Thermodynamics and mechanism of cutinase stabilization by trehalose
Abstract
Trehalose has been widely used to stabilize cellular structures such as membranes and proteins. The effect of trehalose on the stability of the enzyme cutinase was studied. Thermal unfolding of cutinase reveals that trehalose delays thermal unfolding, thus increasing the temperature at the midpoint of unfolding by 7.2°. Despite this stabilizing effect, trehalose also favors pathways that lead to irreversible denaturation. Stopped-flow kinetics of cutinase folding and unfolding was measured and temperature was introduced as experimental variable to assess the mechanism and thermodynamics of protein stabilization by trehalose. The main stabilizing effect of trehalose was to delay the rate constant of the unfolding of an intermediate. A full thermodynamic analysis of this step has revealed that trehalose induces the phenomenon of entropy–enthalpy compensation, but the enthalpic contribution increases more significantly leading to a net stabilizing effect that slows down unfolding of the intermediate. Regarding the molecular mechanism of stabilization, trehalose increases the compactness of the unfolded state. The conformational space accessible to the unfolded state decreases in the presence of trehalose when the unfolded state acquires residual native interactions that channel the folding of the protein. This residual structure results into less hydrophobic groups being newly exposed upon unfolding, as less water molecules are immobilized upon unfolding. © 2008 Wiley Periodicals, Inc. Biopolymers 89: 538–547, 2008.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
An improved knowledge of protein stabilization would significantly enhance the feasibility of some biological processes. Addition of compatible solutes, the so called osmolytes (low-molecular weight compounds which accumulate intracellularly as a response to stress conditions) has been a widely used strategy for protein stabilization. These compounds are effective in stabilizing membrane structure and pharmaceutical preparations, decreasing biological damage at low temperatures, and stabilizing nucleic acids and protein structure during freezing.1, 2 Stabilization of cells and biological macromolecules has been reviewed elsewhere.3, 4 Mechanistic information about the effects of these solutes on folding/unfolding pathways might give additional insight on protein folding mechanisms. The sugar trehalose (α-D-glucopyranosyl α-D-glucopyranoside) is one of the most prevalent compatible solutes in nature. It is a non-reducing disaccharide produced in cyanobacteria and bacteria under moderate and severe stress, as well as in yeast under severe stress.5 Trehalose is among the most chemically unreactive sugars and its strong stability is a result of the very low energy of the glycoside oxygen bond joining the two hexose rings.
Xie and Timasheff have proposed that protein stabilization by trehalose at room temperature is determined by an increase in preferential exclusion of trehalose upon unfolding.6, 7 This mechanism shows a predominant effect of the solute on the unfolded state. The immediate domain of the protein is poorer in trehalose than the bulk solvent for both folded and unfolded state, but the effect is stronger for the unfolded state, and therefore this state is more destabilized than the folded state.8 Recently, Bolen and coworkers have shown that this preferential exclusion is achieved due to unfavorable peptide backbone-osmolyte interactions (the osmophobic effect) as the interactions between amino acid side chains and osmolytes favor protein unfolding.9-12 As a consequence of these unfavorable peptide backbone-osmolyte interactions, more compact protein species are favored resulting in a shift towards the native state and towards more compact species within native-state ensembles.13
Cutinase is an enzyme displaying lipolytic activity (EC 3.1.1.3.) with a molecular weight around 22.5 kDa and an isoelectric point of 7.6.14 Trehalose stabilizes the enzyme cutinase against thermal unfolding.15 It decreases the equilibrium constant for the reversible unfolding of cutinase by shifting the equilibrium towards the folded state with a concomitant increase of the Tm value. Trehalose also stabilizes cutinase against chemical induced unfolding.16 It increases the free energy gap between the folded and unfolded states, increasing thus the midpoint of unfolding induced by guanidine hydrochloride. To characterize in more detail and to gather additional insight on the thermodynamics and mechanism of cutinase stabilization by trehalose, the effect of temperature was introduced and conjugated with the effect of trehalose. Both thermal denaturation and chemical induced unfolding of cutinase were studied in the presence of trehalose as changes in temperature led to different extents of irreversibility but chemical induced unfolding is reversible. Kinetics of chemically induced unfolding/refolding was determined using the stopped-flow technique. In this work, the stabilizing effect of trehalose was clearly assigned to the reversible unfolding of cutinase; and the mechanism of stabilization by trehalose relies on an increased compaction of the unfolded state that channels the folding of the protein and results in less hydrophobic groups being exposed upon unfolding.
MATERIALS AND METHODS
Cutinase from Fusarium solani was cloned and expressed in Escherichia coli WK-6 strain, a kind gift from Corvas International N.V. (Gent, Belgium). The recombinant enzyme has 197 residues [molecular mass 22,500 Da, pI 7.6]14 and was produced and purified to a lyophilized powder of >95% purity (w/w) using an osmotic shock to disrupt cells followed by acid precipitation and two anionic exchange chromatographic steps.17 Cutinase solutions were prepared in acetate buffer 50 mM, pH 4.5, according to a molar extinction coefficient (ε280) of 10,810 M−1 cm−1.
Guanidine hydrochloride (GdnHCl) was obtained from Gibco/BRL Life Technologies (ultrapure) and Trehalose from Sigma (>98.5%).
Thermal Unfolding Studies
Thermal unfolding of cutinase at pH 4.5 was monitored by fluorescence emission using a spectrofluorimeter (Model MPF-3, Perkin-Elmer) with 90° geometry. Protein samples were heated and cooled at approximately constant rates of 1.5°C/min and 4.0°C/min, respectively, using an auxiliary water bath. The temperature of the protein solution was measured to an accuracy of ±0.1°C using a thermometer (Model 51K/J, Fluka) immersed in the cell. The single tryptophan residue of cutinase (Trp69) was selectively excited at 296 nm and emission was measured at 335 nm. Upon cutinase unfolding, the tryptophan residue moves away from the quenching effect of the disulfide bond between Cys31 and Cys109 and the quantum yield increases.18 Slit widths of 2.5 and 5 nm were used for excitation and emission light, respectively. A narrow excitation slit was necessary to prevent cleavage of the disulfide bridge noted earlier.19 Small degree of disulfide bond cleavage cannot be totally discarded but their possible occurrence was deducted when the linear dependence of fluorescence intensity of folded cutinase on temperature was taken into account (see data analysis).
Stopped-Flow Kinetics
Kinetic experiments were carried out on an Applied Photophysics SX-18MV stopped-flow reaction analyzer (Applied Photophysics, Leatherhead, Surrey, U.K.) over the temperature range from 20 to 45°C at 5°C intervals. Cutinase and GdnHCl solutions were mixed in a 1:10 ratio, respectively, to give a final protein concentration of 4 μM and the desired denaturant concentration. Whenever trehalose was used, it was present in both solutions. Unfolding was induced by mixing cutinase in buffer with the appropriate GdnHCl solution, and refolding was induced by mixing unfolded cutinase in 2M (for the kinetics in the absence and presence of 0.3M trehalose) or 2.2M GdnHCl (for the kinetics in the presence of 0.6M and 1.0M trehalose) with the appropriate GdnHCl solution. Excitation was always at 280 nm, and emission was detected above 315 nm using a glass filter.
Data Analysis
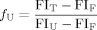 (1)
(1)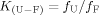 (2)
(2)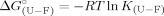 (3)
(3)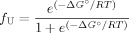 (4)
(4)Kinetics of unfolding/refolding using GdnHCl as denaturant were fitted according to Scheme 116:
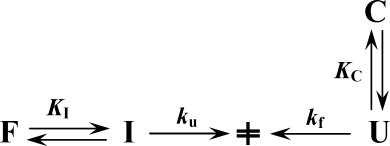
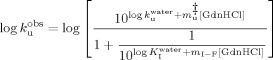 (5)
(5)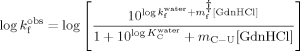 (6)
(6)Equations (5) and (6) were used to fit kinetic data of unfolding and refolding, respectively, using the Levenberg-Marquardt algorithm included in the nonlinear least-squares fitting capability of the Origin® software, version 6.1.
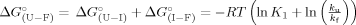 (7)
(7)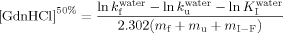 (8)
(8)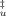 value (fractional accessible area that gives the average degree of exposure of transition state relative to that of the unfolded state) was calculated according to Eq. (9):
value (fractional accessible area that gives the average degree of exposure of transition state relative to that of the unfolded state) was calculated according to Eq. (9):
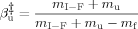 (9)
(9)Compactness of the transition state ensemble equals that of the unfolded state for β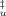 = 1 and that of the folded state for β
= 1 and that of the folded state for β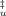 = 0.
= 0.
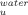 versus temperature for each trehalose concentration and fitting the curves with Eq. (10)20:
versus temperature for each trehalose concentration and fitting the curves with Eq. (10)20:
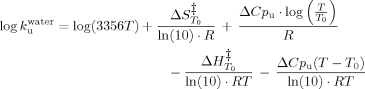 (10)
(10) ), activation entropy (ΔS
), activation entropy (ΔS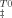 ), and the activation heat capacity (ΔCpu). T0 was 298.15 K and the term for vibrational frequency, which is conventionally used in the analysis of simple chemical reactions, has been replaced with the factor 3356T.21 Changes in partial molar Gibbs free energy of unfolding from I to U state as a function of temperature were obtained using Eq. (11).22
), and the activation heat capacity (ΔCpu). T0 was 298.15 K and the term for vibrational frequency, which is conventionally used in the analysis of simple chemical reactions, has been replaced with the factor 3356T.21 Changes in partial molar Gibbs free energy of unfolding from I to U state as a function of temperature were obtained using Eq. (11).22
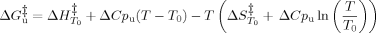 (11)
(11)RESULTS AND DISCUSSION
Thermal Unfolding of Cutinase
Unfolding of cutinase induced by increasing temperature was studied by fluorescence emission (see Figure 1). Parameters which characterize the transition from the folded to unfolded state are shown in Table I. At pH 4.5, cutinase unfolds with a Tm of 49.3°C and transitions were well fitted by a two-state mechanism. Stability of cutinase increases with increasing trehalose concentration, as revealed by the increase in temperature at the midpoint. The Tm value increases 7.2°C in the presence of 1M of trehalose. The presence of high concentrations of this solute leads also to high irreversibility despite some scatter in the data. In the presence of 1M of trehalose only 3.4% of cutinase molecules refold upon cooling. A 10-fold increase in protein concentration did not affect the Tm value, unlike the situation at pH 8.0, which is much closer to its isoelectric point of 7.6.23 Cutinase tends to unfold at 49°C and 52°C in the absence and in presence of 0.5M of trehalose, respectively, within the cutinase concentration range of 4–40 μM (Table II). However, irreversibility increased from 37 to 71% as cutinase concentration was increased from 4 to 40 μM as expected if aggregation of the unfolded state occurs at pH 4.5.24 Moreover, trehalose favors this aggregation mechanism since an increase in irreversibility was observed in the presence of trehalose. Therefore, in opposition to the stabilizing effect on the reversible unfolding, trehalose clearly favors irreversible denaturation of the unfolded state, including aggregation. Several other studies report increased protein aggregation in the presence of osmolytes. Aggregation of unfolded lysozyme seems to be favored by glycine-based osmolytes.25 The refolding yield of aggregation-prone citrate synthase was compromised at high polyol concentration.26 Prevention of protein aggregation in vivo should rely mostly on molecular chaperones not on osmolytes.27

Thermal unfolding of cutinase (4 μM) at pH 4.5 in the absence (•) and in the presence of 0.5M (▴) and 1M (▪) of trehalose. Refolding was achieved upon cooling (empty symbols) and the fraction of irreversibility was estimated according to Eq. (1). Lines were calculated according to Eq. (4) assuming a two-state mechanism.
| Trehalose (M) | Tm (°C) | Irreversibility (% fU After Cooling) |
|---|---|---|
| 0 | 49.3 ± 0.8 | 36.9 ± 18 |
| 0.25 | 50.9 ± 0.1 | 17.8 ± 1.1 |
| 0.50 | 52.0 ± 0.4 | 57.6 ± 12 |
| 0.75 | 54.5 ± 0.3 | 34.9 ± 2.1 |
| 1 | 56.5 ± 1.7 | 96.6 ± 5.8 |
- Irreversiblility at 25°C was calculated according to Eq. (1).
| Protein(μM) | Tm (°C) | Irreversibility (% fU) | ||
|---|---|---|---|---|
| Buffer | 0.5M Trehalose | Buffer | 0.5M Trehalose | |
| 4 | 49.3 ± 0.8 | 52.5 ± 0.8 | 36.9 ± 18 | 57.6 ± 12 |
| 10 | 49.4 ± 0.4 | 53.4 ± 0.5 | 8.50 ± 3.7 | 34.2 ± 16 |
| 20 | 48.9 ± 0.4 | 52.6 ± 0.4 | 27.9 ± 3.7 | 63.3 ± 6.9 |
| 30 | 49.8 ± 0.3 | 52.5 ± 0.7 | 60.2 ± 16 | 86.6 ± 7.8 |
| 40 | 49.9 ± 0.5 | 52.6 ± 0.6 | 71.1 ± 13 | 95.7 ± 6.0 |
Chemically Induced Unfolding of Cutinase
The effect of trehalose on the stabilization of cutinase was clearly assigned above to the reversible unfolding step. Unfolding induced by chemical denaturants such as guanidine hydrochloride (GdnHCl) is typically totally reversible and therefore was used to address the effect of trehalose. Kinetics of unfolding/refolding of cutinase induced by GdnHCl was measured here at different temperatures. Unfolding of cutinase reveals only one phase but refolding shows clearly two phases.16 Chevron plots omitting the slow phase of refolding are shown in Figure 2.
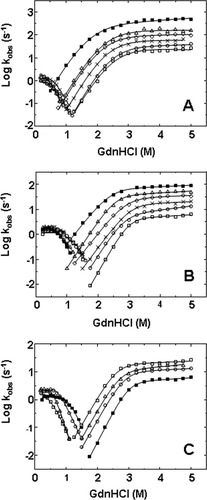
Chevron plots of the fast phase of refolding and unfolding of cutinase at pH 4.5 obtained at different temperatures in the absence (A) and in the presence (B) of 1M of trehalose. Kinetic data was obtained 20°C (□), 25°C (○), 30°C (×), 35°C (⋄), 40°C (▵), and 45°C (▪). Refolding at 45°C in the absence of trehalose was not detected as at this temperature cutinase unfolds in the presence of very low GdnHCl concentrations (see Figure 1). (C) Chevron plot of cutinase at 20°C in 0M (□), 0.3M (▵), 0.6M (○), and 1.0M (▪) of trehalose. Unfolding data conforms to an unfolding intermediate accumulating during the dead time of the experiment [solid lines are the fits using Eq. (5)].16 Refolding data conforms to a compact intermediate accumulating off-pathway during the dead time of the experiment [solid lines are the fits using Eq. (6)].
Neither changes in temperature nor the presence of trehalose change significantly the pattern of the chevron plot. The observable rate constants for refolding and unfolding do not meet at the transition region confirming the presence of a stable intermediate within the transition region.16 This effect is even more clearly observed at 45°C (Figures 2A and 2B). Moreover, the plot deviates from the simple V-shaped curve on both refolding and unfolding limbs. This rollover pattern has been ascribed to folding and unfolding intermediates that accumulate transiently at low and high denaturant concentrations.28, 29 The plateau observed for the unfolding of cutinase points to the accumulation of an intermediate in the dead time of the experiment (Scheme 1). An on-pathway intermediate could describe the rollover pattern and increased trehalose concentrations or temperature variations do not change the pattern of the unfolding limb, showing that the intermediate is still accumulating. On the contrary, the shape of the refolding limb changes in the presence of trehalose. The accumulation of an off-pathway intermediate during refolding (Scheme 1) causes the rollover pattern observed in the refolding limb. The presence of this compact off-pathway intermediate becomes more evident in the presence of high trehalose concentrations. Curves at different trehalose concentrations converge at low GdnHCl concentrations and a positive slope can even be observed at low GdnHCl concentrations in the presence of 0.6M and 1M of trehalose (Figure 2C). The positive slope indicates that the transition state exposes more surface area than the kinetic ground state. An over compact intermediate that has to unfold (leading to a positive slope) to give the transition state likely appearsoff-folding pathway.16, 30 More recently, a small degree of cutinase aggregation was shown to occur at low guanidine concentrations interfering with the slope of the chevron plot.31
On the basis of the identification of intermediate states in the unfolding and refolding pathway of cutinase,16 the chevron plots shown in Figure 2 were fitted as described in material and methods to analyze the effect of temperature and trehalose in the respective rate constants. The rate constants of refolding are much less sensitive to temperature and to the presence of trehalose than the unfolding data, as they tend to merge specially at low GdnHCl concentrations. Analysis of the effect of temperature and trehalose on the kinetics of cutinase was then focused on unfolding data.
No conclusions can be drawn regarding the effect of trehalose on the formation of the intermediate that exists in the unfolding pathway as K values are scattered at different trehalose concentrations. Temperature, however, clearly favors the unfolding intermediate as K
values are scattered at different trehalose concentrations. Temperature, however, clearly favors the unfolding intermediate as K increases with temperature leading to a decrease in the free energy change between the folded and the intermediate state (Figure 3A). However, the intermediate is also destabilized by temperature relatively to the transition state ensemble (TSE) of unfolding as the rate constant of unfolding (k
increases with temperature leading to a decrease in the free energy change between the folded and the intermediate state (Figure 3A). However, the intermediate is also destabilized by temperature relatively to the transition state ensemble (TSE) of unfolding as the rate constant of unfolding (k ) increases with temperature (Figure 3B). In contrast, trehalose significantly decreases the rate constant of unfolding. Stabilization of cutinase by trehalose was mainly due to a decrease in the unfolding rate constant which characterizes the transition from the I intermediate to the TSE of unfolding. The assignment of the main effect of trehalose to this step provides a good opportunity to analyze the effect of osmolytes on a specific transition in a protein unfolding pathway. The logarithm of k
) increases with temperature (Figure 3B). In contrast, trehalose significantly decreases the rate constant of unfolding. Stabilization of cutinase by trehalose was mainly due to a decrease in the unfolding rate constant which characterizes the transition from the I intermediate to the TSE of unfolding. The assignment of the main effect of trehalose to this step provides a good opportunity to analyze the effect of osmolytes on a specific transition in a protein unfolding pathway. The logarithm of k decreases linearly with trehalose concentration independently of temperature, meaning that the transition state is destabilized (relative to I) (Figure 3B). This destabilization leads to a linear increase in the midpoint of the global transition from F to U which includes the transition from F to I (KI) and kf besides ku (Figure 3C). The midpoint increases linearly by a factor of 0.72 ± 0.2 M per molar of trehalose.
decreases linearly with trehalose concentration independently of temperature, meaning that the transition state is destabilized (relative to I) (Figure 3B). This destabilization leads to a linear increase in the midpoint of the global transition from F to U which includes the transition from F to I (KI) and kf besides ku (Figure 3C). The midpoint increases linearly by a factor of 0.72 ± 0.2 M per molar of trehalose.
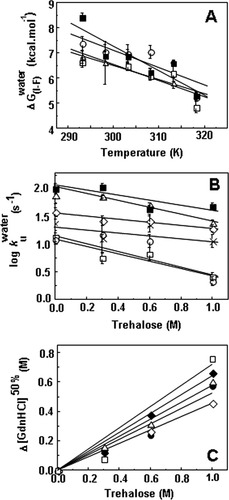
(A) Effect of temperature in the free energy change between the folded (F) and the intermediate state (I) for 0M (□), 0.3M (▵), 0.6M (○), and 1.0M (▪) of trehalose. Values were calculated using ΔG0 = −RT lnK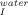 , with the K
, with the K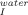 values obtained from Eq. (5). Slopes were −71.9 ± 15 kcal mol−1 K−1 with correlation coefficients within the range of 0.84–0.99. (B) Effect of trehalose on the rate constant of unfolding from the on-pathway intermediate to the transition state at 20°C (□), 25°C (○), 30°C (×), 35°C (⋄), 40°C (▵), and 45°C (▪). Slopes were −0.52 ± 0.21 s−1M−1 with correlation coefficients within the range of 0.64–0.98. (C) Effect of trehalose in the [GdnHCl]50% value for the global pathway between F and U at 20°C (□), 25°C (•), 30°C (♦), 35°C (⋄), and 40°C (▵). Values were calculated assuming a three-state process as described in materials and methods [Eq. (8)]. The mid-point increases by 0.72 ± 0.2 M per molar of trehalose (linear fits with correlation coefficients in the range 0.78–0.99.
values obtained from Eq. (5). Slopes were −71.9 ± 15 kcal mol−1 K−1 with correlation coefficients within the range of 0.84–0.99. (B) Effect of trehalose on the rate constant of unfolding from the on-pathway intermediate to the transition state at 20°C (□), 25°C (○), 30°C (×), 35°C (⋄), 40°C (▵), and 45°C (▪). Slopes were −0.52 ± 0.21 s−1M−1 with correlation coefficients within the range of 0.64–0.98. (C) Effect of trehalose in the [GdnHCl]50% value for the global pathway between F and U at 20°C (□), 25°C (•), 30°C (♦), 35°C (⋄), and 40°C (▵). Values were calculated assuming a three-state process as described in materials and methods [Eq. (8)]. The mid-point increases by 0.72 ± 0.2 M per molar of trehalose (linear fits with correlation coefficients in the range 0.78–0.99.
Increased Compactness of TSE and Unfolded State Induced by Trehalose
The parameter m contains information on the amount of exposure of amino acids to solvent.22 Values of m can be combined to give the fractional exposure of amino acids in the TSE relative to that of unfolded state in the so-called β-value. Figure 4A shows that the absolute value of exposure to solvent in the TSE given by the sum (mI–F + mu) decreases in the presence of trehalose, assuming that the folded state has a fixed compactness. Indeed, this reasoning assumes a constant compactness of the folded state which seems to be a valid assumption as it is not reasonable that trehalose increases amino acid exposure in the folded state (the alternative explanation to a decreased value of the sum mI–F + mu). The opposite would be possible as sucrose was shown to increase compactness of the folded state of the ribosomal protein S6.32 On the other hand, the fractional exposure relative to that of the unfolded state given by the β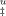 parameter increases with trehalose concentration meaning that the TSE becomes closer to the unfolded state (Figure 4A). The only explanation for this behavior is to evoke an increased compactness of the unfolded state. Trehalose induces an increased compactness of the TSE of unfolding but increases more significantly the compactness of the unfolded state as schematically depicted in Figure 4B. This would also seem logical in view of the general tendency of trehalose and other stabilizers to favor compact states. This is also entirely in accord with the Hammond postulate as the TSE shifts toward the unfolded state with the additional stabilization induced by trehalose. An increased compactness of the unfolded state of the ribosomal protein S6 induced by trehalose was previously reported in total agreement with the results reported here.33 Increased compaction of TSE for the small protein S6 was not detected probably because TSE of S6 is more compact than that of cutinase. Values of β
parameter increases with trehalose concentration meaning that the TSE becomes closer to the unfolded state (Figure 4A). The only explanation for this behavior is to evoke an increased compactness of the unfolded state. Trehalose induces an increased compactness of the TSE of unfolding but increases more significantly the compactness of the unfolded state as schematically depicted in Figure 4B. This would also seem logical in view of the general tendency of trehalose and other stabilizers to favor compact states. This is also entirely in accord with the Hammond postulate as the TSE shifts toward the unfolded state with the additional stabilization induced by trehalose. An increased compactness of the unfolded state of the ribosomal protein S6 induced by trehalose was previously reported in total agreement with the results reported here.33 Increased compaction of TSE for the small protein S6 was not detected probably because TSE of S6 is more compact than that of cutinase. Values of β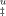 for S6 are lower (0.2–0.4) than for cutinase (0.4–0.5). Several other proteins were shown to increase their compactness in the presence of osmolytes. Osmolyte-driven contraction of reduced and carboxyamidated RNase, used as a model of the unfolded ensemble, was reported.34 The osmolyte trimethylamine N-oxide induced a condensed structure in the unfolded human glucocorticoid receptor.35 An early intermediate in the folding pathway of barstar was shown to be more structured in the presence of osmolytes.36 Denatured equine ferricytochrome C acquires a collapsed conformation in the presence of sugars.37 Sucrose restricts conformational fluctuations and induces a more ordered native conformation of the interleukin 1 receptor antagonist.38
for S6 are lower (0.2–0.4) than for cutinase (0.4–0.5). Several other proteins were shown to increase their compactness in the presence of osmolytes. Osmolyte-driven contraction of reduced and carboxyamidated RNase, used as a model of the unfolded ensemble, was reported.34 The osmolyte trimethylamine N-oxide induced a condensed structure in the unfolded human glucocorticoid receptor.35 An early intermediate in the folding pathway of barstar was shown to be more structured in the presence of osmolytes.36 Denatured equine ferricytochrome C acquires a collapsed conformation in the presence of sugars.37 Sucrose restricts conformational fluctuations and induces a more ordered native conformation of the interleukin 1 receptor antagonist.38
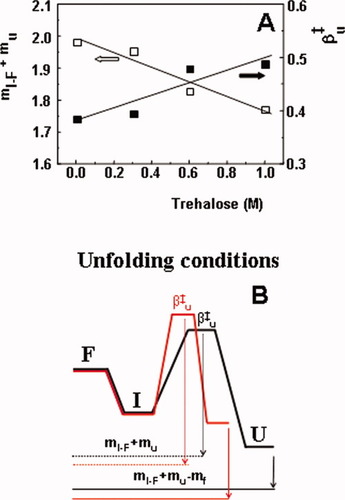
(A) Effect of trehalose in the compactness of the transition state of unfolding. The absolute increase in the accessible area between the folded and transition state can be obtained by the sum mI–F + mu and the relative compactness of the transition state to that of the unfolded state is given by β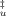 . Values of (mI–F + mu) and β
. Values of (mI–F + mu) and β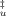 at each trehalose concentration were calculated by averaging m values at different temperatures and they were fitted with linear regressions (correlation coefficients of 0.94 and 0.85, respectively). (B) Schematic representation showing the effect of trehalose on the compaction of TSE and unfolded state. Trehalose (red lines) increases the compaction of TSE (mI–F + mu decreases) but increases more significantly the compaction of the U state (mI–F + mu − mf decreases more significantly) leading to a shift of TSE closer to the U state (β
at each trehalose concentration were calculated by averaging m values at different temperatures and they were fitted with linear regressions (correlation coefficients of 0.94 and 0.85, respectively). (B) Schematic representation showing the effect of trehalose on the compaction of TSE and unfolded state. Trehalose (red lines) increases the compaction of TSE (mI–F + mu decreases) but increases more significantly the compaction of the U state (mI–F + mu − mf decreases more significantly) leading to a shift of TSE closer to the U state (β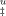 increases being 1 if TSE equals the amino acid exposure of the unfolded state).
increases being 1 if TSE equals the amino acid exposure of the unfolded state).
Thermodynamics and Mechanism of Cutinase Stabilization by Trehalose
The introduction of temperature as a new variable in addition to denaturant concentration has allowed a full thermodynamic analysis of the unfolding of the intermediate I. Values for the activation enthalpy, entropy, and heat capacity were extracted by fitting the log k versus temperature [Eq. (10)] and were plotted in Figure 5. As expected, at 25°C the intermediate shows an enthalpic barrier to unfolding counteracted by an entropic contribution. Trehalose increases both the activation enthalpy and entropy but the former increases more significantly leading to a slope smaller than 1 in a plot of T0ΔS
versus temperature [Eq. (10)] and were plotted in Figure 5. As expected, at 25°C the intermediate shows an enthalpic barrier to unfolding counteracted by an entropic contribution. Trehalose increases both the activation enthalpy and entropy but the former increases more significantly leading to a slope smaller than 1 in a plot of T0ΔS versus ΔH
versus ΔH (inset in Figure 5A). The gap between activation enthalpy and entropy increases by 0.7 kcal mol−1 per molar of trehalose, explaining the destabilization of the TSE (slow down of unfolding) with the increase in trehalose concentration. Trehalose induces the phenomenon of entropy–enthalpy compensation known as the concomitant increase in ΔS and ΔH or as a linear correlation between ΔS and ΔH.39 Trehalose was shown to increase ΔS and ΔH for several other proteins.40 The enthalpy change that favors folding results from the disruption of internal interactions within the protein (positive sign) and from the hydration of the groups that are buried in the native state and become exposed to the solvent on unfolding (negative sign).41 The gain in enthalpy resulting from loss of van der Waals interactions and hydrogen bonds upon unfolding might be more significant in the presence of trehalose if osmolytes increase the packing density of the folded state.41 Indeed, we have observed that the single tryptophan residue of the ribosomal protein S6 becomes more buried in the presence of high concentrations of sucrose, indicating an increased compactness of the folded state.32 Tightening of the native state of azurin in the presence of 2M of sucrose was also shown to occur.42 The folded state, however, cannot increase its compactness largely as globular proteins are generally close-packed.22
(inset in Figure 5A). The gap between activation enthalpy and entropy increases by 0.7 kcal mol−1 per molar of trehalose, explaining the destabilization of the TSE (slow down of unfolding) with the increase in trehalose concentration. Trehalose induces the phenomenon of entropy–enthalpy compensation known as the concomitant increase in ΔS and ΔH or as a linear correlation between ΔS and ΔH.39 Trehalose was shown to increase ΔS and ΔH for several other proteins.40 The enthalpy change that favors folding results from the disruption of internal interactions within the protein (positive sign) and from the hydration of the groups that are buried in the native state and become exposed to the solvent on unfolding (negative sign).41 The gain in enthalpy resulting from loss of van der Waals interactions and hydrogen bonds upon unfolding might be more significant in the presence of trehalose if osmolytes increase the packing density of the folded state.41 Indeed, we have observed that the single tryptophan residue of the ribosomal protein S6 becomes more buried in the presence of high concentrations of sucrose, indicating an increased compactness of the folded state.32 Tightening of the native state of azurin in the presence of 2M of sucrose was also shown to occur.42 The folded state, however, cannot increase its compactness largely as globular proteins are generally close-packed.22
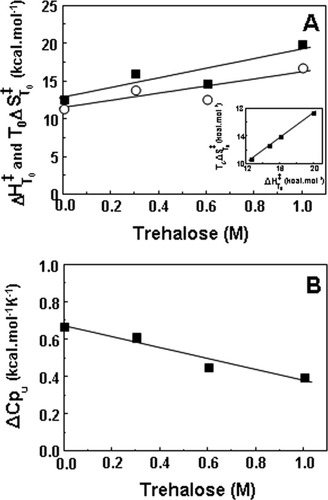
Effect of trehalose on the thermodynamics of unfolding from the intermediate (I) to the transition state of unfolding at 25°C (T0 = 298.15 K). Plots of the rate constant of unfolding (k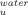 ) versus temperature were fitted to Eq. (10) to obtain the activation enthalpy (ΔH
) versus temperature were fitted to Eq. (10) to obtain the activation enthalpy (ΔH ) (▪) and entropy (TΔS
) (▪) and entropy (TΔS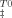 ) (○) plotted in (A) and the activation heat capacity (ΔCpu) plotted in (B). Solid lines correspond to linear fits meaning that enthalpic and entropic contributions increase by 6.4 ± 2.3 and 5.7 ± 2.1 kcal mol−1 per molar of trehalose, respectively. The activation heat capacity decreases by 0.29 kcal mol−1 K−1 per molar of trehalose. Correlation coefficients were within the range 0.79–0.94. Inset in Figure 5A shows the linear relationship between TΔS
) (○) plotted in (A) and the activation heat capacity (ΔCpu) plotted in (B). Solid lines correspond to linear fits meaning that enthalpic and entropic contributions increase by 6.4 ± 2.3 and 5.7 ± 2.1 kcal mol−1 per molar of trehalose, respectively. The activation heat capacity decreases by 0.29 kcal mol−1 K−1 per molar of trehalose. Correlation coefficients were within the range 0.79–0.94. Inset in Figure 5A shows the linear relationship between TΔS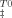 and ΔH
and ΔH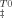 at different trehalose concentrations with a slope of 0.89 and a correlation coefficient of 0.99.
at different trehalose concentrations with a slope of 0.89 and a correlation coefficient of 0.99.
The entropic contribution favors unfolding, being thus dominated by the increase in conformational entropy of the protein. The net change in standard entropy upon unfolding, however, is a balance between the increase in conformational entropy of the protein molecule and a decrease in the entropy of water molecules bound to freshly exposed protein surfaces (the opposite of the hydrophobic effect that favors folding). As discussed earlier, trehalose increases compactness of the unfolded state; and therefore the increase in the entropic contribution induced by trehalose cannot rely on the increase in conformational entropy. It has to be reasoned based on a less significant decrease in the entropy of water molecules bound to exposed protein surfaces when trehalose is present. In the presence of trehalose, fewer water molecules are tied down around the exposed hydrophobic groups and therefore the entropic contribution increases. The increase in the enthalpic contribution induced by trehalose can also be explained, if less water molecules solvate freshly exposed hydrophobic surfaces upon unfolding as the loss in enthalpy resulting from these interactions is smaller.41 Interestingly, urea which is known to have exactly the opposite effect of protecting osmolytes (preferential binding instead of preferential exclusion) was shown to decrease both ΔH and ΔS of unfolding but, since ΔH decreases more significantly than ΔS, the destabilizing effect is primarily enthalpic.43
The activation heat capacity decreases with the increase in trehalose concentration (Figure 5B). Several other studies report indeed a decrease in ΔCp in the presence of osmolytes25, 40 whereas an increase was observed in some other cases.44, 45 The heat capacity of unfolded states is particularly high, mainly because of the water molecules bound to newly exposed hydrophobic groups.46-48 A small activation heat capacity in the presence of trehalose will then be expected if less water molecules are immobilized around exposed hydrophobic groups as pointed out elsewhere.40
Once the activation heat capacity is known, the temperature dependence of ΔS‡ and ΔH‡ and thus of ΔG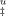 can be analyzed (Figure 6A). Despite some uncertainty resulting from fitted values of ΔCpu, the dependence of ΔG
can be analyzed (Figure 6A). Despite some uncertainty resulting from fitted values of ΔCpu, the dependence of ΔG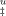 on temperature indicates that trehalose stabilizes cutinase by increasing the free energy change of the TSE of unfolding regardless of temperature. In other words, the TSE of unfolding is destabilized by trehalose relatively to the intermediate I by a small amount, but that amount of free energy remains for a large range of temperatures, leading to an increase in the Tm value (Figure 6B). Tm values for the unfolding transition calculated based on kinetic data using GdnHCl as denaturant are larger than those measured by thermal unfolding under equilibrium conditions (Table I and Figure 6B). This is expected as Tm values measured under equilibrium conditions reflect the global transition from F to U and can be shifted to lower values because of irreversible pathways. The Tm values calculated based on kinetics reflect only the second step of the unfolding pathway, i.e., the transition from I to U. The comparison between Tm values obtained for the unfolding induced by temperature and GdnHCl validates the full thermodynamic analysis carried out for the effect of trehalose on the stability of cutinase.
on temperature indicates that trehalose stabilizes cutinase by increasing the free energy change of the TSE of unfolding regardless of temperature. In other words, the TSE of unfolding is destabilized by trehalose relatively to the intermediate I by a small amount, but that amount of free energy remains for a large range of temperatures, leading to an increase in the Tm value (Figure 6B). Tm values for the unfolding transition calculated based on kinetic data using GdnHCl as denaturant are larger than those measured by thermal unfolding under equilibrium conditions (Table I and Figure 6B). This is expected as Tm values measured under equilibrium conditions reflect the global transition from F to U and can be shifted to lower values because of irreversible pathways. The Tm values calculated based on kinetics reflect only the second step of the unfolding pathway, i.e., the transition from I to U. The comparison between Tm values obtained for the unfolding induced by temperature and GdnHCl validates the full thermodynamic analysis carried out for the effect of trehalose on the stability of cutinase.
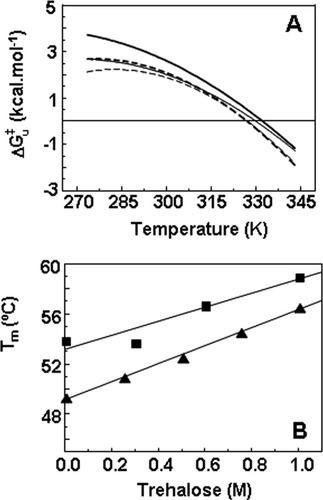
(A) Free energy profiles for unfolding from the intermediate state (I) to the unfolded state of cutinase in the absence (dashed line) and in presence of 0.3M (thick dashed line), 0.6M (solid line), and 1.0 M (thick solid line) of trehalose. Curves of ΔG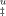 were obtained with Eq. (11). (B) Effect of trehalose in the Tm values of cutinase. Transition from folded to unfolded state (U–F) (▴) was measured by equilibrium fluorescence experiments (Figure 1, Table I) and Tm values for the transition from the intermediate to the TSE (I → ‡) (▪) were calculated for ΔG
were obtained with Eq. (11). (B) Effect of trehalose in the Tm values of cutinase. Transition from folded to unfolded state (U–F) (▴) was measured by equilibrium fluorescence experiments (Figure 1, Table I) and Tm values for the transition from the intermediate to the TSE (I → ‡) (▪) were calculated for ΔG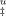 = 0, based again on Eq. (11). Solid lines correspond to linear fits with correlation coefficient in the range 0.9–1.0.
= 0, based again on Eq. (11). Solid lines correspond to linear fits with correlation coefficient in the range 0.9–1.0.
The conceptual framework proposed by Timasheff coworkers to explain protein stabilization by osmolytes states that at room temperature osmolytes are excluded from the protein domain (preferential exclusion), and thus the solvent within the protein domain is richer in water than the bulk solvent.7, 8 This preferential exclusion of solute destabilizes the protein according to the Wyman linkage theory but since the unfolded state displays a larger surface area exposed to solvent, the preferential exclusion is dominant for the unfolded state.49 Therefore, the unfolded state is more destabilized than the folded state and the latter is favored. Apparently, this mechanism may contradict the decrease in ΔCp observed for several proteins in the presence of osmolytes.25, 40 If the protein domain is richer in water than the bulk solvent (preferential hydration), it might be difficult to rationalize a decrease in ΔCp which should be expected if less water molecules are immobilized around newly exposed hydrophobic groups. However, preferential hydration is a net measure of preference by the protein for water or osmolyte and does not correlate to the effective number of water molecules that hydrate the protein.49 The number of water molecules that hydrate the protein can decrease in the presence of solute despite the protein being preferentially hydrated.
Most of the studies have neglected the possibility that the presence of osmolytes would change the protein structure, especially the unfolded state as indicated here and in other studies.12, 33-35, 37, 42, 50 Reasoning according to the new view of protein folding based on funnels or energy landscapes,51 the conformational space accessible to the unfolded state decreases in the presence of osmolytes when the unfolded state acquires residual native interactions that channel the folding of the protein. Osmolytes may channel folding along particular pathways by preferentially stabilizing one or more structural components of an ensemble.13, 36 Osmolytes also make a contribution to solve the Levinthal paradox by conferring residual structure to the unfolded state. The acquisition of residual structure results into less newly hydrophobic groups exposed upon unfolding. Fewer water molecules are immobilized upon unfolding leading to an increased enthalphy and entropy change and a decreased ΔCp change. In the presence of osmolytes, the existence of residual native interactions in the unfolded state might assume more importance than the hydrophobic effect as a driving force to folding. The proof that increased compaction of the unfolded state relies on residual native interactions was previously gathered when we have shown that the more stable mutants of S6 are the most stabilized by trehalose.33 The driving force for the acquisition of residual native interactions is the burial of the peptide backbone (the so-called osmophobic effect) which can be as important as the hydrophobic effect, as 55% of newly exposed groups upon protein unfolding belong to the peptide backbone.9, 12




