. (2013) that found no significant association between the presence of LA structures in patients with a previous history of stroke versus controls. Additionally, there was no significant prevalence of thrombi in patients with both a LA structure and atrial fibrillation versus controls. Based on the lack of thrombi found in our data, and overall low association of LA structures with atrial fibrillation in previous studies (Ko et al., 2013; Troupis et al., 2012), the previous theorized risk of arrhythmia and thrombus formation may be rare. It is possible that ECG-gated CT angiography used to visualize the LA structures is not sensitive enough in identifying possible thrombi in these small LA structures (Killeen et al., 2010).
In the recent Left Atrial Appendage Occlusion Study (LAAOS III) by Whitlock et al. (2021) they discovered that surgical closure of aLAA decreased the chance of systemic embolism or ischemic stroke by 2.2% absolute risk reduction.
This study was limited by variations in definitions of LA structures. A unified definition and more research using more standardized criteria will ensure accurate results. Furthermore, there is a lack of standardized methods in the included studies that is a potential source of bias. Characteristics of the structures could not be differentiated due to less sensitive methods. However, the overall prevalence of LA structures elucidated by our meta-analysis is clinically relevant as it provides a reference for physicians when operating on or manipulating within the heart. Further research should focus on the histopathology of these structures so that proper characteristics can be detailed. In addition, dynamic studies examining the contractile properties and electrical activity would determine the functionality of these structures and elucidate if they are at increased risk of causing arrhythmia or thrombus formation.
5 CONCLUSION
In conclusion, our study found a pooled prevalence of left atrial diverticulums and aLAAs of 28.8%. Specifically, total prevalence of left atrial diverticulums was 29.8% and of aLAAs was 5.5%. The most common location of both LA structures was anterosuperior and the most common shape was cystic. In addition to providing precise information on the morphology of these structures, our research highlights two important clinical aspects. Thrombi may be found in 0.2% of additional LA structures, thus the previously theorized risk of thrombus formation in left atrial diverticulums and aLAAs may be rare. The presence of aLAA and/or left atrial diverticulum should not predispose to atrial fibrillation. These findings are consistent with previous research and provide a more thorough understanding of additional LA structures. Data presented in our present study is essential when considering the etiology and pathophysiology of analyzed structures as well as the possible complications during clinical procedures.