Complex biopolymeric systems at stalk/epicuticular wax plant interfaces: A near infrared spectroscopy study of the sugarcane example
Abstract
Naturally occurring macromolecules present at the epicuticular wax/stalk tissue interface of sugarcane were investigated using near infrared spectroscopy (NIRS). Investigations of water, cellulose, and wax-cellulose interrelationships were possible using NIRS methods, where in the past many different techniques have been required. The sugarcane complex interface was used as an example of typical phenomena found at plant leaf/stalk interfaces. This detailed study showed that sugarcane cultivars exhibit spectral differences in the CHn, water OH, and cellulose OH regions, reflecting the presence of epicuticular wax, epidermis, and ground tissue. Spectrally complex water bands (5276 cm−1 and 7500–6000 cm−1) were investigated via freeze-drying experiments which revealed sequentially a complex band substructure (7500–6000 cm−1), a developing weak H-bonding system (∼7301 cm−1), and strong H-bonding (∼7062 cm−1) assigned to water—cellulose interactions. Principal component analysis techniques clarified complex band trends that developed during the desorption experiment. Bands from wax-free stalk were minimized in the 4327–4080 cm−1 region (CHn vibrational modes associated with long chain fatty compounds), while bands from the stalk tissue (particularly lignin and moisture) became more pronounced. This work is a comprehensive guide to similar studies by scientists involved in a variety of plant and fiber research fields. © 2009 Wiley Periodicals, Inc. Biopolymers 91: 642–651, 2009.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
The cellulose polymer forms the backbone of macro-structures derived from natural or processed plant sources. In its natural state, cellulose is in close contact with many other large and small, naturally occurring, molecules on or within the leaves, stems, and roots of a plant. Common essential molecules in this context include water, hemicellulose, lignin, and proteins as well as the trace elements. Additionally, the primary plant surfaces are complex matrices consisting of cutin and waxes, which serve as interfaces between the plant and the environment. Recently, it has been shown that such surface substances play a significant role in the resistance of plants to disease.1-3 Within the plant substrate, cellulose, hemicellulose, and water interact forming a complex chemical matrix.
Although cellulose is an important component of plants and has many applications, our understanding of its nature remains incomplete. Cellulose is a linear polymer composed of β-(1-4)-D-glucopyranose units in a 4C1 conformation.4, 5 Naturally occurring cellulose I contains two co-existing phases—cellulose Iα (triclinic) and cellulose Iβ (monoclinic).6 These are present in varying proportions depending upon the origin of the biopolymer. Higher plants contain predominantly the cellulose Iβ phase of the biopolymer.4-6
Cellulose in its various forms has been studied extensively with the use of vibrational spectroscopy.4, 7, 8 Both mid infrared (IR) and near infrared (NIR) spectroscopy have been applied to investigate the degree of crystallinity, the type of hydrogen bonding, and the relative amount of crystalline modification in cellulose.8, 9
A recent mid IR study investigated the interactions between dry microcrystalline cellulose (MCC) and absorbed water.4 The IR spectra were collected from wet MCC during a controlled drying experiment, and an absorbance decrease was noted with increased drying time in the 3700–3000 cm−1 region (assigned to the OH stretching modes of cellulose and water) and the 1700–1600 cm−1 region (attributed to the OH bending mode of water). Isosbestic points were observed for the latter region and two types of water were proposed to be present in equilibrium: the bulk water and the water with weak OHwater···OHwater hydrogen bonding resulting from interaction with MCC OH groups. The drying process was viewed as a disturbance of the equilibrium. In the OH stretching region (3700–3000 cm−1), similar observations were made, and were reflected in a wave number shift. For instance, the 3412 cm−1 band involved with the O6H6···O3′ interaction shifted to higher wave number (8 cm−1). In a similar NIR study on water adsorption with MCC,10 the first overtone of the OH stretching vibration of cellulose was reported inthe 7200–6000 cm−1 region and the combination of symmetric and antisymmetric OH stretching modes of vibration (ν1 + ν2 mode) of water was also observed. In the 7500–7200 cm−1 region, the combination of the first overtone of the CH stretching mode and the CH deformation mode of cellulose bands was reported.9 The band assignments were supported by PCA loadings profiles. In addition, an adsorption isotherm involving water activity and moisture content (wt %) was constructed. The physical data collected were from the drying specimen just before its sampling by NIR. Various stages of the adsorption process were identified.
In general, the aerial surfaces of plants are covered with a wax layer that is primarily a waterproof barrier, which is not present on the MCC. This layer also provides protection against environmental stresses. Epicuticular crystalline structures of wax overlay an amorphous layer, which often gives the plant surface a gray appearance.11 Sugarcane wax is different to that of many other plants because of the predominance of long-chain aldehydes ranging from C26 to C36,1, 12, 13 triglycerides,14 long-chain alcohols, and alkanes.1
Thus, a composite NIR spectrum is obtained from the complex interface where the epicuticular wax layer and the plant tissue meet. This poses a substantial challenge for spectral interpretation.
Lignin is also present in plant tissue. It fills the spaces in the cell wall between cellulose, hemicellulose, and pectin components and confers mechanical strength to the cell wall and therefore the entire plant. Lignin is defined as a member of a group of vegetable compounds that are insoluble in 72% sulfuric acid, and contain phenylpropanoid residues as building blocks.14 There are many substituent/functional groups and substructures in lignin, including aliphatic and phenolic OH, CO, COOH, CH3, OCH3, α,β CC, furan, and interunit COC and CC linkages.15
Hemicellulosic polysaccharides are defined as those plant cell-wall polysaccharides that are not solubilized by water or chelating agents but are solubilized by aqueous alkali.16 In dicotyledonous and non-graminaceous monocotyledonous plants, the principal hemicellulose polysaccharide is xyloglucan. However, in the cell walls of the Gramineae, of which sugarcane is a member, the principal hemicellulose is glucuronoarabinoxylan, with only small amounts of xyloglucan present.16, 17
This article describes extensive NIR investigations of the complex biopolymeric system at the stalk tissue/epicuticular wax interface of sugarcane. While sugarcane is of particular interest for our work, the interface system, in this case, is a specific example of the phenomena found on leaf/stalk parts of plants in general. Thus, this study is of much broader significance and application.
There are many different sugarcane cultivars, and in this work, we have used as an example, the cultivar Q124, because it has been well regarded commercially with consistently high yields and reasonable resistance to diseases. We compared the spectral properties of Q124 with those of cultivar Q90, another example of a well-performing commercial clone. The work has been carried out with the use of NIR spectroscopy, which with the aid of an optical probe, facilitated spectral collection at-field, directly from the cane stalk as required. The work also links in with the recent studies on MCC using mid IR and NIR techniques.5, 9 Of particular interest is the comparison of spectra between high purity cellulose and those sampled from the surface of the sugarcane. Thus, we compared band assignments of the NIR spectra from the interface of sugarcane with those found in the pure cellulose model studies, as well as with other sources from the literature. Since pore water is of considerable importance in the model system reported previously9 and is also vital for plant biology in general, NIR spectra were measured from sequentially dried sugarcane samples over the 11,000–4000 cm−1 range and the spectral results of the complex and heavily overlapping NIR spectra were interpreted. In addition, we compared these band assignments with those found in the spectra of sugarcane surfaces from which the epicuticular wax has been removed by a suitable solvent. Interpretation of the complex spectra collected was assisted by chemometrics.
EXPERIMENTAL SECTION
Sugarcane Samples
Sugarcane stalk samples of Q90 and Q124 were collected from the BSES Limited Pathology Farm (Woodford, Australia). Healthy and undamaged stalks were selected, cut in half and tagged. Immediately after cutting, the cane was prepared for the different treatments to be applied.
Samples for NIRS of Epicuticular Wax/Stalk Interfaces
The upper leaf sheaths of the stalk were removed with a scalpel to expose the nodal and internodal areas beneath (Figure 1a). Appropriate sampling sites were defined by counting down between the nodes 13 and 16 from the top, which generally provided samples for analysis that had been well protected from the environment.
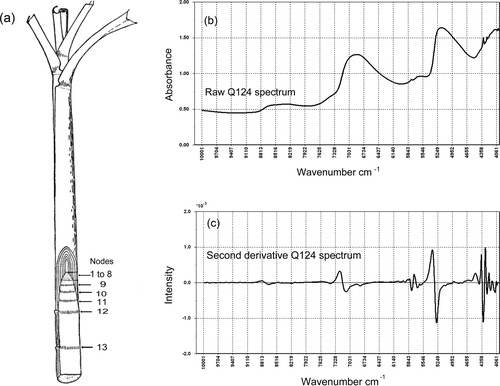
Sugarcane stalk schematic showing the individual nodes, nodes 1–8 are part of the growing point or apical bud (1a). A typical near infrared absorbance spectrum (1b) and the 2nd derivative spectrum (1c) of the stalk surface of cultivar Q124.
Samples for the Progressive Drying Experiment
Ten 4 × 4 mm squares of sugarcane stalk tissue with a reasonably constant depth (2 mm) were excised from the waxy areas between nodes 15 and 16, taking due care not to disrupt the wax coverage. The weight of each sample was obtained immediately using a Mettler AE 240 analytical balance. Sugarcane samples for the freeze drying experiment were placed without pre-freezing, in a freeze dryer (Lindner and May, Brisbane, Australia) operating at −70°C and 10 Torr vacuum and samples were withdrawn at 0 min (fresh), at 5 and 10 min and thereafter, every 10 min until 60 min drying, with a further two samples were taken at 120 and 240 min. After removal from the freeze drier, each sample was placed into a desiccator and reweighed within a few minutes. The weighed sample was analyzed by NIRS immediately.
FT-NIR Instrumental Conditions and Data Collection
All NIR spectra were collected using a Multi-Purpose Analyzer, Fourier transform spectrometer (Bruker) equipped with an indium gallium arsenide (InGaAs) detector. Eighty scans were co-added for each spectrum at a spectral resolution of 16 cm−1. All spectra were presented as −log10(R/Ro), where R and Ro are the intensity signals from a sugarcane sample and from a Spectralon background material, respectively. All raw spectra were normalized (Min–Max normalization) using OPUS Spectroscopy Software, Version 6.
NIR Sampling of the Epicuticular Wax/Stalk Interface
The fiber optic probe was clamped in a laboratory retort stand, and the appropriate portion of the cane stalk was carefully positioned underneath the probe. All samples were scanned in the spectral range 4000–11,000 cm−1.
NIR Sampling of the Freeze Drying Experiment
The NIR probe was set up as described in the Section “NIR Sampling of the Epicuticular Wax/Stalk Interface.” Each dried sugarcane sample was positioned on a clean glass Petri dish, which was located on a vertically movable platform. The platform was raised until the sample was just in contact with the tip of the fiber optic probe, at which point a spectrum was collected over the wavelength range of 4000–11,000 cm−1.
NIR Sampling of the Stalk Without Epicuticular Wax
The probe was set up as in the Section “NIR Sampling of the Epicuticular Wax/Stalk Interface” and a spectrum was taken from the appropriately collected fresh sugarcane sample. The surface of this sample was wiped with hexane (Mallinckrodt Baker, Phillipsburg, NJ) to remove the epicuticular wax and allowed to dry before the cane was scanned again. This process was repeated several times until the epicuticular wax signal from the original spectrum could not be discerned, indicating that the wax had been completely removed.
An NIR difference spectrum was produced using the initial spectrum with epicuticular wax intact, and the final spectrum from which the wax had been completely removed.
RESULTS AND DISCUSSION
The normalized NIR spectrum (Figure 1b and 2nd derivative, Figure 1c) sampled from the stalk surface of Q124 displayed broad overtone and combination bands. The band assignments (Table I) were made with respect to the 2nd derivative spectrum, which showed improved spectral detail. The effective IR radiation penetration depth based on a sample of an MCC powder has been reported to be 2.5 mm according to the variable layer thickness method.18 Assuming a similar penetration depth for the sugarcane sample, the IR beam penetrates beyond the thickness of the wax layer structure (10 μm) and the epidermal layer (25–75 μm).19 Thus, the IR beam should penetrate into the cell layer structure of the stalk where the ground tissue and vascular bundles are located. This infers that the spectra obtained should be a composite of the responses of these three noted structural features of sugarcane. Thus, a spectrum will contain responses from epicuticular wax as well as cellulose, hemicellulose, lignin, and water present in the stalk tissue.
| Functional Group | Observed Wavenumber cm−1 | Literature Value cm−1 | Overtone | Structure |
|---|---|---|---|---|
| CH | 4080 | 3937–4065 | CH bend | Fatty compounds |
| CH | 4185 | 4201 | CH stretch | Fatty compounds |
| CH2 | 4253 s | 4252 | CH2 2nd bend | Fatty compounds |
| CH | 4327 s | 4347 | CH bend 2nd OT | Fatty compounds |
| OH/CO | 4406 w | 4405 | OH/CO combination | Lignin |
| CH/CO | 4560 w | 4545 | CH/CO combination | CO |
| COO− | 4761 | 4761 | Asym COO− stretch 3rd OT | Cellulose |
| CO | 5093 w | 5181–5076 | CO stretch 2nd OT | CO |
| H2O | 5151–5273 | 5154 | OH bend 2nd OT | H2O |
| OH/CO | 5502 | 5494 | OH/CO combination | Cellulose |
| CH/HOH | 5591 | 5617 | CH stretch/HOH deformation | Cellulose |
| CH | 5664 m | 5665 | CH stretch 1st OT | Fatty compounds |
| CH | 5784 m | 5797 | CH stretch 1st OT | Fatty compounds |
| CH2 | 5840 | 5865 | CH stretch 1st OT | Fatty compounds |
| CH3 | 5909 | 5899 | CH stretch 1st OT | |
| CH3 | 6688 | 6688 | CH stretch 1st OT | Cellulose |
| OH | 6823 | 6823 | OH stretch 1st OT | |
| OH | 7108 | 7142 | OH stretch 1st OT | |
| OH | 7251 | 7270–7220 | OH antisymmetric and symmetric stretch combination | Water |
| CH | 7301 | 7380–7330 | CH combination | |
| CH | 7427 | 7380–7326 | CH combination | |
| CH | 8218 w | 8230 | CH/CH combination | |
| CH2 | 8570 w | 8571 | CH stretch 2nd OT | |
| CO | 8677 w | 8620 | CO stretch 4th OT |
- Blank spaces in the structure column imply that the origin of the absorbance band within the complex plant tissue matrix is unclear.
- w, weak; m, medium; s, strong band intensity.
The following band assignments refer to Figure 1b (the raw absorbance spectrum containing heavily overlapping bands), Figure 1c (the second derivative spectrum with markedly improved spectral definition) and Table I. All band frequencies have been taken from the second derivative spectrum, although a number of these bands are rather weak. The characteristic cellulose band at 4761 cm−1 is associated with the COO− antisymmetric stretching vibrational mode of the 3rd overtone.15 The band observed at 4406 cm−1 (combination OH stretching/CO stretching mode) is reported as the characteristic wave number for lignin.15 The strongest band in the region at 4327 cm−1 is attributed to the CH bending, 2nd overtone mode of the fatty compounds present predominantly in the epicuticular wax. Other bands associated with CH vibrational modes are presented in Table I. Carbonyl group vibrational modes are present at 4560 cm−1 (CH/CO combination associated with an aldehyde), 5093 cm−1 (CO stretching, 2nd overtone), and 8570 cm−1 (CO stretching, 4th overtone). An intense broad band attributed predominantly to the OH bending, 2nd overtone of water between 5151 cm−1 and 5273 cm−1 is present. Other interactions including the OH stretching/HOH deformation combination of cellulose and water are reflected in this heavily overlapped region and will be further investigated. Additionally, the OH antisymmetric stretch and OH symmetric stretch combination of water is present at 7251 cm−1.20 Bands associated with OH vibrational modes are present at 5502 cm−1 (OH stretching/CO stretching, 2nd overtone combination bands of cellulose) and 5591 cm−1 (CH stretching/HOH deformation of cellulose), with other bands associated with OH vibrational modes indicated in Table I.
This set of band assignments was compared with that from a typical, normalized spectrum taken from Q90 (refer Figure 2). The objective here was to compare a significantly different sugarcane cultivar to investigate if there are any major NIR spectral changes. However, it was not intended in this work, to compare these cultivars in detail. Rather, we focused on an investigation of the typical properties of Q124 and the interpretation of the complex spectral responses derived from it.
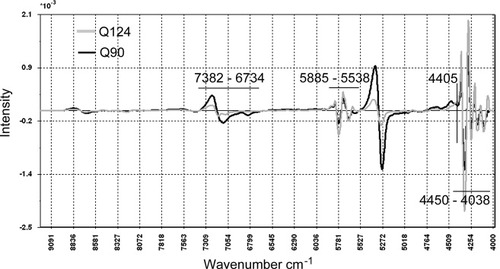
Overlay of 2nd derivative spectra from cultivars Q124 and Q90.
For both cultivars, the typical 2nd derivative spectral profiles are similar. However, it is noted that the CH bands attributable to fatty compounds in the 4450–4038 cm−1 region from Q90 are generally more intense, with the exception of the band at 4405 cm−1, which has been attributed to the lignin absorption.15 This is a simple reflection of the increased wax coverage for the Q90 stalk surface. The bands observed between 5885–5538 cm−1 behave similarly, although again, the Q90 profiles are generally more intense. These bands include the CH stretching/HOH deformation of cellulose at 5591 cm−1. However, the OH stretch/CO stretching combination at 5502 cm−1 shows the opposite behavior, with the water bands of the Q124 spectrum being more intense. The same behavior is also observed in the 5273–5151 cm−1 region attributed to OH stretching/HOH deformation combination and similarly in the 7382–6734 cm−1 range. Thus, it would appear that some of the molecular species which may help to discriminate the sugarcane cultivars are the CHn-rich compounds, especially those found on the plant surfaces such as epicuticular waxes, as well as the H-bonding in the water-cellulose matrix near the surface of the stalk.
Desorption Drying Curve
To support the interpretation of the moisture dependent NIR spectra collected as part of the freezing drying experiment, the moisture loss for each stalk sample with respect to drying time was calculated. The moisture content of fresh sugarcane was obtained experimentally using samples that had been oven dried for 24 h at 60°C.21 The obtained value of 79% moisture agreed well with that from many other plant products.22 All fresh samples in this work, were assumed to contain a moisture content of 79% by weight, and this reference data point was used to construct a desorption drying curve as a function of time (refer Figure 3).
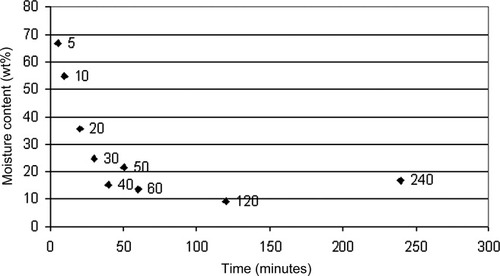
A desorption drying curve of sugarcane stalk tissue.
This plot could be roughly divided into two stages, 0–60 min (fresh) and 60–240 min. During the 0–60 min period, the bulk water present in the sugarcane sample was rapidly removed as the relatively weak hydrogen bonding network between the frozen water–water molecules was broken, and the desorption curve decreased rapidly. This behavior began to slow down after the first 10 min as the stronger intermolecular water–water forces and water–cellulose bonding began to manifest their presence. The desorption process effectively reached a plateau after about 60 min, and indicates an approximate moisture content of 12% at equilibrium. Many freeze drying studies refer to freezing and unfreezing types of water, which have been discussed in detail by Wolfe et al.23 and Hong et al.24 Unfreezing water is generally referred to as the water which remains unfrozen below a specified temperature. It is suggested that it contains some water which involves short and long range interfacial interactions with, in this work, mainly the cellulose polymer, as well as some water which is trapped, but not bound, in the structure on the cellulose/stalk. Studies involving freeze drying of raw vegetables22 indicate that quantities of unfreezing water are commonly around 10–15% w/w. Thus, it would appear that the remaining 12% water in the sugarcane samples analyzed here could be classified as the unfreezing water.
Near Infrared Spectra of Freeze-Dried Samples
An overlay of normalized NIR spectra of the progressively freeze-dried sugarcane samples (Figure 4a) showed a complex, asymmetric, broad band with a peak at 5181 cm−1, which decreased in intensity over the 4-h freeze-drying period. Simultaneously, a shoulder developed at 4774 cm−1. In addition, the weak peak at 5581 cm−1 also decreased and effectively disappeared towards the end of the drying period. In general, this behavior is consistent with the loss of water as a function of the drying time, and is similar to the behavior previously observed with MCC.10 However, comparison of the bands is considerably more difficult than in the MCC study as the sugarcane-cellulose system is much more complex. Further, the maximum amount of water adsorbed by MCC is about 15%,10 which corresponds approximately to the amount of unfrozen water observed in the sugarcane samples after 60 min of freeze drying. Thus, the two studies do not overlap, but in general, some similar trends of spectral behavior may be expected, and hence, the MCC work is used as a convenient reference point. Therefore, band assignments in this work, although being guided by the literature noted, will necessarily involve some degree of interpretation.
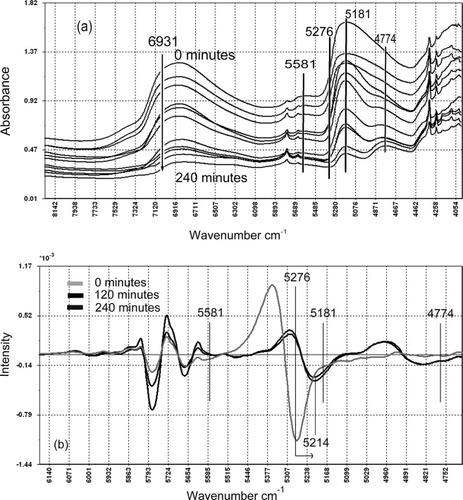
(a) Overlay of the 10 normalized moisture dependent NIR spectra of sugarcane stalk samples progressively dried from 0 to 240 min. (b) Overlay of the normalized 2nd derivative moisture dependent NIR spectra of the initial (0 min drying time) and final two spectra (120 and 240 min).
As the complex, broad band at 5181 cm−1 decreased with drying time, the developing shoulder formed a distinct band peaking at 4774 cm−1. This corresponds well with the asymmetric COO− stretch, 3rd overtone of cellulose.15 Figures 4a and 4b show a significant spectral shift in the 5400–5000 cm−1 region during the freeze-drying treatment. The peak at 5276 cm−1 identified in the raw normalized spectra (Figure 4a) displayed significant band narrowing at longer drying times. The second derivative spectra of the 0, 120, and 240 min drying time samples show a progressive shift from 5276 cm−1 to 5214 cm−1 (Figure 4b). This may be attributed to the OH stretching/HOH deformation combination of water involved in the disruption of bulk water (OHwater···OHwater) H-bonds. The gradual shift to lower wave numbers indicate a weakening of the OH water bond, and, hence, a strengthening of the H-bonding between the water molecules. It would appear that the water absorption band stopped shifting after 120 min, which could be attributed to the H-bonding in the trapped unfrozen water as discussed earlier and the contribution of the OH stretching/HOH deformation combination band (5181 cm−1).15
Normalized NIR spectra of the progressively freeze-dried sugarcane samples (Figure 4a) were also studied over the 7500–6000 cm−1 region. In general, the raw spectra revealed a complex, broad asymmetric band at 6931 cm−1, which is commonly associated with the water modes of vibration [combined symmetric and antisymmetric OH stretching modes of vibration (v1 + v2 mode)].10 A detailed spectral investigation of this region as a function of freeze-drying time broadly supported the interpretation of the 5276 cm−1 region discussed above. In general, there was a decrease in spectral intensity as a function of drying time. Band allocations in this region (Table I) include the first overtone of the OH stretching vibration and the OH antisymmetric and symmetric stretch combination. The 2nd derivative spectra of the most extreme sugarcane samples depicted on the desorption curve [0 min drying time and 240 min drying time (Figure 5a)] showed a strong band at 7108 cm−1 present in the spectrum of the fresh sugarcane, but, after 240 min drying, only two weak bands were present at 7309 cm−1 and ∼6985 cm−1. The broad peak at 7108 cm−1 is consistent with the more specific assignment of the OH stretch, associated with the free OH groups on the glucose unit.25 The weak shoulder at 7301 cm−1 is assigned to a CH3 combination. However, a more detailed appreciation of the band changes may be made from the 2nd derivative spectra (Figure 5b), where the progress of the band shifts with freeze drying time is displayed. This heavily overlapping spectral region was investigated further with the use of PCA.
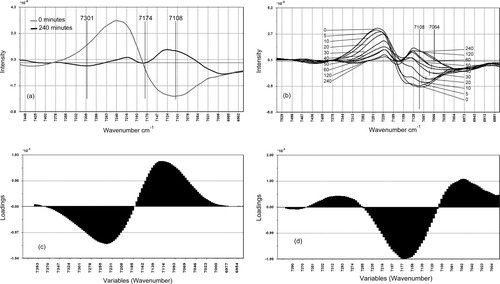
(a) Overlay of the 0 and 240 min moisture dependent normalized 2nd derivative NIR stalk spectra. (b) Overlay of the 10 normalized 2nd derivative moisture dependent NIR spectra of the progressive freeze drying experiment. (c) PC1 Loadings versus variables plot. (d) PC2 Loadings versus variables plot.
Principal Component Analysis
- i
Drying times 0–10 min (negative PC1 and positive PC2 values).
- ii
20 min (negative PC1 and negative PC2 values).
- iii
30–40 min (positive PC1 and negative PC2 values).
- iv
50–240 min (positive PC1 and positive PC2 values).
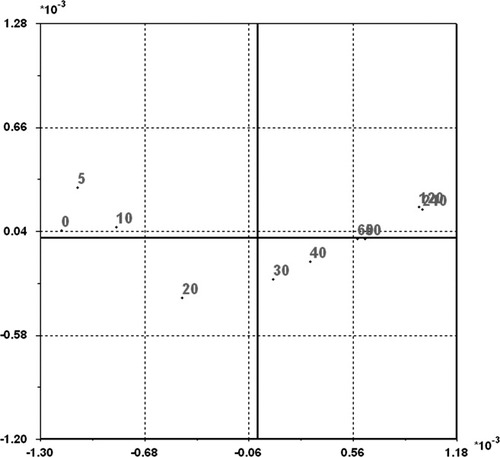
PC1 and PC2 score plot of the NIR spectra shown in Figure 4. The numbers in the plot indicate drying the time of each sample in minutes.
This produces a parabolic PCA scores plot which, in general, reflects the changes in interactions at the water/sugarcane interface during the desorption process as sampled by NIR spectroscopy. Overlays of the two 2nd derivative spectra obtained at 0 and 240 min drying time (Figure 5a), and of all 10 2nd derivative spectra of the progressively dried samples (Figure 5b) in the region between 7401 and 6938 cm−1, reflect these changes.
- i
the PC1 loadings plot (Figure 5c) discriminates the spectral objects from the 30–240 min drying range by a positive peak at 7108 cm−1, while the negative objects are separated by the negative loadings with a minimum at 7251 cm−1;
- ii
the PC2 loadings plot (Figure 5d) discriminates the spectral objects with positive scores by two well spaced positive loadings bands with maxima at 7301 cm−1 and 7062 cm−1, respectively, while the objects with negative scores are associated with a high value loadings peak at 7174 cm−1.
The PC1 loadings plot reflects the changes in the complex spectral band structure (maximum at 7108 cm−1, Figure 5c) as the desorption of water occurs during the freeze-drying process. As drying proceeds, the major bulk (or frozen) water band decreases in intensity. This is manifested firstly in the decrease of the 2nd derivative spectral wing observed at ∼7251 cm−1. This can be assigned to a combination of OH antisymmetric stretch and OH symmetric stretch modes of vibration of water.20 There is also an accompanying decrease in intensity of the 7108 cm−1 band, which possibly can be attributed to the free hydroxyl groups on the cellulose (free OH, cellulose, stretch first overtone20) but, more likely, it is a more complex band involving some features of the remaining water as well. The PC1 loadings plot illustrates and supports this rationalization [note (i) above].
The spectral overlays (Figure 5b) indicate the progressive emergence of a weak band at 7301 cm−1, which is attributed to the CH3 stretching plus bending combination modes of vibration,20 and is reflected by the positive loadings values on PC2 in the 7301 cm−1 region. A significantly large negative loadings value associated with the CH2 stretching combination at 7174 cm−1 region was also observed. Second derivative spectra over the total drying period exhibit an apparent progressive shift from 7108 to 7062 cm−1 (Figure 5b). The band is most likely to be a complex overlay of the remaining water and cellulose sub-bands. As the water desorption process continues, the underlying band structure is progressively revealed and the band maximum shifts as the band intensity contributions change. The PC2 loadings plot (Figure 5d) reflects this spectral change with positive loadings values around 7064 cm−1.
A possible rationalization of the behavior of these bands is that when most of the bulk water has been desorbed (after about 30 min), the small amount of remaining unfrozen water may be H-bonded in two ways: (i) either more strongly with the highly polar OH groups of the cellulose, or (ii) more weakly with, for example the CHn groups.15 For such an interpretation, the changes of the weak band at 7301 cm−1, as illustrated by the appearance of a small loadings band on PC2, could be attributed to the presence of weak H-bonding interactions referred to above in (ii).26 On the other hand, the progressive spectral shift from 7108 cm−1 to 7062 cm−110 indicates a weakening of the cellulose OH bond,27 and, hence, supports the notion that a relatively strong H-bond is present at the cellulose and the remaining water interface. A corresponding argument was proposed by Watanabe et al.,27 for the behavior of water in pure MCC, and it appears that the processes that are spectrally monitored by NIR in the more complex sugarcane system studied in this work are similar.
Fatty Compound Investigation
The NIR spectrum sampled from the stalk reflects a very complex matrix, which includes contributions from the epicuticular wax and the underlying plant tissue. The contribution of the epicuticular wax component of this composite spectrum (wax/rind interface of fresh stalk tissue) was investigated by scanning a fresh stalk tissue sample with the epicuticular wax intact, and then sampling a spectrum from the same sample which had been cleaned with hexane (refer Figure 7). These spectra indicated the band shifts and intensity changes related to the removal of the epicuticular wax. All bands in the 4327–4080 cm−1 region displayed a decrease in intensity, and are predominantly attributed to the CH (4080, 4185, and 4327 cm−1) and CH2 (4253 cm−1) vibrational modes associated with the long chain fatty compounds found in the epicuticular wax.15 However, the band at 4406 cm−1 (OH stretching/CO stretching combination) has increased and is attributed to lignin, which is predominantly in the epidermis of the stalk. This becomes more accessible for NIR sampling after the removal of the epicuticular wax and, therefore, produces a stronger lignin response. Other CHn vibrational stretching modes which support this conclusion and also decrease in intensity, appear at 5664 and 5784 cm−1. They are attributed to CH2 and CH3 stretching 1st overtones, respectively.15 Smaller losses in band intensity are noted in the 2nd overtone stretching region at 8218 and 8677 cm−1.15
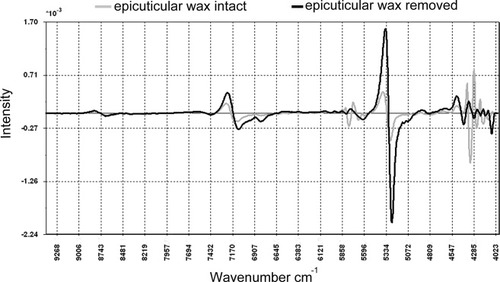
Overlay of 2nd derivative NIR spectra from Q124 with and without epicuticular wax.
Removal of the wax layer also provides better spectral access to other components of the sugarcane. Thus, spectral intensities increase in the 5151–5273 cm−1 region which is attributed predominantly to the OH bending, 2nd overtone of water. Additionally, the removal of the epicuticular wax resulted in a simplification of the complex band, which loses spectral contributions from the epicuticular wax. This manifests itself in an apparent spectral shift of 27 cm−1 to lower wavelength which is somewhat closer to the value of the OH bending vibrational mode of water at 5154 cm−1.15 Similarly, increased spectral band intensities are noted at the cellulose OH stretching 1st overtone at 6688 cm−1 and a significantly more intense OH stretching 1st overtone at 6823 cm−1. A spectral shift of 5 cm−1 to lower wavelength, plus a significant increase in band intensity, was observed at 7108 cm−1 (OH stretching 1st overtone). Interestingly, the CH stretching combination at 7301 cm−1 has also increased in intensity. This supports the view expressed in the above discussion of the nature of the bulk water in the sugarcane sample that this band is associated with frozen water—plant tissue rather than with the interactions of the epicuticular wax.
CONCLUSION
- 1
Typical spectra from the fresh epicuticular wax/stalk tissue interface of two commercial sugarcane cultivars could be discriminated on the basis of: (i) the CHn region, reflecting the contributions from the cellulose and the epicuticular waxes, and (ii) the spectral region associated with H-bonding in the water-cellulose matrix.
- 2
The desorption drying curve decreased asymptotically, leveling off after 60 min, and indicated that ∼12% (w/w) trapped unfrozen water remained. A progressive NIR spectral study of this drying process showed a broad complex band (5181 cm−1; OH bending mode of water) which decreased with increased drying time. The major bulk (or frozen) water band (7500–6000 cm−1) decreased in intensity, progressively revealing the underlying spectral structures. A suggested rationalization of the progressive spectral changes was that after the bulk water desorption, the remaining unfrozen water may be H-bonded in two ways: (a) more strongly with the highly polar OH groups of the cellulose and (b) more weakly with, for example, the CHn groups of cellulose.
- 3
For experiments where NIR spectra were obtained with and without the epicuticular wax layer present, the CHn band intensities from the fatty wax—free stalk were not observed and those attributed to the stalk tissue became pronounced. Other observations from the same sample suggest that frozen water is related to plant tissue rather than with the epicuticular wax, which point to the view that the wax and stalk behave largely independently.
- 4
This study demonstrated that NIRS techniques can produce similar results to studies, which in the past, required data collection by several separate costly techniques. Now one relatively common technique, i.e., NIRS, aided by chemometrics, allows the study of moisture, cellulose, and inter-relationships between wax and cellulose at plant interfaces. The article may be used as a comprehensive guide in such studies by scientists involved in a variety of plant and fiber research fields.




