Investigating the effects of peptoid substitutions in self-assembly of Fmoc-diphenylalanine derivatives
Funding information: This work was supported by the National Science Foundation (DMR-1148836).
Abbreviation of article: Self-assembly and hydrogelation of the Fmoc-PhePhe dipeptide is investigated by systematic substitution of the Phe residues with N-benzyl glycine (Nphe) peptoid units. It was found that at least one Phe residue is required for self-assembly into one-dimensional fibril networks that elicit hydrogelation. These finding reinforce the importance of backbone hydrogen bonding and side chain geometry in promoting the self-assembly of ultrashort peptides.
Abstract
Low molecular weight agents that undergo self-assembly into fibril networks with hydrogel properties are promising biomaterials. Most low molecular weight hydrogelators are discovered empirically or serendipitously due to imperfect understanding of the mechanisms of self-assembly, the packing structure of self-assembled materials, and how the self-assembly process corresponds to emergent hydrogelation. Herein, the mechanisms of self-assembly and hydrogelation of N-fluorenylmethoxycarbonyl diphenylalanine (Fmoc-PhePhe), a well-studied low molecular weight hydrogelator, is probed by systematic comparison with derivatives in which Phe residues are replaced by corresponding N-benzyl glycine peptoid (Nphe) analogs. Peptoids are peptidomimetics that shift display of side chain functionality from the α-carbon to the terminal nitrogen. This alters the hydrogen bonding capacity, the side chain presentation geometry, amide cis/trans isomerization equilibrium, and β-sheet potential of the peptoid relative to the corresponding amino acid in the context of peptidic polymers. It was found that amino acid/peptoid hybrids Fmoc-Phe-Nphe and Fmoc-Nphe-Phe have altered fibril self-assembly propensity and reduced hydrogelation capacity relative to the parent dipeptide, and that fibril self-assembly of the dipeptoid, Fmoc-Nphe-Nphe, is completely curtailed. These findings provide insight into the potential of low molecular weight peptoids and peptide/peptoid hybrids as hydrogelation agents and illuminate the importance of hydrogen bonding and π–π interaction geometry in facilitating self-assembly of Fmoc-Phe-Phe.
1 Introduction
Low molecular weight agents that undergo fibril self-assembly to form entangled hydrogel networks are promising materials for applications in biomedicine, energy, and photovoltaics.1-4 Functionalized amino acids and dipeptides are a privileged class of low molecular weight hydrogelator.5, 6 The Fmoc-Phe-Phe (Fmoc-FF) dipeptide, derived from the shortest self-assembling motif of the β-amyloid peptide, is among the most widely studied supramolecular hydrogelators from this class of molecule.7-14 Self-assembly of this simple peptide system is mediated by a potent combination of hydrophobic, π–π, and hydrogen bonding interactions that result in formation of one-dimensional fibrils that under the proper conditions exhibit emergent hydrogelation.12, 15-19
The rational design of next-generation supramolecular hydrogels requires more detailed understanding of the relationship between the mechanisms of self-assembly and how self-assembly properties correlate to emergent hydrogel character. Herein, we report efforts to gain insight into the importance of hydrogen bonding and the geometry of π–π interactions in Fmoc-FF self-assembly and hydrogelation. Specifically, we systematically replaced Phe residues in Fmoc-FF with N-benzyl glycine peptoid (Nphe) derivatives (Figure 1). Peptoids are peptidomimetics in which the side chain functionality of the monomer units is shifted from the α-carbon to the nitrogen.20 Peptoid monomer units are constitutional isomers of the corresponding amino acids that possess altered hydrogen bonding capacity. In addition, the different geometry of side chain presentation also changes cis/trans isomerism about the amide bond and the β-sheet potential in the context of polyamide polymers.21-25 Amphipathic peptoid polymers have been shown to form self-assembled structures that differ from their peptide counterparts.26 While peptides with alternating hydrophobic and hydrophilic sequence patterns self-assemble into one-dimensional fibrils, corresponding peptoid sequences instead assemble into two-dimensional sheets, most likely due to the lack of a constraining hydrogen bond network within the peptoid assemblies.26 The consequences of site-specific peptoid incorporation into self-assembling peptide sequences is not clear.
Chemical structures of Fmoc-Phe-Phe (Fmoc-FF) derivatives
We thus compared the self-assembly behavior of Fmoc-FF to derivatives in which the Phe residues are systematically replaced with the corresponding Nphe peptoid residue (Figure 1). Using conditions that have been previously used to affect Fmoc-FF self-assembly and hydrogelation27-32 we observed the peptide/peptoid hybrids (2 and 3) undergo one-dimensional self-assembly into fibrils, but with altered hydrogelation potential relative to the parent Fmoc-FF compound. In contrast, the dipeptoid Fmoc-Nphe-Nphe (4) failed to assemble into fibrils altogether. Based on previous examples of self-assembling peptoids, we anticipated that self-assembly of dipeptoid 4 may be diverted into pathways leading to two-dimensional sheet structures due to the lack of amide hydrogen bonding, but 4 was found to form only amorphous aggregates.26 These studies indicate that self-assembly and hydrogelation of Fmoc-FF depend on both a constraining amide hydrogen bond network and ideal presentation of aromatic functionality to facilitate stabilizing intermolecular π–π interactions. Alteration of either of these properties by peptoid substitutions impairs efficient one-dimensional self-assembly and hydrogelation.
2 Materials and methods
2.1 Materials
Amino acids and organic solvents were purchased commercially and used without further purification. Synthetic details and characterization of the compounds 1–4 as shown in Figure 1 are reported in the accompanying Supporting Information (Schemes S1–S3, Figures S1–S9). Water used for the experiments was purified using a filtration system prior to use (Barnstead NANOpure, 0.2 μm filter, 18 Ω filtration system). Thin layer chromatography (TLC) was performed using silica gel (40 μm particle size).
2.2 Solvent switch method for gelation
Each compound 1-4 was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 247 mM. Aliquots of these DSMO solutions were then diluted into water to a final compound concentration of 5 mM in 2% DMSO/H2O (v/v).
2.3 HCl titration self-assembly experiments
These experiments were conducted as described by Saiani and co-workers.33 Appropriate masses of Fmoc-FF (1) and derivatives 2-4 as shown in Figure 1 were measured and suspended in 2 mL of water to obtain 10 mM concentrations. Sodium hydroxide (0.5 M) was added to the samples with mixing until pH 10.5 was achieved. All compounds were fully dissolved at this pH. Next, 10 µL of 0.085 M of HCl was sequentially added to each sample with mixing and heated to 80°C after each addition. The heated samples were cooled back to room temperature using a water bath. The pH values were measured before and after cooling after each sequential addition of HCl aliquots. The pH differences were within ±0.3 units, and the pH values after cooling were deemed to be the true values for reporting. A calibrated VWR Symphony SB70P pH probe with a 4.75 mm × 15 mm VWR probe (Ag/AgCl reference system, 3 M KCl electrolyte) was used for pH measurements. The probe was calibrated with three buffer standard solutions (4.01, 7.00, 10.00). The number of moles of HCl was plotted against pH to determine pH transitions that might be indicative of assembly events.
2.4 Gelation conditions using GdL
Fmoc-FF was measured and suspended in 2 mL of nanopure water then 667 µL of 0.1 M NaOH was added to the sample and it was vortexed, sonicated, and heated to 60°C to aid dissolution (final concentration of peptide/peptoid compounds was 18 mM). The sample was then added to 5 mg (10 mM) or 15 mg (30 mM) of glucono-δ-lactone (GdL). The solutions were allowed to stand for 12 h to enable complete hydrogel formation (for samples that were competent to form hydrogels). Under these conditions, compounds 2-4 do not form self-supporting hydrogels but precipitate from solution. Therefore, a modified gelation method was utilized using reduced GdL concentrations. Compounds 2–4 were suspended in 1.5 mL of water (34 mM). Then 500 µL of 0.1 M NaOH was added to each sample which were then mixed by vortex, sonication, and heating to 60°C to aid dissolution of the compounds. A GdL solution (1 mg/mL) was prepared and then immediately added to the NaOH solutions of compounds 2-4 to form final concentration of compounds of 18 mM (800 µL of the GdL solution was added to a final GdL concentration of 1.6 mM). After addition of GdL solution, the resulting mixtures rapidly mixed and allowed to stand for 12 h to enable hydrogelation to proceed to completion. The final concentration of compound in each solution was 18 mM and the pH of these solutions was 7.1–7.6.
2.5 Circular dichroism spectroscopy
Circular dichroism (CD) spectra were recorded using a Prodata Chirascan 202 circular dichroism spectrometer. A 0.1 mm path length quartz cuvette was used. Self-assembled samples were prepared and utilized as described above (pH 6.8–6.9). Spectra were collected at 25°C from 350 to 190 nm with a 1.0 nm step, 1.0 nm bandwidth, and 3 s averaging time per step.
2.6 FT-IR spectroscopy
FT-IR spectra were obtained using a Shimadzu 8400 FT-IR spectrometer. Self-assembled samples were prepared and utilized as explained above at pH 6.8–6.9. An aliquot (100 μL) of each sample was placed on 25 mm × 4 mm CaF2 plates (International Crystal Laboratories). Background and absorbance measurements were taken using the Happ-Genzel method from 1550–1750 cm−1 with a 4 cm−1 resolution for 1024 scans.
2.7 NMR spectroscopy
NMR spectra were recorded using Brüker Avance-400 MHz and 500 MHz spectrometers. 1H and 13C NMR chemical shifts are reported as δ with reference to TMS at 0 ppm for 1H NMR and solvent for 13C NMR. See Supporting Information for 1H and 13C spectra. To compare the extent of self-assembly of the variants, samples were prepared at 2 mM concentration at pH 10.5 and pH 6.8–6.9. Samples were prepared in D2O as reported in the procedures above until the desired pH was obtained and then placed in NMR tubes. The NMR tubes were then fitted with the same internal capillary tube containing 24 mM of DMF in D2O used as the external standard. The amount of soluble monomer at pH 6.8–6.9 (unintegrated into aggregate assemblies) was obtained by comparative integration of diagnostic peaks against the external standard and compared to the sample at pH 10.5 in which the samples remain unassembled. Under solution-state NMR conditions, signal from assembled fibril is lost due to anisotropic effects and line broadening.
2.8 Transmission electron microscopy
Images were obtained using a Hitachi 7650 transmission electron microscope with an accelerating voltage of 80 kV. Samples of compounds in different self-assembled states and solvent conditions (10 µL) were applied directly onto 100 mesh carbon coated copper grids and allowed to stand for 1 min. Excess sample was then carefully removed via capillary action using filter paper and the grids were stained with uranyl acetate (10 μL) for 2 min. Excess stain was also removed by capillary action and the grids were allowed to air-dry for 15 min.
2.9 High performance liquid chromatography
High performance liquid chromatography (HPLC) analysis of compound hydrophobicity was performed using a Shimadzu liquid chromatograph LC 2010A HT over a reverse phase C18 column (Gemini 5U 110A, 250 × 4.6 mm) with elution gradient of 10 mL min−1 in acetonitrile/water with 0.05% TFA. The eluent was monitored by UV absorbance at 215 and 254 nm.
2.10 Powder X-ray diffraction measurements
Experiments were performed on Scintag PAD-X theta-theta diffractometer with CuKα radiation (1.54 Å wavelength, 45 kV, 40 mA). The step size was 0.02 degrees with a scan rate of 0.5 degrees/minute. Self-assembled samples were prepared and utilized as explained above at a pH of ∼6.9. The samples were then applied to a glass slide and allowed to dry before powder X-ray diffraction data were obtained for the materials. The crystals formed by Fmoc-FF were collected using a spatula and allowed to air dry for powder X-ray diffraction analysis.
2.11 Rheology
Rheological measurements were conducted using a TA Instruments AR-G2 rheometer. A 20 mm parallel plate geometry equipped with a solvent trap filled with water was used for the experiments. Gels were prepared in glass vials about 12 h prior to each experiment. The gels were then carefully applied to the Peltier plate. The experiments were performed using about 500 µL of sample with a 1.4 mm gap size operating in oscillatory mode. Strain sweep experiments were performed at 25°C from 0.01–100% strain at a frequency of 6.283 rad s−1. Frequency sweep experiments were then performed from 0.1 to 100 rad s−1 at 0.1% strain at 25°C. Reported storage (G′) and loss (G″) modulus values are the average of at least three separate experiments with error reported as the standard deviation about the mean.
3 Results and discussion
The Fmoc-FF dipeptide has been shown to form fibril hydrogel networks under a number of conditions. A “solvent-switch” method in which the peptide is dissolved at high concentrations in a DMSO stock solution that is then diluted into water triggers self-assembly and hydrogelation.34 We first used these conditions to compare self-assembly of compounds 1-4. Each compound was dissolved in DMSO at 247 mM; these solutions were then diluted into unbuffered water to a final concentration of 4.9 mM in 2% DMSO/H2O (v/v). Under these conditions, Fmoc-FF formed self-supporting, transparent hydrogels while the peptoid-containing compounds 2-4 precipitated as amorphous aggregates (Figure S10A, Supporting Information).
These initial findings clearly indicated reduced self-assembly potential for the peptoid analogs, although it was not clear whether this was due to an inherent alteration of self-assembly propensity, or whether this might be due to sensitivity to gelation conditions or subtle differences in solubility. To assess differences in hydrophobicity, we conducted an HPLC analysis of each compound using identical stationary phase, mobile phase, and gradient conditions (Figure 2). The peptoid containing analogs are more hydrophobic than the parent dipeptide as indicated by longer comparative retention times (with dipeptoid 4 being the most hydrophobic).35-37 These differences in hydrophobicity are not so dramatic that self-assembly of compounds 2-4 should be curtailed on this basis alone.

HPLC retention times obtained for compounds 1-4 under identical mobile and stationary phase conditions to assess hydrophobicity
We subsequently explored other conditions to compare self-assembly of these compounds 1-4. A “pH-switch” method is commonly used to initiate self-assembly of low molecular weight peptide and amino acid-derived hydrogelators.33, 38, 39 Fmoc-FF can be dissolved in water without organic cosolvents at high pH (10.5). Reduction of the solution pH by titration with protic acids such as HCl or by slower acidification by hydrolysis of glucono-δ-lactone (GdL) reduces the charge of the C-terminal carboxylate, eliminating Coulombic repulsion between charged molecules and promoting self-assembly and hydrogelation.38 Fmoc-FF has been reported to form homogenous hydrogels using GdL hydrolysis to acidify basic solutions of the dipeptide.32 Accordingly, we compared self-assembly of 1-4 by dissolution of each compound at 18 mM in basic aqueous solutions (pH 10.5). All compounds were freely soluble under these conditions. Solid GdL (30 mM) was then added to slowly acidify the solutions as a function of GdL hydrolysis. As expected, Fmoc-FF formed homogenous self-supporting hydrogels within 12 h of GdL addition (Figure S10B, Supporting Information). Peptoid-containing analogs 2-4 once again failed to self-assemble, but merely precipitated (Figure S10B, Supporting Information).
The final pH of the solutions above after GdL hydrolysis was ∼4. We next considered whether self-assembly of the peptoid-containing analogs might occur more efficiently at pH values that differ from that of Fmoc-FF. Saiani and co-workers have shown that acid titration of Fmoc-FF in basic solutions exhibits two distinct pKa shifts pH values of 9.5–10.2 and 6.2–5.2, significantly higher than the predicted carboxylic acid pKa of ∼3.5.33 These pKa transitions corresponded to self-assembly of Fmoc-FF, with self-assembly occurring most efficiently under pH conditions at or just below the transition values.33 We repeated these acid titration experiments to determine where the pKa transitions exist for compounds 1-4 to understand what optimal pH conditions for assembly might be. Each derivative was dissolved in basic aqueous solutions at pH 10.5 at 10 mM concentrations. To these solutions, aliquots of HCl were sequentially added and the number of moles of HCl added was plotted against pH to determine the pKa transition values for each compound (Figure 3, see Materials and Methods for detailed procedures).
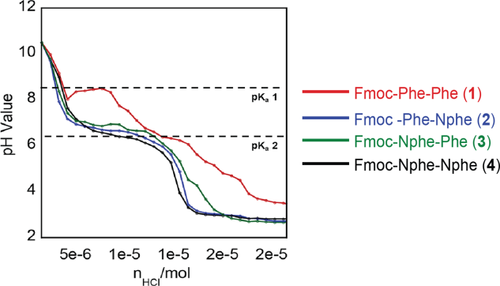
HCl titration experiment for compounds 1-4 at 10 mM concentration (pH vs. moles of HCl added)
In addition to changes in pH as aliquots of HCl were added, it was observed that physical changes occurred in the samples. As solution acidity increased, solutions of all compounds increased in turbidity (beginning a pH of ∼7.5) and solutions of compounds 1-3 increased in viscosity, indicating possible self-assembly events. Compound 4 alone did not increase in viscosity as the pH was lowered. As shown in Figure 3, Fmoc-FF showed two apparent pKa transitions at pH values of 8.5 and between 7–6.5, consistent with previous reports.33, 38 In contrast, compounds 2-4 exhibited only a single pKa transition. By applying a second derivative function to the titration curves in Figure 3, The pKa transitions were found to be at pH 6.7 for compound 2, pH 6.9 for compound 3 and pH 6.7 for compound 4. At pH values lower than 6, precipitation resulting in phase separation of the precipitate from the water layer occurred. It is significant that these are different from that of Fmoc-FF in that only a single pKa transition is observed. This suggests that peptoid-containing derivatives may differ from Fmoc-FF in terms of ideal conditions for self-assembly. The single pKa transition at ∼6.8 is still significantly elevated from the theoretical pKa of ∼3.5 for the C-terminal carboxyl group, suggesting that self-assembly events are occurring and an ideal pH at which to trigger self-assembly.
Based on these findings, the self-assembly behavior of all the Fmcoc-FF derivatives was reassessed by examining solutions of all compounds at pH values near each pKa transition. Transmission electron microscopy (TEM) images were taken at various pH intervals based on the pH titration data. Similar to previous reports, TEM images of Fmoc-FF solutions at pH 10.5 contained no fibrils (Figure 4A). Solutions at pH 8.8, above the first pKa transition for 1, contained small fibrils of 3.9 ± 0.8 nm in diameter. As solution pH became increasing more acidic, the fibrils that were observed in these solutions became increasingly wider. At pH 7.5 (below the first pKa transition) and at pH 7 (above the second pKa transition), the observed fibrils were 8–18 nm in diameter due to apparent lamination of the smaller fibrils observed at pH 8.8 (Figure 4B–D). Finally, solutions at pH 5.4 (below the second pKa transition) contained fibrils 20–35 nm in diameter (Figure 4E). These results are consistent with previous reports.33 It should be noted that under these conditions, gelation was not observed although the solutions containing fibrils did increase in viscosity.

TEM images of compounds 1-4 at various pH values that mark pKa transition states according to pH titration. (A) 1 at pH 10.5, (B) 1 at pH 8.8 (above pKa transition 1), (C) 1 at pH 7.5 (below pKa transition 1), (D) 1 at pH 7 (above pKa transition 2), (E) 1 at pH 5.4 (below pKa transition 2), (F) 2 at pH 10.5, (G) 2 at pH 7.4 (above pKa transition), (H) 2 at pH 5.7 (below pKa transition), (I) 3 at pH 10.5, (J) 3 at pH 7.4 (above pKa transition), (K) 3 at pH 5.6 (below pKa transition), (L) 4 at pH 10.5, (M) 4 at pH 7.5 (above pKa transition), (N) 4 at pH 5.7 (below pKa transition)
Solutions of peptoid analogs 2-4 were also examined at pH values above and below the single observed pKa transition. At pH 10.5, no fibrils were observed (Figure 4A,F,I, L) in any of these solutions. At pH 7.4, which is above the first pKa transition, solutions of 2 and 3 contained fibrils 14 ± 3 nm and 23 ± 4 nm in diameter, respectively (Figure 4G,J), while variant 4 only formed amorphous or spherical aggregates (Figure 4M). At pH 5.7 (below the pKa transition), solutions of 2 and 3 continued to show fibrils similar to those observed at pH 7.4, but the fibrils were more heavily clustered (Figure 4H,K). At pH 5.7, solutions of compound 4 still contained only amorphous or spherical aggregates with no evidence of fibril formation. Under these conditions, the samples that showed evidence of fibril assembly increased in viscosity, but failed to form self-supporting hydrogels.
Thus, peptoid/peptide hybrids (2 and 3) retain the ability to form one-dimensional fibril assemblies similar to Fmoc-FF while the Fmoc-dipeptoid 4 appears to be unable to assemble into one-dimensional fibrils under these conditions, favoring assembly into amorphous or spherical structures. Saiani and co-workers have suggested that the primary driving force behind the self-assembly of Fmoc-dipeptides is a combination of hydrophobic and aromatic π–π interactions between the Fmoc and side chain groups, while hydrogen bonds play only a secondary role.15, 40 Our data is only partially consistent with this contention. Each analog assessed herein that retains the capacity to form at least one intermolecular hydrogen bond interaction via the amide residues (compounds 1-3) maintains the capacity to form one-dimensional fibrils. In contrast, dipeptoid 4 lacks the ability to form these hydrogen bonds and it fails to assemble. All compounds have identical aromatic content, although the geometry of presentation for these aromatic groups is altered in the peptoid-containing analogs. This suggests that (1) hydrogen bonding may be more important than previously thought in defining 1D fibrils of Fmoc-FF, and (2) the spatial positioning of aromatic groups is also important in mediating formation of stabilizing intermolecular π–π interactions in the context of one-dimensional fibrils.
We next compared the efficiency with which each compound becomes incorporated into aggregate structures. Self-assembly is an equilibrium process and a simplified equilibrium state involved fibrils in equilibrium with monomer. All self-assembling peptides have characteristic equilibrium points and characterizing the amount of monomer in solution at equilibrium can allow us to comment on relative self-assembly proclivity for a given peptide.41 Solution-state NMR can be used to assess self-assembly equilibrium since signal from unassembled monomer is easily detected and signal from material incorporated into fibrils is lost due to line broadening effects.42-44 Comparative integration analysis against an external standard of samples in which a monomer state is maintained and samples in which assembly has occurred allows quantitative comparison of the amount of monomer that has been assimilated into fibrils.
Accordingly, we used solution-state NMR to compare samples of each compound (2 mM) at pH 10.5 (monomeric, unassembled) and pH 7 (self-assembled fibrils observed) (Figure 5). A common external standard of DMF in D2O in a capillary tube was used for comparative signal integration in each set of samples (see Materials and Methods for the detailed protocol). Figure 5 shows 1H NMR of compounds 1-4 at pH 10.5 (top spectrum in each panel) and pH 6.8–6.9 (bottom spectrum in each panel). This data shows strong signal in each sample at pH 10.5, consistent with the previous pH study indicating that no fibrils were observed in TEM images at this pH. At pH 7, signal intensity is reduced. For the Fmoc-FF 1 sample, signal intensity at pH 7 is reduced by almost 100% relative to the pH 10.5 sample (Figure 5A), indicating highly efficient self-assembly of this dipeptide, with 2 mM of 1 (out of a total [1] of 2 mM) incorporated into fibril. The extent of signal reduction for peptide/peptoid hybrids 2, 3, and 4 is 32%, 74%, and 32%, respectively, correlating to ∼0.64–1.48 mM of compound incorporated into fibril under these conditions (Figure 5B–D). Thus, self-assembly efficiency is somewhat impaired by peptoid substitutions into the Fmoc-FF dipeptide. The reduction in monomer quanitity by Fmoc-Nphe-Nphe dipeptoid 4 is due to the nonfibrillar aggregation formed.
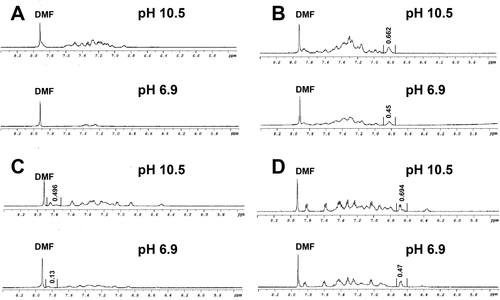
1H NMR spectra of compounds 1-4 at 2 mM (D2O) and at pH 10.5 (nonfibril) and pH 6.9 (fibril). An external sample of DMF in D2O (labeled) is included for comparative integration to assess the amount of monomer incorporated into fibril at pH 6.9 (fibril signal is lost to line broadening effects). (A) Compound 1, (B) compound 2, (C) compound 3, (D) compound 4 at 2 mM in pH 10.5 and pH 6.9 in D2O using 24 mM DMF as external standard
Spectroscopic analysis was used to compare secondary structural characteristics for assemblies of compounds 1-4. Infrared (IR) spectroscopy in the amide region was performed as described in the experimental section in samples of each compound at pH 6.8–6.9, near the pKa transition at which abundant fibrils are observed for compounds 1-3. The IR spectrum of the Fmoc-FF dipeptide 1 shows distinctive β-sheet character (Figure 6A) with signals at ∼1620 cm−1, characteristic of amide I β-sheet,33 from 1580 to 1590 cm−1, representative of amide II β-sheet, and from 1680 to 1695 cm−1, indicative of the Fmoc-derived carbamate carbonyl.45-48 The IR spectrum of 1 is consistent with previously reported spectra. IR spectra of peptoid-containing analogs 2-4 were also obtained, although these are more difficult to interpret since characteristic peptoid IR signals are not well characterized. Hybrid 2 showed similar signals to Fmoc-FF including a shifted amide I stretch at 1630 cm−1. Variant 3 and 4 however, showed significantly shifted peaks, including an IR stretch at ∼1640 cm−1 which is often characteristic of random structure in peptides, although the characteristic amide II and the carbamate stretches of the peptides are still retained by both variants.49-51
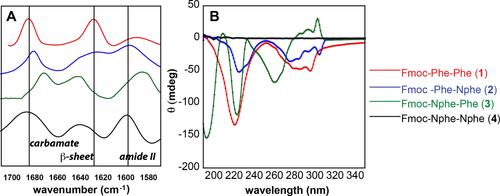
(A) IR spectra for Fmoc-FF and peptide/peptoid hybrids at pH 7. (B) CD spectra for Fmoc-FF and peptide/peptoid hybrids at pH 7
CD spectra were also obtained to further assess structure in solutions of compounds 1-4 (Figure 6B). Similar to the past reports, the CD spectrum of the Fmoc-FF peptide had absorbances at 220 nm (β-sheet) and from 270–310 nm, which has been attributed to Fmoc-Fmoc stacking interactions.52 Crystals of Fmoc-FF obtained by Adams and co-workers indicate a parallel alignment of molecules with hydrogen bonds existing between the amide hydrogen with adjacent carbonyl oxygen (NH…O) and π–π interactions between neighboring benzyl side chain groups (phenyl-phenyl) and Fmoc groups (Fmoc-Fmoc).53 This CD data is consistent with previous data and with interactions that can be justified in the CD spectrum. Hybrid compound 2 shows a similar CD pattern pattern to that of Fmoc-FF, with absorbances at 224 nm and 270–310 nm. This is consistent with the IR data for these compounds that supports a similar packing structure. The CD spectrum of compound 3 differs from those of compounds 1 and 2, with strong absorbances at 193 nm and 223 nm and a weak absorbance at 209 nm. Additional signals at 260 nm and from 270–310 nm (positive, possibly Fmoc-Fmoc) are also observed. The electronic structure of assemblies of 3 that give rise to this data appears to be more dominated by the initial peptoid residue than that of the amino acid. The CD spectrum of dipeptoid 4 showed no significant absorbances. Compound 4 is achiral, and this data may indicate that there are no significant assembled structures in these samples or that any existing aggregates of compound 4 have opposite chirality that are negated in the spectrum. Interestingly, the CD and IR data seem to indicate that the residue following the Fmoc group has the strongest effect on self-assembly outcomes; Fmoc-Phe-Nphe (2) appears similar in every regard to Fmoc-FF, while Fmoc-Nphe-Phe (3) exhibits similar fibrils, but with shifted spectral signals.
Similar to reports by Adams and co-workers, we were able to obtain spherulite crystals from Fmoc-FF solutions that were allowed to stand after the pH titration experiments.53 These self-assembled samples were kept at pH 6.9 for 1 month before the spherulite crystals appeared. The crystals, which were thin needle structures, were of insufficient quality for high-resolution X-ray diffraction analysis. However, powder X-ray diffraction (PXRD) was performed on these crystals (Figure 7A) and compared with PXRD data obtained dried fibrils of Fmoc-FF (10 mM, pH 6.9) (Figure 7B). PXRD distance patterns can also be directly compared with published wide angle X-ray scattering (WAXS) data reported by Ulijn and co-workers on materials obtained by from pH titration experiments.52 Fmoc-FF PXRD data for spherulite crystals and dried fibrils showed similar reflections at 2.5, 4.2, and 6.5 Å for crystals and at 2.8, 4.2, 6.4, Å for dried fibrils (Figure 7A,B). Previous reports of these materials by Adams and co-workers indicated reflections at 3.4, 3.8, 4.2, 4.9, 15, and 12.6 Å; our data showed some similar reflections at 4.2, 4.9, and 3.6 Å and other distinct reflections at 5.4, 6.5, 7.7, and 13.2 Å (Figure 7A).53 WAXS data for Fmoc-FF fibrils reported by Ulijn showed reflections at 26, 16, and 4.3 Å where distances from 4 to 5 Å were reasoned to arise from spacing commonly seen between β-sheet peptides and 16 Å distances as spacing between Fmoc fluorene motifs.52 Our PXRD reflections at 4.2, 4.9, and 13.2 Å may also be attributed to β-sheet and fluorene spacings, with subtle variations due to differences in fibrillization conditions.
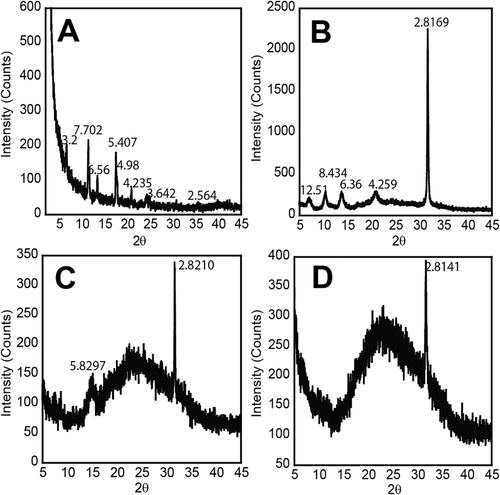
PXRD data for (A) spherulite crystals formed by aqueous Fmoc-FF (10 mM, pH 6.9) samples after 1 month, (B) Fmoc-FF 1 dehydrated fibrils (10 mM, pH 6.9), (C) compound 2 (10 mM, pH 6.9), (D) compound 3 (10 mM, pH 6.9)
PXRD data were also obtained with fibrils of compounds 2 and 3 formed at pH 6.8–6.9 to compare these reflection patterms to those of the parent molecule (Figure 7C,D). The fibrils were allowed to air dry before data acquisition. The PXRD data for hybrids 2 and 3 showed broad weak reflections, with the only strong reflection peak at 2.8 Å (Figure 7C,D, respectively). The reflection at 2.8 Å is very similar to the Fmoc-FF dipeptide. This distance may arise from the intermolecular hydrogen bonding in the assembled network. The weaker signal is also probably a reflection of the lower degree of assembly in these derivatives, as was described in the solution state NMR experiments previously.
It is clear from these studies that the conditions for self-assembly is optimal near pKa transition regions of the C-terminal carboxylic acid (Figure 3).33, 52 However, the self-assembly studies reported herein resulted fibril networks with hydrogel properties only in the case of Fmoc-FF. Thus, we next explored alternative assembly conditions to assess whether peptoid hybrids 2 and 3 are able to form hydrogel networks. Assembly of 1-3 by adjusting the pH to ∼7 by titration with HCl resulted in high viscosity solutions, but mixtures became inhomogenous, with pockets of gel and fluid intermixed. Previous published studies have indicated that the highest quality Fmoc-FF gels are formed using pH modification methods in which the hydrolyzed products of glucono-δ-lactone (GdL) have been utilized as the proton donor since this slows the rate at which the pH is lowered.54 While we explored the use of GdL in the our initial attempts to promote assembly of compounds 1-4, only Fmoc-FF effectively formed gels under these conditions (18 mM Fmoc-FF, 30 mM GdL).32, 54 The peptoid-containing derivatives merely precipitated under these conditions. We reasoned that this precipitation occurred because the high concentration of GdL under these self-assembly conditions gave final pH values near 4 and the most efficient assembly of the peptoid derivatives was found to occur at pH 7.
Based on this reasoning, we hypothesized that hydrogelation of compounds 2 and 3 may be affected by modifying the pH of basic solutions of these compounds with lower concentrations of GdL so that the final solution pH is ∼7. We found that when GdL concentrations as low as 1.6 mM were used in basic solutions of hybrids 2 and 3 (18 mM), the final solution pH was between 7.1 and 7.6 and self-supporting hydrogels were formed (Figure S10C, Supporting Information). In contrast, Fmoc-FF was able to form hydrogels over a wider range of values as low as 4.29 (30 mM GdL) and as high as 7.5 (10 mM GdL). Thus, ideal hydrogelation of the peptoid hybrid compounds required much more precise control over the solution pH, with optimal hydrogelation occurring near the observed pKa transition at pH ∼7.
The viscoelasticity of the resulting hydrogels of compounds 2 and 3 was characterized using oscillatory rheology. These experiments indicate that Fmoc-FF forms relatively rigid hydrogels compared to the weaker gels formed by compounds 2 and 3 (Figure 8). Strain sweep experiments were used to define the linear viscoelastic region for the gels (Figure 8A) to facilitate frequency sweep experiments in this linear viscoelastic region (Figure 8B). Frequency sweep experiments were conducted to compare the storage (G′) and loss (G″) moduli for each gel. Compound 2 formed gels with G′ values of around 288 Pa and G″ values of 50 Pa, while compound 3 formed gels with G′ value of around 530 Pa and G″ values of 110 Pa (Figure 8B). In comparison, Fmoc-FF (compound 1) formed significantly stronger gels with G′ values of 5720 Pa and G″ values of 1411 Pa at pH 4 and gels with G′ values of 1719 Pa and G″ values of 720 Pa at pH 7 (Figure 8A). This data is consistent with reports previously described, where Fmoc-FF formed gels with comparable rigidity at both pH values.38 The weaker gelation capacity of compounds 2 and 3 is most likely due to a lower density of fibrils in these solutions based on an equilibrium that is shifted toward monomer as described in the solution-state NMR experiments previously.
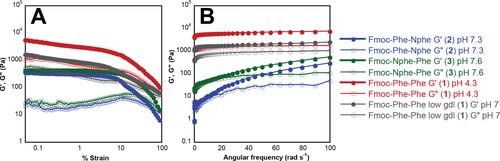
(A) Rheological strain sweep experiments for hydrogels of compounds 1-3 at pH 7 (1-3) and at pH 4 (1). (B) Rheological Frequency sweep data obtained for hydrogels formed by compounds 1–3 under various pH conditions
4 Conclusion
Collectively, these studies provide insight into the fundamental interactions that drive self-assembly of the Fmoc-FF dipeptide into fibrils. While aromatic π–π interactions are clearly a core stabilizing interaction for self-assembly of Fmoc-FF, the observed self-assembly properties of peptoid-containing analogs indicate that subtle changes spatial arrangement of side chain presentation can have strong effects on self-assembly. Thus, π–π interactions are limited to a fairly limited range of geometries if they are to mediate effective self-assembly. Hydrogen bonding also appears to play more than just a secondary role in stabilizing or directing Fmoc-FF self-assembly. This is apparent based on the observation that Fmoc-Nphe-Nphe (4), which lacks intermolecular hydrogen bonding capacity, fails to undergo one-dimensional fibril self-assembly altogether, whereas the peptide/peptoid hybrids 3 and 4 retain the ability to undergo self-assembly. Hydrogen bonding is thus not just a secondary stabilizing interaction, but a critical property for self-assembly of Fmoc-dipeptides.
These studies also highlight important remaining questions in the development of low molecular weight supramolecular hydrogelators derived from functionalized amino acids and peptides. First, it is not readily apparent why Fmoc-FF undergoes two elevated pKa transitions (at ∼8.5 and from 6–7) relative to the predicted pKa of the C-terminal carboxyl group (∼3.5) when adjusting pH from basic to acidic conditions whereas the peptoid containing analogs undergo only a single pKa transition at ∼7. The elevated pKa transitions are clearly a function of altered hydrophobic enviroment as self-assembly occurs on protonation of the C-terminal carboxylate. The unique pKa transition profile of Fmoc-FF belies a mechanistic peculiarity of this self-assembling peptide that is not clearly understood. Also, while these studies clearly indicate that hydrogelation in many cases relies on identification of ideal gelation conditions, the relationship between self-assembly propensity and emergent hydrogel formation remains mysterious. Bridging the gap between serendipity and rational design in the development of next-generation supramolecular hydrogelators with tailored properties for specific applications will require additional insight into these persistent questions.
Acknowledgments
This work was supported by the National Science Foundation (DMR-1148836). We gratefully acknowledge Karen Bentley (URMC Electron Microscope Research Core) for her assistance in the use of TEM and SEM instruments, Dr. William Brennessel for his assistance with X-ray diffraction analysis, and Dr. Maura Weathers (Cornell Center for Materials Research) for assistance with powder X-ray diffraction experiments.




