Influence of chondroitin sulfate on collagen gel structure and mechanical properties at physiologically relevant levels
Abstract
The ability to alter collagen organization could lead to more physiologically relevant scaffolds for tissue engineering. This study examined collagen organization in the presence of polysaccharide and the resulting effects on viscoelastic properties. Fibrillogenesis in the presence of chondroitin sulfate (CS) resulted in changes in the collagen network organization with an increase in void space present. The increased void space caused by CS addition correlated with a decreased stiffness of the collagen gel. These changes occurred with physiologically relevant ratios of collagen to CS, at physiological pH and ionic strength, and without a decrease in the amount of collagen incorporated into fibrils. The addition of dextran, an uncharged polysaccharide, yielded no change in network void space or mechanical properties. Changes in fibril diameter caused by CS or dextran were not correlated with mechanical properties. The results of this study demonstrate that collagen organization can be modified by the addition of GAG, leading to altered matrix mechanical properties. © 2008 Wiley Periodicals, Inc. Biopolymers 89: 841–851, 2008.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
Type I collagen is the most abundant protein in the body and is the primary component of many tissues, such as tendon, skin, vessels, and bone. The organization of collagen in each of these tissues leads to the vast differences in their mechanical properties.
Collagen has the ability to self assemble in vitro to form collagen fibrils similar to those found in vivo.1 The ability to control the organization of collagen has potential application throughout tissue engineering since collagen can provide a natural substrate for many different types of tissue. Structural and mechanical properties of these reconstituted matrices can be controlled to an extent by the adjustment of pH and ionic strength during fibrillogenesis,2-7 but these methods are not suitable for tissue engineering applications in which cells will be present during fibrillogenesis. The organization of collagen in vivo is in part due to its interactions with other components in the tissue, so it is reasonable to attempt to alter collagen matrices with biocompatible materials.
Physical interactions between molecules may play a large role in structural organization of a tissue. The strength of physical interactions between glycosaminoglycans (GAGs) and peptide are strong enough to form a physical gel network.8 We therefore wanted to examine these interactions between GAGs and collagen near physiological ratios.
It is known that GAGs can interact with collagen electrostatically at physiologic conditions.2, 9-13 Many studies have begun to correlate the effects of GAGs on collagen fibril diameter and rate of fibrillogenesis. A summary of such work is presented in Table I. It is evident that much of the data are conflicting, as is true for most reports on any effects of GAGs on collagen fibrillogenesis. These conflicts in many cases are due to variation in collagen source, pH, ionic concentration, GAG molecular weight, and collagen to GAG ratios. This necessitates very controlled studies and conspicuous reporting of all such variables when reporting data so that comparison between studies can be made.
| Study | HA | Hep | CS | |||
|---|---|---|---|---|---|---|
| Size | Rate | Size | Rate | Size | Rate | |
| Lelu15 | + * | |||||
| Salchert45 | * | * | − | * | ||
| Tsai33 | * | + | ||||
| Guidry13 | + − | |||||
| Brightman28 | + | + | ||||
| Stamov7 | + | + − | ||||
| Bierbaum46 | + − | + | ||||
| Douglas23 | − | |||||
| Obrink4 | + | + | + | |||
| Mathews3 | − * | − | − * | |||
| Wood12 | − | + | ||||
| Xin14 | − | |||||
- + indicates an increase in fibril size or rate of fibrillogenesis. − indicates a decrease. * indicates no change observed. HA = hyaluronic acid. Hep = heparin.
The effect of GAGs on collagen fibrillogenesis affects both fibril diameter and mechanical properties of the resulting gel2, 14, 15; however, there is a lack of information correlating changes in the mechanical strength with changes in the network structural characteristics of a collagen gel. To obtain structural information, there is a trade-off between sample processing and image resolution. Confocal reflectance microscopy (CRM) and multiphoton microscopy can be used to image the gels in a naturally, hydrated state, but do not provide the resolution necessary to examine fine fibrillar structure. Electron microscopy (EM) can be used to examine individual fibrils but requires fixation and dehydration of the samples, with a subsequent loss in morphological features of the gel network. In contrast, cryo-scanning electron microscopy (cryoSEM) allows the resolution necessary to obtain fine, fibrillar detail, while retaining structural aspects of the network. Therefore, we used a combination of microscopy techniques to correlate changes in collagen gel structure with mechanical characteristics due to addition of GAG.
3D collagen scaffolds were examined in the presence of chondroitin-6-sulfate (CS), a GAG prevalent throughout the body, at ratios similar to that seen in the vasculature.16-21 Dextran was also examined to compare the effects of adding CS with those of adding an uncharged polysaccharide to collagen. The structures of the two molecules are shown in Figure 1. We hypothesized that CS interactions with collagen would affect the morphological characteristics of the collagen gels, which could be correlated to the resulting viscoelastic properties.
Structures of the repeating units of chondroitin sulfate (CS) and dextran.
MATERIALS AND METHODS
Materials
Acid solubilized collagen was either isolated ourselves according to previously described methods22 or purchased from BD Biosciences (Franklin Lakes, NJ). In both cases, the source of the collagen was rat tail, and similar trends were seen between both collagens. Chondroitin-6-sulfate (shark cartilage) and dextran were purchased from Sigma-Aldrich (St. Louis, MO) and had average molecular weights of 20 and 25 kDa, respectively.
Gel Preparation
Stock collagen was kept at a concentration of 5 mg/mL in 20 mM acetic acid. The acidic collagen solution was mixed on ice with 10× phosphate buffered saline (PBS) (1× PBS contained 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.76 mM KH2PO4), 1M NaOH, and 1× PBS on ice to give a final pH of 7.4 and salt concentration of 164 mM. Gelation occurred by heating the sample to 37°C. Polysaccharide, either CS or dextran, was solubilized in 1× PBS at a concentration of 10 mg/mL and added to the solution as part of the 1× PBS solution immediately prior to heating. Gels had a final concentration of 4 mg/mL type I collagen and 0.2 mg/mL CS or dextran, resulting in 5% polysaccharide to collagen content by weight, a weight ratio similar to that seen in the vasculature.
Collagen and Polysaccharide Incorporation into Fibrils
The amount of collagen incorporated into the gels was determined using a method previously described.23 Samples were allowed to gel for 24 hours at 37°C before spinning at 10,000g for 15 min. The amount of supernatant collected from each sample was recorded, and then diluted 4× with 20 mM acetic acid. This solution was then tested for the presence of collagen using the modified Lowry assay (Pierce, Rockford, IL) according to the manufacturer's protocol. Known concentrations of collagen diluted in 20 mM acetic acid were used to compose a standard curve for each assay, and collagen content in the supernatant was compared to this curve.
Additionally, the diluted supernatant was tested for the presence of CS and dextran. CS was detected using a dimethylmethylene blue (DMMB) assay.24 Briefly, DMMB (Sigma, St. Louis, MO) was mixed in water with 40 mM NaCl, 40 mM glycine, and HCl to yield a solution with pH 3.0. Supernatant (25 μL) was mixed with 200 μL of the DMMB solution, and absorbance was measured at 525 nm. Results were compared with a standard curve made with CS. To determine if salt concentration affected the results of the DMMB assay, CS standard curves were made in 1× PBS, 5× PBS, 10× PBS, and 20 mM acetic acid. No differences were found between the standard curves made with the different solutions. DMMB measures sulfated sugars, so dextran could not be measured using this assay.
Dextran was detected by using a phenol sulfuric acid assay.25 An 80% phenol solution was prepared in water and 4 μL was added to 150-μL supernatant. Concentrated sulfuric acid at a volume of 360 μL was then added directly to the solution. The solution was allowed to incubate for 10 min at room temperature before vortexing. The solution was then pipetted in triplicate into a 96-well plate and incubated for 10 min at 30°C. The absorbance was measured at 490 nm and compared with a standard curve composed of known dextran concentrations subjected to the same treatment.
Time to Gelation
The affect on rate of fibrillogenesis was measured using turbidity. The collagen solutions were prepared on ice as described above, and 100-μL aliquots were pipetted into a 96-well plate. The absorbance of the solution was measured at 313 nm every 30 sec at 37°C in a Spectromax M5 plate reader (Molecular Devices, Sunnyvale, CA).
Gel Formation for Microscopy
For all microscopy experiments, collagen gels were made in the same way. Briefly, collagen samples were allowed to gel for 15 min at 37°C before 1× PBS was added to the top of the gel to prevent dehydration of the gels. Gels were then incubated overnight at 37°C.
Confocal Reflection Microscopy
Confocal reflection microscopy (CRM) was utilized to image the collagen fibrils in a hydrated state, as described previously.26 Gels were made in an 8-well LabTek chamber slide and imaged with an Olympus FV1000 confocal microscope using a 60×, 1.4 NA water immersion lens. Samples were illuminated with 488-nm laser light and the reflected light was detected with a photomultiplier tube using a blue reflection filter. All images were taken at a depth of 50–150 μm into the gel to eliminate any edge effects.
Density measurements of individual 2D image slices of the collagen gel were processed in Matlab. A threshold of the gray scale image was chosen and kept constant for all images. The images were converted to black and white to distinguish fibrils from background, and the fraction of each image containing fibrils was calculated.
Scanning Electron Microscopy
Two methods of SEM were examined. First, conventional SEM, in which samples are fixed and dried, was performed. Samples were fixed for 2 hours with 3% glutaraldehyde in 0.1M cacodylate, followed by dehydration with increasing concentrations of EtOH and critical point drying. Samples were then pulled apart to reveal the inside surface of the gel. Samples were mounted with double sided tape and sputter coated with AuPd for 3 min prior to imaging.
Conventional SEM images obtained at 20,000× magnification were used to measure fibril diameter. The diameter of a fibril was determined by drawing a line across the fibril in NIH ImageJ software and converting pixel number to length. Three different images were analyzed per gel type and 20 measurements were made per image.
SEM was also performed in cryo-mode. Collagen solutions were gelled directly in the SEM holders to minimize any dislocations of the gels. The sample holders were transferred to the cryo holder, secured in place with the set screw, and plunged into liquid nitrogen slush. Vacuum was pulled and the sample was transferred to a Gatan Alto 2500 prechamber cooled to −170°C. The sample was fractured with a cooled scalpel to produce a free-break surface, and the samples were sublimated at −85°C for 20–30 min followed by sputter coating for 120 sec with platinum. The sample was then transferred to the microscope cryo-stage (−130°C) for imaging.
All samples were imaged with a FEI NOVA nanoSEM field emission SEM using the TLD (through the lens) or ET (Everhart-Thornley) detector operating at 5-kV accelerating voltage. Two different gels were imaged, and two to four images were taken at different positions in each gel at multiple magnifications to obtain representative images.
Images obtained at the highest magnification (80,000×) were used to measure the diameters of collagen fibrils. Because of the different types of structures seen in these images, three observers blinded to the type of sample in each image performed the measurements. ImageJ software was used to measure the fibril diameter by drawing a perpendicular line across a fibril. Fifteen measurements were made by each observer for each image, and 4 to 7 images of each gel type were analyzed by each observer. The trends and distribution were similar between sample groups for each observer, so all measurements were combined for construction of the histogram.
CryoSEM images obtained at a magnification of 20,000× were used to determine the amount of void space in each image. Thresholding was applied to eliminate background, images were converted to black and white, and the fraction of the image containing fibrils was determined and correlated to the amount of void space present. Additionally, the void size distribution was calculated. These measurements were performed in Matlab and NIH ImageJ software.
Rheology
Rheological analysis was performed on a TA instruments ARG2 rheometer using a 20-mm cone and plate geometry with a 1° angle. Collagen solutions were prepared on ice as described above. The solution was pipetted onto the rheometer stage, which was held at 25°C. The cone of the rheometer was lowered and the stage was then adjusted to 37°C so that gelling occurred directly on the rheometer stage. A solvent trap was employed to reduce effects of dehydration.
Time sweeps were performed using a controlled stress of 0.5 Pa and 1 Hz, values found to be within the linear viscoelastic range of the system. All samples were completely gelled within 5 min. Frequency sweeps were therefore performed after a conditioning step of 5 min at 37°C, using a controlled stress of 0.5 Pa. Complex, storage, and loss moduli were recorded.
Statistical Analysis
Time to gelation, void space, and rheological measurements were statistically analyzed using StatEase Design Expert 7.1. One-way ANOVA between the three different sample types was conducted. Statistical difference is denoted when P < 0.05. The distribution of fibril diameters was assessed using a Kolmogorov-Smirnoff test.
RESULTS
Collagen and GAG Content
Collagen incorporation into gels was measured indirectly by determining the amount of collagen remaining in the supernatant once the fibrils were pelleted by centrifugation. The addition of CS decreased the amount of collagen in the supernatant, indicating a higher amount of collagen incorporated into fibrils (Figure 2A). Dextran had no significant effect on the concentration of collagen in the supernatant.
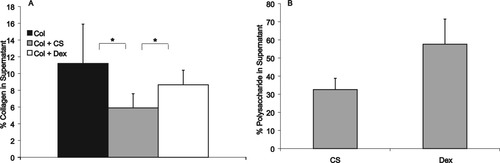
Percent mass of collagen (A), CS, and dextran (B) remaining in supernatant after fibrils were pelleted by centrifugation. * represents P < 0.05. Bars represent mean ± standard deviation. n = 6.
The amount of CS or dextran in the supernatant was also determined using a DMMB or phenol sulfuric acid assay. Approximately 30% of the mass of CS originally added to the solution was found in the supernatant, indicating 70% of the CS was incorporated in the fibril pellet (Figure 2B). In contrast, approximately 60% of the dextran was found in the supernatant. Neither assay detected measureable quantities of polysaccharide in the isolated collagen stock solution (data not shown).
Turbidity
Kinetic data for the three samples are shown in Figure 3. The addition of CS and dextran increased the rate of fibrillogenesis. The time to half max absorbance was calculated for each sample type by determining the midpoint between the minimum and maximum values of absorbance. A line was generated between the two points in which the turbidity value fell to calculate a corresponding time. The time to half max is reported in the legend of Figure 3. This value was significantly different for all sample types, with both polysaccharides decreasing the time to half max.
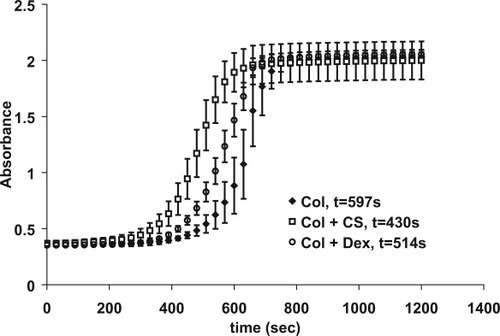
Kinetics of fibrillogenesis. Time to half max absorbance is stated in the legend. All times are significantly different (P < 0.05). Points represent mean ± standard deviation. n = 3.
Confocal Reflection Microscopy
Representative two-dimensional (2D) images obtained from CRM are shown in Figure 4. Visual qualitative analysis of these images revealed that the addition of CS to collagen appeared to result in fewer large fibrils and a larger number of small fibrils. The images of the samples with dextran appeared to show the presence of many very fine fibrils and no large fibrils. The fraction of image containing fibrils is shown in Figure 4D. Despite the qualitative differences in structure between the three different sample types, no significant differences were found between the areas of each image containing fibrils. This result was consistent over a large range of thresholding values.
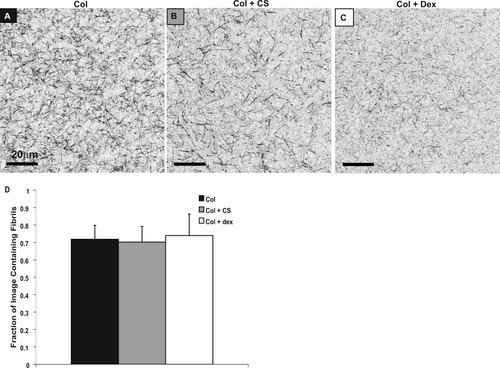
CRM images of collagen (A), collagen + CS (B), and collagen + dex (C). The average fraction of fibrils covering each image was calculated with MatLab (n = 8) with scale bars representing standard deviation (D). No significant differences were noted. Images (A–C) have been gray scale inverted.
Scanning Electron Microscopy
Both conventional SEM and cryoSEM were performed to better assess the microarchitecture of the samples. Representative images from the two methods are shown in Figure 5. No structural differences were observed between the three sample types using conventional SEM (Figure 5A–5C). In fact, because of processing, significant shrinkage of the gels occurred and all fine morphological differences were lost. Fibril diameter was measured and the distribution of fibrils is shown in Figure 6A. A similar fibril diameter distribution was seen for each sample type. No significant differences in the fibril diameter distribution was found using the Kolmogorov-Smirnoff test.
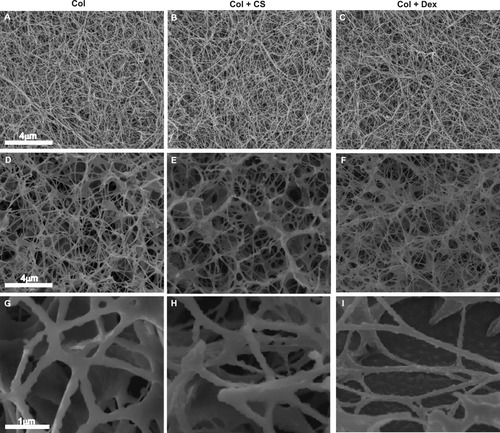
Conventional SEM (A–C) and cryoSEM (D–I) of collagen samples. Conventional SEM does not reveal the differences seen when cryoSEM was performed. The images of col + CS (B, E, H) show larger void spaces and less uniform fiber distribution as compared with collagen only (A, D, G) and collagen + dex (C, F, I), while the collagen only and collagen + dex sample have similar fibril and void space distributions. Bar = 4 μm for images A–F. High magnification cryoSEM images used to compute diameters in Figure 6 (G–I). Bar = 1 μm for images G–I.
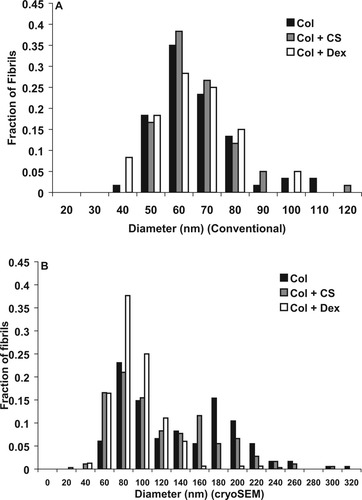
Fibril diameter measured with conventional SEM, n = 60 (A) and with cryoSEM, n > 180 (B). Y-axis represents the fraction of fibrils with each diameter.
CryoSEM (Figure 5D–5F) revealed structural differences between sample types. The samples containing CS contained larger void spaces than the samples with only collagen or containing dextran. Void space, shown in Figure 7A, was quantified by thresholding the images and subtracting the area containing fibrils. Images of samples containing CS contained significantly more void space compared to collagen only or dextran containing samples. This result was consistent over a large range of thresholding values. The size distribution of the void spaces is shown in Figure 7B. A small percentage of the voids were over 1 μm in diameter when CS was present.
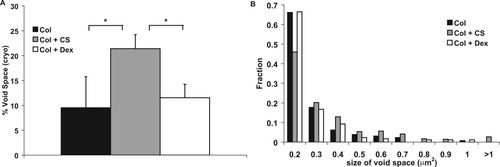
Amount of void space (A) and void size distribution (B) calculated from cryoSEM images. * represents P < 0.05. Bars represent mean ± standard deviation. n = 4.
Higher magnification images obtained with cryoSEM were used to measure the fibril diameters of the samples (Figure 5G–5I). The distribution of fibril diameter is shown in Figure 6B. CryoSEM measurements resulted in a second peak of fibril diameters larger than measured with conventional SEM. This bimodal distribution was present for samples containing only collagen and collagen + CS. The samples containing dextran did not have a bimodal distribution of fibril size. Kolomorogov-Smirnoff tests revealed significant differences between all three sample distributions (P < 0.05).
Rheology
The storage (G′), loss (G″), and complex (G*) moduli for the samples are shown over a frequency range of 0.1 to 10 Hz in Figure 8. The complex modulus is a measurement combining both the storage and loss modulus and is correlated with the stiffness of the sample. These measurements were taken after a five-minute equilibration period, a time that allowed for complete gelation. A representative time sweep indicating time to complete gelation is shown in Figure 8D. This time for complete gelation differs from that shown in the turbidity data because of the different heat profile present in the samples within each experiment.
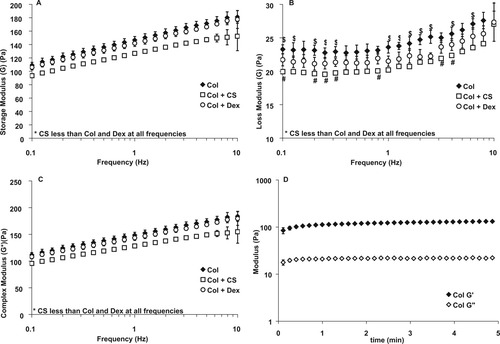
The addition of CS to the collagen decreased the storage modulus (A) and complex modulus (C) significantly at all frequencies, while dextran did not cause a change. The loss modulus of the CS samples was also significantly lower than the samples containing only collagen, but often similar to the samples containing dextran (B). Dollar signs ($) above the curves in (B) denote significant differences between dextran and collagen, while pound signs (#) below the curves denote significant differences between dextran and CS. (n = 3, *, #, $ denotes P < 0.05, points represent mean ± standard deviation). (D) Representative time sweep showing time to complete gelation.
The storage modulus is greater than the loss modulus at all frequencies for all samples, indicating gel-like behavior. The addition of CS significantly decreased both the storage and complex moduli, indicating a more compliant gel. Dextran did not alter the storage or complex moduli (Figure 8 A and 8C). The loss modulus, which measures the viscous gel component, was also significantly reduced by the addition of CS at all frequencies. Dextran significantly changed the loss modulus at certain frequencies, and these differences are denoted in Figure 8B.
DISCUSSION
The importance of polysaccharide–protein interactions are beginning to gain wide attention, but so far the data on the subject has been difficult to compare due to variations in a number of variables (see Table I). This study aimed to examine the effect of CS on the fibril formation and network characteristics of type I collagen at physiological pH and ionic concentration. The weight ratio of GAG found in the vascular is approximately 2–5% GAG (wt/wt).16-21 Based on stock collagen concentrations, the highest collagen content possible was 4 mg/mL. A 5% CS concentration led to a CS concentration of 0.2 mg/mL to 4 mg/mL collagen.
Dextran was used as a control polysaccharide due to its similar structure to GAGs but with the absence of the negative charge conferred upon CS through decoration of the backbone with sulfate and carboxylate groups. In this way, the presence of a relatively stiff polymer chain in each sample was accounted for without introducing the unique charge density and distribution present in GAGs such as CS. A major goal of this study was to correlate any structural changes in fibrillar and network organization with mechanical changes caused by the addition of polysaccharide during collagen fibrillogenesis.
The addition of both polysaccharides resulted in an increase in fibrillogenesis rate, as measured turbidimetrically. The effect of CS on rate of fibrillogenesis depends on if the CS is present before the nucleation phase of growth.3, 4, 11, 12 In the present study, all components were mixed together quickly, and the CS or dextran was present during all phases of fibril growth, with a resulting increase in rate of nucleation. Other studies have correlated an increase in fibrillogenesis rate or a final absorbance value with a decrease in fibril diameter.12, 27 In this study, samples containing dextran had no fibrils with larger diameters. Samples containing CS had a fewer percentage of fibrils with larger diameters, compared with samples containing only collagen. While dextran addition resulted in a greater amount of smaller fibrils, CS addition resulted in the fastest rate of fibrillogenesis. Also, the final turbidity values were similar for all three sample types. These results agree with others in which there is no simple correlation between turbidity measurements and fibril diameter.12, 28, 29
Three imaging techniques (CRM, conventional scanning electron microscopy (SEM), and cryo-SEM) were used to assess the microarchitectural properties of the collagen gels formed in the presence of polysaccharide. CRM has the advantage of requiring no sample processing, allowing fibrils to be viewed in a naturally hydrated state. CRM, however, has the disadvantage of having a resolution lower than that necessary to detect fine details in structure since the theoretical resolution of confocal microscopy is 200 nm, and collagen fibrils can have diameters as small as 30 nm.30 Diameter measurements can be overestimated with CRM because of diffraction artifacts from light reflecting from the fibril edges.31 In this study, CRM was useful in examining qualitative bulk differences in the fibrils. While individual fibrils could not be resolved, this method eluded to differences in fibril diameter that were confirmed with cryoSEM.
Higher resolution EM was utilized to measure the finer structures that we could not resolve with CRM. EM is used widely when examining collagen fibrils and is often used to measure fibril diameter.2, 5, 7, 15, 29, 31-35 In contrast with observations from CRM and measurements from cryoSEM, the distribution of fibril diameter in all three sample types was similar when measured with conventional SEM. Conventional SEM requires fixation and dehydration of samples, which results in substantial shrinkage of the sample. Such artifact is evident as all of the fibrils are seen collapsed upon one another in Figure 5. It is likely then that the fibril diameter results from conventional SEM are due to artifact, as collagen fibrils can be highly porous and can be dehydrated during sample processing.31
CryoSEM revealed much greater structural detail than the other two imaging methods. CryoSEM requires no chemical fixation and no shrinkage of samples by dehydration. Instead, samples are quick frozen at vitreous temperatures to prevent the formation of ice crystals. The free water is then sublimated from the samples, leaving a preserved 3D architecture of fibrils. Other studies have compared the methods of cryoSEM with conventional SEM to show the differences in the resulting structures.34, 36 It has been suggested that cryoSEM yields results similar to those found with CRM observations of fully hydrated gels.31 Our results show that a substantial amount of information is lost in samples prepared for conventional SEM while cryoSEM preserves the microarchitecture and reveals the differences in our samples that could not be fully appreciated with CRM.
Quantitative fibril diameter measurements from cryoSEM validated our qualitative observations from CRM: addition of CS resulted in fewer large collagen fibrils, and addition of dextran resulted in no large fibrils. The decrease in diameter and increase in rate of fibrillogenesis points to the polysaccharides increasing the nucleation sites of the collagen molecules.12, 27 Additionally, the electrostatic interactions between CS and collagen could potentially change the shape of nucleation sites, promoting end-to-end aggregation of collagen molecules.12
CryoSEM additionally provided a more accurate representation of the 3D network of the collagen gel. The structural uniformity of the network was altered with the addition of CS. The amount of void space increased significantly in the presence of CS, while dextran did not have an effect on this parameter (Figure 5D–5F). The addition of CS resulted in a slightly increased amount of collagen incorporation into the fibrils, so this void space cannot be attributed to a smaller amount of polymer present within the network. Additionally, CS addition did not alter the excluded volume of liquid from the gel (data not shown), so the increased void space is not due to CS inflating the network of collagen fibrils. The increased void space present with CS therefore means that the volume fraction of collagen fibrils is decreased in samples containing CS.
The increased void space present in samples containing CS correlated with a significant decrease in the viscoelastic properties of the collagen gel. The decrease in mechanical properties cannot be justified with a decrease in polymer present, since the amount of collagen incorporated into fibrils was not decreased by addition of CS. Other studies have examined the mechanical effects of different GAGs on collagen matrices with varying results, depending on GAG identity, molecular weight, ratio of GAG to collagen, and scaffold formation.2, 14, 15 In many cases, the addition of polymer results in an increase in mechanical properties. For example, the addition of HA2, 14 or DS2 increased the storage modulus of collagen gels; however, no correlations between network structure and mechanical properties with these molecules have been made. Additionally, the differences in GAG identity, GAG molecular weight, ratio of GAG to collagen, the phase of fibril growth the GAG was added, ionic strength, and pH are all factors that are not always clear and could have significant effect on the resulting collagen structure and the strength of interactions between GAG and collagen.
GAGs have a high hydration volume, which is important in the viscoelasticity and permeability of tissues.16, 21, 37-39 In native vessels, it has been reported that digestion of CS chains with chondroitinase ABC results in an altered stress-strain relationship in the vessel wall, which can be correlated to an increase in stiffness (decrease in compliance).40 The addition of CS to our collagen gels led to a decrease in the complex modulus, which correlates with an increase in compliance. Thus, the measurements reported herein agree with in vivo data supporting that CS adds to compliance of tissues.
In our in vitro system, the decrease in gel stiffness with incorporated CS correlated to the greater void space in the network structure. GAGs such as CS play a role in determining the distance between collagen fibrils, and therefore in determining the shape of the ECM.41 While fibril diameter differed between sample types, this parameter does not correlate directly with mechanical strength.42-44 Rather, there is a complex relationship between fibril diameter, fibril length, and fibril volume fraction that contribute to the viscoelastic properties of the material, in addition to physical interactions present between collagen and polysaccharides. Our results demonstrate that CS acts in a way to alter the organization of the collagen matrix so that the fibril volume fraction is decreased and greater void space is present. These changes result in an alteration of matrix stiffness.
It is possible that the influence of CS on the collagen gel could be effective at lower concentrations, so the mass of CS was decreased by an order of magnitude. At this concentration, CS did not affect the stiffness of the type I collagen gel or the kinetics of gelation; however, the structural aspects of the collagen fibrils did differ, but to a smaller extent. It may be possible, therefore, to separate the variables of stiffness and structure by altering the amount of CS incorporation. A comprehensive investigation on the effects of GAG concentration was not feasible for this study, but may be of interest in the future. It is important to note that in any case in which CS is added to affect stiffness or structure of the collagen matrix, cellular activity within these constructs would also be affected by the additional CS biochemical cues.
This study related mechanical characteristics to structural changes in collagen fibril organization caused by the presence of polysaccharide during fibrillogenesis. Polysaccharide can alter the rate of collagen fibrillogenesis, fibril diameter, network organization, and the resulting stiffness of the matrix. These changes occur without an alteration in pH or ionic strength and at collagen to GAG ratios similar to those found in vivo. Therefore, polysaccharides can be used to alter matrix properties in ways that may affect the behavior of cells grown within the matrix. Specifically, CS can be used to alter the stiffness and void space of the matrix. Future studies will examine these biochemical, structural, and mechanical properties of the matrix on cell behavior.
Acknowledgements
The authors thank Debra Sherman for performing all SEM.




