Glycerol-induced folding of unstructured disulfide-deficient lysozyme into a native-like conformation
Abstract
2SS[6-127,64-80] variant of lysozyme which has two disulfide bridges, Cys6-Cys127 and Cys64-Cys80, and lacks the other two disulfide bridges, Cys30-Cys115 and Cys76-Cys94, was quite unstructured in water, but a part of the polypeptide chain was gradually frozen into a native-like conformation with increasing glycerol concentration. It was monitored from the protection factors of amide hydrogens against H/D exchange. In solution containing various concentrations of glycerol, H/D exchange reactions were carried out at pH* 3.0 and 4°C. Then, 1H-15N-HSQC spectra of partially deuterated protein were measured in a quenching buffer for H/D exchange (95% DMSO/5% D2O mixture at pH* 5.5 adjusted with dichloroacetate). In a solution of 10% glycerol, the protection factors were nearly equal to 10 at most of residues. With increasing glycerol concentration, some selected regions were further protected, and their protection factors reached about a 1000 in 30% glycerol solution. The highly protected residues were included in A-, B-, and C-helices and β3-strand, and especially centered on Ile 55 and Leu 56. In 2SS[6-127,64-80], long-range interactions were recovered due to the preferential hydration by glycerol in the hydrophobic box of the α-domain. Glycerol-induced recovering of the native-like structure is discussed from the viewpoint of molten globules growing with the protein folding. © 2009 Wiley Periodicals, Inc. Biopolymers 91: 665–675, 2009.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
Protein folding occurs by passing through different ensembles of conformation.1-3 During the folding process, non-persistent short secondary structures may be associated with each other and their structural freedom may be frozen through cooperative interactions between structural elements. To elucidate the folding process, conformations which are gradually structured during the folding must be studied in detail. Spectroscopic properties such as circular dichroism have been studied to explore kinetic intermediates,4 but can not give detailed information about the conformation in a residue specific manner. NMR spectroscopy is a powerful tool to provide such information, but generally not suitable for kinetic study, although hydrogen exchange labeling in combination with NMR was excellent in characterizing transient intermediate states emerging immediately after dilution of denaturants.5, 6 Since protein folding is an all-or-none transition in nature, only the native conformation was detectable except the unfolded one even at the midpoint of folding transition. It was very difficult to trap an intermediate conformation in equilibrium. As an alternative strategy for investigating conformations gradually structured, we prepared protein derivatives which have variously ordered structures and tried to study the conformations in equilibrium by using NMR spectroscopy. Also it was useful for trapping intermediate conformations to induce a protein derivative unstructured in water to recover the native-like conformation through the preferential hydration by glycerol.
For that end, we have studied various molecular species of disulfide-deficient variants of lysozyme. Four species of 3SS-variants of lysozyme each lacking one of the four disulfide bridges had nearly the same tertiary structure as that of the wild-type lysozyme,7, 8 whereas 2SS-variants containing two native disulfide bridges varied in preserving the tertiary structure, that is, 2SS[6-127,30-115] containing the disulfide bridge Cys6-Cys127 and Cys-30-Cys115 preserved the native-like tertiary structure,9, 10 but 2SS[6-127,64-80] with Cys6-Cys127 and Cys64-Cys80, and 2SS[64-80,76-94] with Cys64-Cys80 and Cys76-Cys94 had no definite tertiary structure in water. In addition, four species of 1SS-variant containing a single disulfide bridge,11 and 0SS-variant lacking all of the disulfide bridges were unstructured judged from their CD and 1H-15N-HSQC spectra. Thus, 2SS-variants are located at the boundary between the native-like and non-native structures. Non-native states of 2SS-variants were characterized by measuring heteronuclear 15N-relaxation rates.12 The subject of the present study is glycerol-induced recovering of the native-like conformation from the non-native states.
Glycerol is well known as a solvent additive to stabilize the folded structure of protein through the preferential hydration, that is, a negative binding of glycerol to the protein surface.13-15 In this article, we studied the effects of glycerol on disulfide-deficient variants of lysozyme losing the ordered structure in water. Glycerol induces them to recover some ordered structure. In a solution of 30 to 70% glycerol, all the variants recovered a helical conformation, but tertiary structures of variants except 2SS[6-127,64-80] remained unstable. 2SS[6-127,64-80] alone could recover not only secondary structures but also a rigid tertiary structure in part. The purpose of the present study is to reveal the processes of recovering such an ordered structure at the atomic resolution. Because of the broadening of resonance lines in 30% glycerol solution, the peak assignment for the HSQC spectrum of 2SS[6-127,64-80] was very difficult, and NOESY spectra could not give useful information about long-range interactions. Therefore, we carried out H/D exchange reactions of amide hydrogens in a solution containing glycerol, and then determined protection factors against H/D exchange for individual residues. In this article, we report the experimental procedures to measure H/D exchange rates of inherently unstructured proteins. For NMR measurements of partially deuterated proteins, a mixture of 95% DMSO-d6 and 5% D2O was used as a quenching buffer for H/D exchange.16, 17
As a result of H/D exchange experiments performed in 0%, 10%, 20%, and 30% glycerol solutions, it was found that protection factors of most residues were nearly equal to 10 in 10% glycerol solution. With increasing glycerol concentration, they increased to about 100 in some selected regions, but in other regions they remained unaltered. Probably this means that structural cooperativity arose among some structural elements and it resulted in the increase in the protection factors of the selected region. Finally, a rigid tertiary structure emerged in the region surrounded by A-, B-, and C-helices centering on Ile 55 and Leu 56. Glycerol-induced recovering of the native-like structure is discussed from the viewpoint of molten globules growing with the protein folding.
MATERIALS AND METHODS
Protein Expression and Purification
Details of the construction of the genes for the 1SS-, 2SS-, and 3SS-variants of hen lysozyme are described in the references published previously.10, 11, 18 The expression and purification of lysozyme variants and uniformly 15N-labeled samples have been described previously.7-9 Concentrations of lysozyme variants were estimated by using A280 = 2.64 for 1 mg/ml protein.
Materials
DMSO-d6 and glycerol-d8 were obtained from Cambridge Isotope Laboratories. Formic acid-d2 and dichloroacetic acid-d2 were purchased from Sigma-Aldrich Corporation. D2O was obtained from Wako Pure Chemical Industries.
CD Measurements
CD spectra of lysozyme variants were obtained on a Jasco J-600 spectropolarimeter (JASCO Corporation) with a thermostatically controlled cell holder. They were measured in 20 mM glycine buffer at pH 3.0 and 4°C by using a cuvette with an optical length of 2 mm or 5 mm for far-UV measurements and 10 mm for near-UV. The cell was defrosted with the flow of dry nitrogen gas below the room temperature. Protein concentrations for near-UV CD spectra were about 25 μM, those for far-UV CD 5 μM.
NMR Experiments
NMR spectra of disulfide-deficient lysozyme were measured in 95% DMSO/5% H2O (or D2O) containing 60 mM dichloroacetic acid at pH* 5.5, where pH* is the uncorrected pH electrode reading. The pH* was adjusted by adding DCl and NaOD. Shigemi NMR tubes matched to DMSO-d6 were used in all of the experiments 2D 1H-15N HSQC spectra19 and 3D NMR spectra were usually measured at protein concentrations of about 0.2 mM and 1 mM, respectively. Data were recorded at 600.13 MHz on a Bruker Avance 600 DRX spectrometer. Solvent signal suppression was achieved by using the WATERGATE20 scheme. Typically, 2D 1H-15N HSQC spectra were collected at 25°C with 1024 × 2048 points in t1 and t2 directions, using uniformly 15N-labeled samples. 3D HSQC-NOESY-HSQC (mixing time of 150 ms) spectra21 were collected with 64 × 64 × 2048 in the t1, t2, and t3 directions. Zero filling was applied before Fourier transformation, and data were processed with a shifted squared sine-bell window function in both dimensions. The DMSO peak was referenced as 2.55 ppm in all spectra.
H/D Exchange of Disulfide-Deficient Variants of Lysozyme
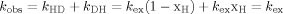
To measure HSQC spectra of partially deuterated proteins, freeze-dried samples were dissolved in a quenching buffer (95% DMSO-d6/5% D2O mixture at pH* 5.5 adjusted with dichloroacetate-d2). This process was carefully performed under an atmosphere of dry nitrogen in a polyethylene glove bag. The first HSQC spectrum was obtained about 40 min after dissolving protein in the quenching buffer, so that this is the dead time (τd). As mentioned later, H/D exchange rates in the quenching buffer were slower than 1/120 per minute for most residues. The ratio of the peak volume, Iex(τd)/Iex(0), was about 72–92%, where Iex(0) and Iex(τd) are a true peak volume and that first observed after τd, respectively. Since the ratio remains constant for each residue in a series of H/D exchange reactions, the observed peak volume, Iex(τd), was plotted as a function of H/D exchange time without the correction due to the dead time.
RESULTS
Structures Induced by Glycerol in Disulfide-Deficient Variants of Lysozyme
Effects of glycerol on disulfide-deficient variants of lysozyme which are unstructured in water were studied by measuring their CD spectra. Figure 1A shows far-UV CD spectra of 1SS[64-80] in solutions containing various concentrations of glycerol. The negative ellipticity at 220 nm, [θ]220, gradually increased with the increase in glycerol concentration. In a solution of 50% glycerol, the value of [θ]220 approached to that of the wild-type lysozyme in water, and exceeded it at 70% glycerol. The dotted line close to the curve d refers to the spectrum of the wild-type lysozyme which was independent of the concentration of glycerol. Far-UV CD spectra of 0SS-variant and 1SS[76-94] were almost identical with those of 1SS[64-80] under all the concentrations of glycerol, and those of 1SS[30-115] were virtually the same as those of 1SS[64-80], although the former was slightly larger than the latter in the negative ellipticity. Figure 1B shows far-UV CD spectra of 2SS[6-127,64-80]. The value of [θ]220 in water was somewhat larger than that of 1SS[64-80], but [θ]220 approached to the same value at 70% glycerol for both 1SS[64-80] and 2SS[6-127,64-80]. Also, far-UV CD spectra of 1SS[6-127] and 2SS[64-80,76-94] were similar to those of 2SS[6-127,64-80], although shapes of CD spectra were slightly different from each other. These data suggest that the addition of glycerol increases the helical contents of all the disulfide-deficient variants studied, and that the helical contents exceed that of the wild-type at 70% glycerol although the helical structure is non-native.
CD spectra of different species of disulfide-deficient variants of lysozyme. All spectra were measured at pH 3.0 and 4°C. (A) Far-UV CD spectra of 1SS[64-80]. Glycerol concentrations are 0% (a), 10% (b), 30% (c), 50% (d), and 70% (e). The dotted line refers to the spectrum of the wild-type protein which was independent of the concentration of glycerol. Those of 0SS-variant and 1SS[76-94] were almost identical with those of 1SS[64-80]. (B) Far-UV CD spectra of 2SS[6-127,64-80]. Glycerol concentrations are 0% (a), 10% (b), 30% (c), and 70% (d). (C) Near-UV CD spectra of various variants in the presence of 50% glycerol and those of wild-type protein as a reference. a: wild-type, b: 2SS[6-127,64-80], c: 1SS[64-80], and d: 2SS[64-80,76-94]. (D) Near-UV CD spectra of 2SS[6-127,64-80] under various glycerol concentrations, a: 50%, b: 30%, c: 10%, and d: 0%.
As shown in Figure 1C, near-UV CD spectra of lysozyme variants except 2SS[6-127,64-80] remained nearly equal to zero even in the presence of concentrated glycerol. 2SS[6-127,64-80] alone could recover the native-like CD spectrum around 280 nm by the addition of glycerol. In Figure 1C, the curve a represents the CD spectrum of the wild-type lysozyme which remained unaltered in water and in 50% glycerol. As shown in Figure 1D, 2SS[6-127,64-80] was completely unstructured in water, but some ordered structure was induced around tryptophan residues by the addition of glycerol, while no definite tertiary structure was observed in other variants such as 0SS, four species of 1SS-variants, and 2SS[64-80,76-94].
To explore the glycerol-induced conformation of 2SS[6-127,64-80] in a residue specific manner, we tried to measure 1H-15N HSQC spectra, in 30% glycerol solution, but it was very difficult to assign the cross-peak to an individual residue because of broadening of resonance lines. Therefore, we carried out H/D exchange reactions of amide hydrogens in a solution containing glycerol, and then determined the H/D exchange rates of individual amide hydrogens by measuring HSQC spectra of deuterated protein in the manner described below. From the protection factors of amide hydrogens against the H/D exchange, we deduced the glycerol-induced conformation of 2SS[6-127,64-80].
NMR Spectra in DMSO/D2O Solution
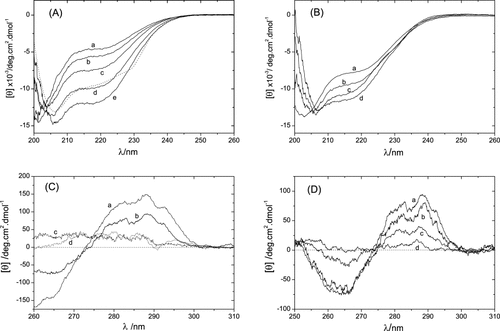
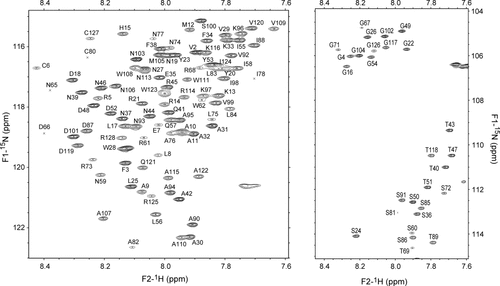
To determine H/D exchange rates of amide hydrogens, H/D exchange reaction must be virtually stopped during the acquisition of NMR signals (about 15 min). In the conventional H/D exchange NMR method, therefore, partially deuterated proteins are rapidly refolded immediately after the H/D exchange reaction to stop the progress of the reaction, then the HSQC spectra of refolded proteins are measured. Since disulfide-deficient variants of lysozyme are inherently unstructured in water, H/D exchange time-constants of almost all amide hydrogens are much faster than 15 min. Therefore, it was impossible to measure their H/D exchange rates by the conventional method. To measure the NMR spectra of partially deuterated proteins, we adopted the mixture of 95% DMSO and 5% D2O at pH* 5.5 adjusted with dichloroacetate-d2 as a quenching buffer, because the minimum exchange rate was found to be about 100-fold reduced in the quenching buffer relative to that in water.16 This solvent system had another advantage that it significantly suppressed broadening of the resonance lines of lysozyme variants and enabled us to obtain well-separated NMR spectra as shown in Figure 2. The peak assignment was readily carried out using 3D HSQC-NOESY-HSQC spectra. Almost all residues were successfully identified on the HSQC spectrum of 2SS[6-127,64-80] except K1, W63, P70, P79, R112, and L129. In addition, the HSQC spectrum of 0SS variant was measured in DMSO/H2O solution. The assigned chemical shifts for lysozyme variant, 0SS and 2SS[6-127,64-80], have been deposited in the BMRB database with accession numbers of 11051 and 11052, respectively. Figure 3 shows the chemical shift differences of amide protons of 2SS[6-127,64-80] from those of 0SS. Actually, large differences were observed near the disulfide bridges existing in 2SS[6-127,64-80].
1H-15N HSQC spectrum of 2SS[6-127,64-80] measured in a mixture of 95% DMSO/5% H2O at pH 5.5 and 25°C. The right figure shows the upper half of the spectrum along the F1-axis, and the left figure the lower half. The cross-peaks of N65, D66, I78, and C80 could be identified but their contour levels were below the threshold.
Chemical shift differences of 1HN of 2SS[6-127,64-80] from those of 0SS-variant. Chemical shift values are significantly influenced in the region of a short loop formed by Cys64-Cys80, while they remain unaltered in other regions except the immediate vicinity of Cys6-Cys127.
H/D Exchange Rates of 2SS[6-127,64-80] in DMSO/D2O Solution During the Acquisition of NMR Spectra
As mentioned earlier, the HSQC spectra of partially deuterated protein after the H/D exchange were measured in the quenching buffer (mixture of 95% DMSO/5% D2O) at 25°C. To see to what extent the H/D exchange progresses during the acquisition of NMR spectra, we actually observed the time course of HSQC spectra in this solvent. The time constants of H/D exchange in the quenching buffer were determined for most amide hydrogens of 2SS[6-127,64-80], and are summarized in Table I. Generally speaking, time constants of most Gly, Ser, and Asn were fast (about 45 to 120 min.), those of Ala were around 150 min, and those of hydrophobic residues were slow ranging from 500 to 1000 min, although the values were dependent on neighboring residues. As a result, H/D exchange time-constants of most residues were longer than 120 min except R5, C6, H15, G16, D18, D48, G49, D52, D87, D101, G102, and D119 with too fast exchange rates.
| Residue | t (min) | Residue | t (min) | Residue | t (min) | Residue | t (min) |
|---|---|---|---|---|---|---|---|
| K1 | F34 | 307 | G67 | S100 | 62 | ||
| V2 | 183 | E35 | 302 | R68 | 266 | D101 | |
| F3 | 207 | S36 | 299 | T69 | 733 | G102 | 18 |
| G4 | 91 | N37 | 79 | P70 | N103 | 117 | |
| R5 | 45 | F38 | 537 | G71 | 179 | G104 | 99 |
| C6 | 30 | N39 | 364 | S72 | 177 | M105 | 271 |
| E7 | T40 | 230 | R73 | 446 | G106 | 239 | |
| L8 | 178 | Q41 | 555 | N74 | A107 | 274 | |
| A9 | 105 | A42 | 205 | L75 | 1226 | W108 | 570 |
| A10 | 92 | T43 | 739 | A76 | 92 | V109 | 1293 |
| A11 | 109 | N44 | 346 | N77 | A110 | 372 | |
| M12 | 174 | R45 | 484 | I78 | 1594 | W111 | 394 |
| K13 | N46 | 360 | P79 | R112 | |||
| R14 | 329 | T47 | 858 | C80 | N113 | 170 | |
| H15 | D48 | 45 | S81 | 359 | R114 | 474 | |
| G16 | G49 | 28 | A82 | 356 | A115 | 305 | |
| L17 | 79 | S50 | 69 | L83 | 554 | K116 | 188 |
| D18 | T51 | 621 | L84 | 668 | G117 | 100 | |
| N19 | D52 | 36 | S85 | 307 | T118 | 108 | |
| Y20 | 554 | Y53 | 272 | S86 | 291 | D119 | 36 |
| R21 | 327 | G54 | 133 | D87 | 19 | V120 | 85 |
| G22 | 137 | I55 | 305 | I88 | 330 | Q121 | 318 |
| Y23 | 160 | L56 | 703 | T89 | 372 | A122 | 124 |
| S24 | 284 | Q57 | 200 | A90 | 205 | W123 | 329 |
| L25 | 568 | I58 | 1814 | S91 | 86 | I124 | 554 |
| G26 | 155 | N59 | 646 | V92 | 363 | R125 | 298 |
| N27 | 154 | S60 | 414 | N93 | 104 | G126 | 142 |
| W28 | 739 | R61 | 975 | A94 | 204 | C127 | 165 |
| V29 | 819 | W62 | A95 | R128 | 302 | ||
| A30 | 222 | W63 | K96 | L129 | |||
| A31 | 97 | C64 | K97 | ||||
| A32 | 109 | N65 | I98 | 260 | |||
| K33 | 225 | D66 | V99 | 548 |
H/D Exchange Reaction of Disulfide-Deficient Variants of Lysozyme in the Absence of Glycerol
H/D exchange reactions of 0SS- and 2SS[6-127,64-80]-variants were carried out at 4°C and pH* 3.0 in the absence of glycerol. After various time of 5, 10, 30, 60, 120, and 480 min, the exchange reaction was stopped by freeze-drying the protein, then the partially deuterated protein was dissolved in the quenching buffer for the measurements of HSQC spectra. Figure 4a shows some examples of H/D exchange reactions of amide hydrogens. H/D exchange time-constants were determined as described in Methods. In Figure 4a, the best-fitting curves for F34, V92, I55, and W108 had time-constants of 34, 50, 66, and 163 min, respectively.
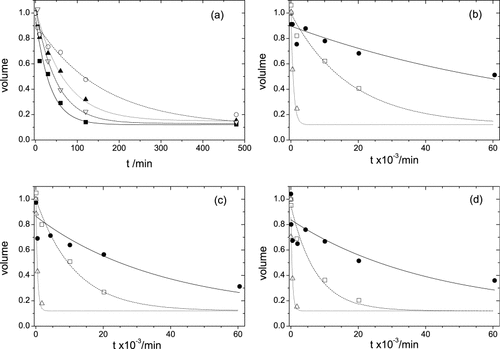
H/D exchange reactions for amide hydrogens of some residues. The peak-volume of each amide hydrogen is normalized by using that measured in H2O solution, and plotted against the time of H/D exchange reaction. (a) H/D exchange reactions in 20 mM formate buffer. W108(○), I55(▴), V92(▿), F34(▪). The baseline of exchange reactions was observed close to 0.1. (b), (c), and (d) H/D exchange reactions of some amide hydrogens in the presence of glycerol; 10% glycerol (Δ), 20% (□), 30% (•). F34 (b), I55 (c), V92 (d). Exchange time-constants were analyzed with the baseline of 0.12.
However, the time-constants of some residues could not be precisely determined for some reasons. Amide hydrogens of R5, S6 (in 0SS), H15, G16, D18, D48, G49, D52, D87, D101, G102, and D119 exchanged so fast even in the quenching buffer that the calculation of their peak volumes was difficult. In the case of residues located near the disulfide bridges in 2SS[6-127,64-80], the peak-heights remarkably diminished due to the peak broadening as shown in Figure 2, so that we could not determine the exchange rates of C6, E7, W63, C64, N65, D66, G67, C80, and S81. Further, time-constants of some residues could not be precisely determined because of an overlap of cross-peaks. For example in 2SS[6-127,64-80], Y20, L83, and I124 overlapped each other, and the cross-peaks of A10 and A76, L17 and N93, N19 and N74, A95 and Q57, K96 and I55, could not be separated from each other because of an insufficient resolution of cross-peaks, which was inevitable to shorten the acquisition time of NMR spectra.
Besides 2SS[6-127,64-80], H/D exchange time-constants for the residues of 0SS-variant were determined and are shown in Figure 5 together with those of 2SS[6-127,64-80]. The exchange rates of the same residue in the two variants were quite similar to each other. In Figure 6, exchange time constants of 0SS-variant measured in 20 mM formate buffer are compared with those expected in a random coil model, which are predicted according to Bai et al.22 The results indicate that time constants in the formate buffer, τfor, are significantly faster than Bai's predicted values, τpre. Bai's experimental data were obtained in 0.5M KCl to shield possible charge effects, whereas our experiments had to be performed in 20 mM formate buffer to avoid the aggregation of disulfide-deficient variants. Since the difference in salt concentrations seems the cause of the disagreement,23 H/D exchange reactions of 0SS-variant were measured in a solution containing 4.0M GuHCl. As shown in Figure 6, the time constants in 4.0M GuHCl, τgdm, were nearly equal to Bai's predicted values. These data suggest that neither 0SS nor 2SS[6-127,64-80] has any structure preventing amide hydrogens from being exchanged. Although the helical contents of 2SS[6-127,64-80] were somewhat larger than those of 0SS-variant judged from their far-UV CD spectra shown in Figures 1A and 1B, in the absence of glycerol, two disulfide bridges existing in 2SS[6-127,64-80] do not appear to promote the formation of partially folded structures.

H/D exchange time-constants of each amide hydrogen in 2SS[6-127,64-80] and 0SS in 20 mM formate buffer solution at pH* 3.0 and 4°C. Profiles of time-constants versus the amino acid sequence are similar to each other between 2SS- and 0SS-variants. In the absence of glycerol, the constraint on the backbone conformation due to two disulfide bridges existing in 2SS[6-127,64-80] has little influence on the H/D exchange rates, although the helical contents of 2SS[6-127,64-80] in water were somewhat larger than those of 0SS-variant, judged from their Far-UV CD spectra.
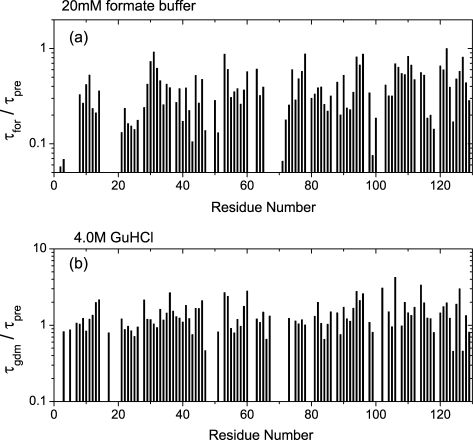
Differences in H/D exchange rates of 0SS-variant between in 20 mM formate buffer and in 4.0M GuHCl solution. (a)τfor/τpre, where τfor is a time constant in formate buffer, and τpre Bai's predicted one. (b) τgdm/τpre, where τgdm is a time constant in 4.0M GuHCl solution. τfor/τpre ranges from 0.2 to 0.5, whereas τgdm/τpre is nearly equal to 1.0.
H/D Exchange Reaction of Disulfide-Deficient Variants of Lysozyme in the Presence of Glycerol
H/D exchange reactions of 2SS[6-127,64-80] were performed in solutions containing 10, 20, and 30% glycerol. Figures 4b, 4c, and 4d show the H/D exchange reactions of F34, I55, and V92, respectively, in the presence of glycerol. In a solution of 10% glycerol, H/D exchange reactions were carried out for 30 min, 1.0, 8.0, and 30 h at pH* 3.0 and 4.0°C. Exchange reaction was somewhat delayed compared with that in water, but it finished within 30 h for almost all residues. When the concentration of glycerol increased from 10 to 20%, exchange reaction definitely slowed down in the regions of L8-R14, W28-F34, F38-N39, Q41, Y53-R61, L83-S85, T89, S91-S100, W108, W111, and W123-R125. In the presence of 30% glycerol, HSQC spectra of partially deuterated samples were measured after each H/D exchange reaction was carried out for 1.0, 8.0, 32, 72, 168, 336, and 1008 h. The peak volume of 42 residues diminished fast, but more than 30 cross-peaks still remained definitely even after 168 h. In the case of 30% glycerol, time-constants of H/D exchange were determined only for residues whose peak volume was enough detectable after 8.0 h. For other residues whose exchange reaction almost finished in 8.0 h, time-constants were not estimated anymore.
Protection factors against the H/D exchange reaction in a glycerol solution were determined for individual amide hydrogens as the ratio of τobs to τfor, where τobs and τfor are time constants of exchange reaction measured in a glycerol solution and in a formate buffer solution, respectively. Figure 7 shows the protection factors of each residue in 10, 20, and 30% glycerol solutions. In a solution of 10% glycerol, protection factors uniformly increased to about 5 to 10 at most residues. It is likely that non-native secondary structures are induced anywhere along the polypeptide chain, but the conformation appears non-persistent, that is, to be easily broken and rebuilt again. With the increasing concentration of glycerol, some selected regions grow the protection factors, but other regions do not any more. In highly protected regions, secondary structure may be frozen by the tertiary structure induced by glycerol. Most highly protected regions are V9-M12, V29-F34, I55-I58, L83-L84, T89, and V92-V99, and their protection factors reached about 1000 in 30% glycerol solution. Strictly speaking, the cross-peaks of K96 and I55, those of A95 and Q57, and those of I124 and L83 overlapped each other, so we could not determine their time constants separately. A common value was assigned as the protection factors for these residues.
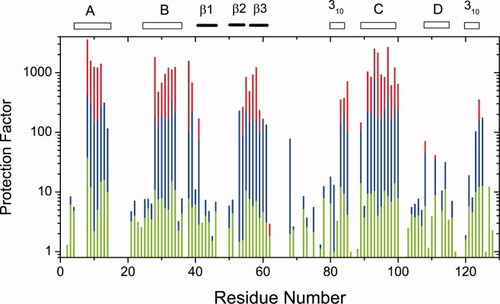
Profiles of protection factors versus amino acid sequence. Green: 10% glycerol, Blue: 20% glycerol, Red: 30% glycerol. The locations of structural elements in the wild-type lysozyme are shown by short bars along the horizontal axis.
In H/D exchange reactions of L8, W28, F38, N39, Q41, Y53, G54, N59, S60, S85, S91, N93, A94, and S100, a fast phase of exchange reaction was observed within an hour and followed by a slow phase. The signal amplitude of the fast phase was close to a half of total one for these residues. That is to say, such a residue was weakly protected in some ensemble of structures, while it was strongly protected in another one. Tertiary structures induced in concentrated glycerol solution might be inhomogeneous with a very slow inter-conversion rate between ensembles. These residues were placed on the periphery of highly protected regions. In general, such a fast phase was also observed for highly protected residues mentioned earlier, but the amplitude of the fast phase was relatively small. In Figure 7, the protection factors of the slow phase are plotted for all residues.
In the native structure of the wild-type lysozyme, A-helix including A9-M12, B-helix including V29-F34, and C-helix including T89-V99 enclose the side chains of Ile 55 and Leu 56. Also, Q57 and I58 are located in the interface between two domains in the native structure. Therefore, the amino acid residues whose protection factors reach 1000 appear to form a native-like rigid structure with centering on Ile 55 and Leu 56. In Figure 8, the highly protected region is represented by sticks-and-balls on the ribbon diagram of the native structure of lysozyme. This region looks like a pivot of lysozyme folding with three helices and three β-strands anchored together. Also, it is suggestive that protection factors of F38, N39, and L83-S85 which are located near the pivot in the native structure also approach 1000. On the other hand, protection factors increased to 100, but did not exceed it in the regions of D-helix (residues 108–115) and C-terminal 310-helix (residues 120–124). Protection factors were limited to 10 in the region of β1- and β2-strands (residues 41–54), and other regions corresponding to irregular structures of the native lysozyme. The preferential hydration seems insufficient to freeze the structural freedom of these regions because of the lack of cooperativity in interactions among residues.
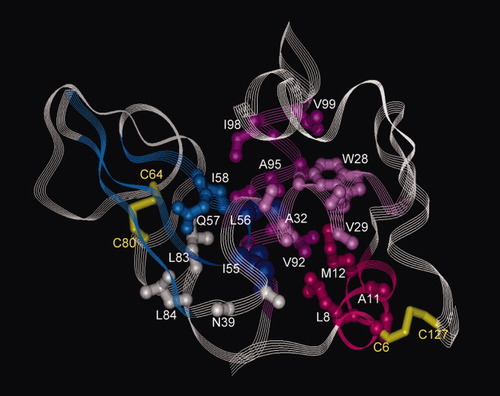
Highly protected regions in the glycerol-induced structure of 2SS[6-127,64-80]. Highly protected residues are represented by sticks-and-balls on the ribbon diagram of the native structure of lysozyme. Ile 55 (I55) and Leu 56 (L56) are colored dark blue, and Q57 and I58 light blue. Residues in helix A (L8 to M12) are colored crimson red, those in helix B (W28 to F34) pink, and those in helix C (S91 to V99) magenta. F38, N39, L83, and L84 are colored white. Some side chains are omitted to avoid a crowd of residues.
DISCUSSION
Preferential Hydration by Glycerol
The effect of solvent additives on protein stability can be interpreted in terms of the solvation properties of protein. In general, large cosolvent molecules are excluded from the solvation shell near the protein's surface because of volume exclusion, and protein is said to be preferentially hydrated.13, 14 It was experimentally shown that glycerol resulted excluded from the surface of wild-type lysozyme in water/glycerol mixtures, confirming that lysozyme was preferentially hydrated.24 The protein surface area in contact with the solvent tends to be minimized by negative binding of glycerol. In most of the previous studies on the effects of glycerol, proteins were originally in the native state and the preferential hydration further stabilized them preventing the exposure of buried groups to the solvent.18, 25, 26
In the present study, the disulfide-deficient variant, 2SS[6-127,64-80], is inherently unstructured in pure water. Preferential hydration by glycerol induces 2SS[6-127,64-80] to recover a native-like tertiary structure in a part of molecule. [θ]220 of 2SS[6-127,64-80] was relatively large at low concentrations of glycerol compared with that of 0SS- or 1SS-variants, but it only increased gradually in the range of 20 to 70% glycerol. At the early stage below 10% glycerol, the helical contents of 2SS[6-127,64-80] may increase easily at most of residues, but they will soon reach saturation. In such an early stage, it seems likely that the helical contents are relatively large but the protection factors remain low. Protection factors of about 10 mean that the protected form of a given amide merely persists nine times longer than the exposed one. Suppose each residue independently changes its conformation, the probability that several consecutive residues are in a protected form is low. As a result, a helical conformation is not persistent and short secondary structures only float around along the polypeptide chain. In the case of 0SS- or 1SS-variants of lysozyme, [θ]220 increased with the increase in concentration of glycerol, and it approached to the same value as in 2SS[6-127,64-80] at 70% glycerol, but the protection factors for H/D exchange of 1SS[64-80] and/or 1SS[76-94] variants remained low (around 10) even in 70% glycerol solution. These observations are similar to those for 2SS[6-127,64-80] in 10% glycerol solution. The conformation of 2SS[6-127,64-80] in this stage looks like a disordered one rich in helices.
With increasing concentration of glycerol, the preferential hydration seems to induce weakly protected segments in 2SS[6-127,64-80] to associate with each others through hydrophobic interactions. If the cooperativity gets to work among some structural elements, a rigid tertiary structure may emerge and protection factors rapidly increase to reach 1000 in the part of molecule. In 20 to 30% glycerol solution, the cooperativity seems to work among residues centering on Ile 55 and Leu 56 surrounded by A-, B-, and C-helices, while β1- and β2-strands, D-helix and C-terminal 310-helix appear to be independent from the former region.
An Early Stage of Lysozyme Folding and the Role of Disulfide Bridges
Although the helical contents of 0SS- or 1SS-variants increased due to the preferential hydration by glycerol, the effect of glycerol alone was insufficient for these variants to fold successfully. For example, 1SS[64-80] with a single disulfide bridge Cys64-Cys80 was rich in helices but still remained non-persistent judged from the protection factors. The stability of glycerol-induced structure in 2SS[6-127,64-80] suggests that Cys6-Cys127 plays an important role for the formation of the stable α-domain structure, because Cys64-Cys80 is far from the α-domain itself. However, 1SS[6-127] containing only one disulfide bridge Cys6-Cys127 could not recover the tertiary structure even in 70% glycerol as well as 1SS[64-80]. Besides Cys6-Cys127, either Cys30-Cys115 or Cys64-Cys80 is necessary to keep the α-domain stable.
When both Cys6-Cys127 and Cys30-Cys115 were present, 2SS[6-127,30-115] maintained the native-like structure even in the absence of glycerol.9 As pointed out in our previous article, many side-chains are closely packed within the hydrophobic box in the α-domain, and many long-range NOE contacts were detected between side-chain and main-chain protons. The α-domain structure was never like a molten globule, rather close to the native one. On the other hand, when Cys64-Cys80 coexists with Cys6-Cys127, the tertiary structure of the α-domain was lost in pure water, but recovered in 30% glycerol solution. The preferential hydration by glycerol seems to reinforce the hydrophobic box enclosed by A-, B-, and C-helices. What is the role of Cys64-Cys80 in 2SS[6-127,64-80]? Besides the preferential hydration by glycerol, the constraint on the backbone conformation by Cys64-Cys80 is necessary for 2SS[6-127,64-80] to stabilize the glycerol-induced structure. Our previous studies on 3SS-variants of lysozyme showed that Cys64-Cys80 made a large contribution toward maintaining the β-sheet structure.8 Suppose Cys64-Cys80 is removed from 2SS[6-127,64-80], it may lead β-sheet to fluctuate significantly, so that the cooperativity between two domains may weaken, and the side-chains of Ile 55 and Leu 56 may come out the pocket surrounded by A-, B-, and C-helices. As a result, the glycerol-induced structure in the α-domain may be disrupted simultaneously. Probably Cys64-Cys80 is necessary for side-chains of Ile 55 and Leu 56 to anchor at the hydrophobic box in the α-domain.
The glycerol-induced structure in 2SS[6-127,64-80] is rather close to a molten globule. At relatively low concentration of glycerol, it looked like a disordered structure rich in helices. With increasing glycerol concentration, a rigid tertiary structure emerged but long-range NOE contacts were not detected because of the resonance-line broadening. These are the characteristics of the molten globule. Further, it is intriguing that the glycerol-induced structure in 2SS[6-127, 64-80] is similar to that of 2SS[6-127,30-115]. In particular, residues centering on Ile 55 and Leu 56 were highly protected against the H/D exchange in both 2SS-variants. The difference between them is that the hydrophobic box looks like a molten globule in the former, and a crystalline structure in the latter, and that the C-terminal region including D-helix and 310-helix is flexible in the former, and rigid in the latter. It implies that the structural motif common to both 2SS[6-127,64-80] and 2SS[6-127,30-115], in which residues centering on Ile 55 and Leu 56 form a native-like persistent structure through hydrophobic interaction as shown in Figure 8, is crucial to the lysozyme folding.
The wild-type lysozyme is a protein for which folding has been studied in great detail by combination of different techniques.5 When the folding was initiated by dilution of denaturants from 6M GuHCl, it was complex with a variety of distinct steps. At the intermediate stage of folding, most highly populated species was the α-domain folding intermediate, in which the α-domain formed a highly stable structure with a hydrophobic box, while the β-domain was still unstable and rapidly fluctuating.6 The glycerol-induced structure in 2SS[6-127,64-80] is close to the α-domain folding intermediate of the wild-type in most of characteristics. Thus, it seems a molten globule at equilibrium. It is likely that a molten globule grows from a disordered form to an ordered one during the folding. At the early stage of molten globule such as in 10% glycerol, it may be a disordered conformation rich in helices, while a stable native-like structure may emerge in a part of molecule at the late stage such as in 30% glycerol. If that is the case, the late molten globule is still distinguished definitely from the native state by the global cooperative transition between them.
Acknowledgements
The authors thank Dr. S. Iimura and Dr. K. Yutani for their valuable discussion. The assigned chemical shifts for lysozyme variants, 0SS and 2SS[6-127,64-80], were deposited in the BMRB database with an accession number of 11051 and 11052, respectively.




