Glycodynamers: Dynamic analogs of arabinofuranoside oligosaccharides†
Dedicated to the memory of Elkan, a long-standing friend, a thoughtful scientist, a trustful colleague, and a warm human being.
Abstract
Dynamic analogs of α-(1→5)-D-oligoarabinofuranosides were prepared by oxime polycondensation. These equilibrium polymers were characterized by 1H NMR studies, NMR DOSY, and MALDI mass spectrometry. Their reversibility under mild acidic conditions was demonstrated by component exchange reaction followed by 1H NMR studies. Their size and composition can be tuned by component exchange emphasizing their constitutionally dynamic character. They represent dynamic versions of biopolymers, biodynamers. © 2007 Wiley Periodicals, Inc. Biopolymers 89: 486–496, 2008.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
INTRODUCTION
Equilibrium polymers of supramolecular type are widely found in nature, and their dynamic properties are crucial for their biological functions. The examples of actin filaments and microtubules that contribute to the cytoskeleton of eukaryotes show the importance of the adaptive character of such polymers whose size and localization are in constant evolution according to the cell needs. The dynamics of these supramolecular polymers control many biological processes such as cell motion or division.1-3
Dynamic processes concerning the constitution of both molecular and supramolecular entities have been integrated and rationalized on the chemical level by the introduction of the constitutional dynamic chemistry (CDC) concept.4, 5 CDC takes advantage of reversible chemical linkages (covalent or noncovalent) to generate mixtures of molecular or supramolecular constituents resulting from the recombination of the reversibly connected components, dynamic combinatorial libraries (DCLs).6-9 These combinations are present in proportions that are specific to the thermodynamic equilibrium of the system, and therefore, various physical or chemical factors can affect the relative proportions of the members, i.e. the composition of the DCL. These perturbations may lead to adaptation through amplification of one or more constituents of the library by recombination of its components. This approach was used successfully namely for the generation of synthetic receptor molecules6-9 as well as for the discovery of biologically active substances selected by the biological target itself under the pressure of recognition.7 Physical factors like temperature, pH10 or electric field11 have also been shown to affect the distribution of the DCL, illustrating their potential as stimuli responsive and adaptive chemical entities. Note that a constitutional dynamic library may be, usually is, but need not be, combinatorial. It stresses the dynamics of the system, which is a prerequisite for recombination of components into “libraries” of constituents.
Implementation of these principles in material science, and in particular in polymer chemistry led to the development of “dynamers,” i.e. dynamic polymers, that encompass molecular or supramolecular equilibrium polymers as they result from the reversible connection of monomers via either covalent or noncovalent linkages.12-14 Such equilibrium polymers present the ability to undergo changes in their constitution, length, and sequence even after polymerization, and may thus be considered a “living polymers.” These modifications can be triggered by physical or chemical stimuli like temperature,15-17 addition of a catalyst18 or of a template.19 Dynamers are thus smart, adaptive materials. Moreover, changes in the molecular constitution of the polymer can induce a modification of the properties (such as mechanical20, 21 or optical19, 21) of the material.
Dynamers based on components of biological nature may generate dynamic analogs of natural polymers, biodynamers, that offer the possibility to combine the functional properties (recognition, catalysis) of naturally occurring polymers with the adaptive behavior of constitutional dynamic systems. Therefore, in view of the prospects offered, the incorporation of biologically relevant moieties into dynamic polymers deserves close scrutiny.22, 23 In addition to proteins, and nucleic acids, polysaccharides represent a third class of biopolymers. Oligo- and polysaccharides are ubiquitous in living organism and are involved in a wide range of biological or pathological events like immune response, inflammation, cancer, cell adhesion or cell–cell recognition.24-26 They also possess a wide range of material properties going from gels for hyaluronic acid to highly resistant crystalline fibers for cellulose.27
“Static” analogs of polysaccharides have been prepared using different methodology including acetylene coupling,28, 29 click-chemistry,30-33 peptide coupling,34 hemiaminal,35 and acetal formation36, 37 or derivatization of natural polysaccharides.38, 39
Extending our work on dynamers12-15,19-21 to the biopolymer area,23 we were interested in designing dynamic analogs of natural polysaccharides. Such biodynamers may be of three types: (1) main-chain, resulting from polycondensation of saccharide residues through reversible reactions; (2) side-chain, where the saccharide residues are either (a) appended on a dynamic main chain or (b) reversibly grafted on a non-dynamic main chain; (3) “doubly dynamic,” incorporating both main chain and side-chain dynamics.
Of special interest, as reversible condensation reaction, is the formation of an imine-type double bond from an amino group and a carbonyl group. Classical “static” polymers incorporating carbohydrate-based monomers have been obtained by reductive amination40 and poly(acylhydrazone) formation.39, 41
As reducing sugars are among the only biomolecules that contains an aldehyde function, oxime ligation,42 in particular, has been employed for saccharide and oligosaccharide labeling with fluorescent dyes,43 or peptide decoration.44-47 This type of junction has also been used for polymer preparation.48 It is important to note that the potentialities offered by the reversible character of the oxime bond has neither been explicited nor exploited in these studies. It is only recently that the reversibility properties of this chemical link were implemented for glycopeptides labeling and isolation.49 Ditopic saccharide monomers yield reversible polymers with a bis-boronic acid22 and a dynamic library of cyclic sugar oligomers has been obtained via acylhydrazone formation.50
Here we describe the application of the oxime condensation reaction to the generation of main-chain dynamic polysaccharide analogs (Type 1 above).
RESULTS AND DISCUSSION
Model System
We first investigated the dynamic properties of the oxime junction on a model compound 2 prepared in one step from glucosamine 1 (Figure 1).
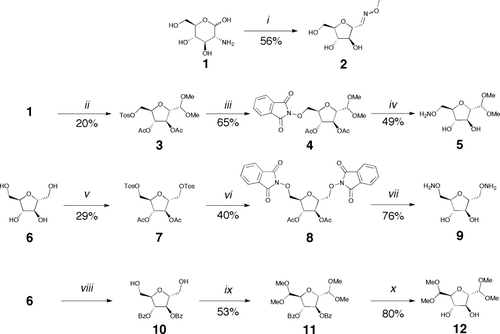
Synthesis of the model oxime 2 and the monomers 5, 9, 12. Reagents and conditions: i 1. NaNO2, H2O 2. MeONH2, K2CO3, H2O; ii 1. NaNO2 2. trimethylorthoformate, p-toluenesulfonic acid, MeOH; 3. tosyl chloride, pyridine 4. Ac2O, pyridine; iii N-hydroxyphtalimide, DBU, DMF; iv butylamine, MeOH; v 1. tosyl chloride, pyridine 2. Ac2O, pyridine; vi N-hydroxyphtalimide, DBU, DMF; vii butylamine, MeOH; viii 1. Trityl chloride, pyridine 2. benzoyl chloride, pyridine 3. 80% aqueous acetic acid; ix 1. TEMPO, trichloroisocyanuric acid, CH2Cl2 2. trimethylorthoformate, p-toluenesulfonic acid MeOH; x NaOMe, MeOH.
D-2,5-Anhydromannose is accessible in one step from commercial D-glucosamine 1 and constitute an excellent model for sugar-aldehydes. We prepared the O-methyl oxime 2 of this aldehyde and investigated its reversibility properties. Oxime formation was fast as it took 15 min at pD = 4 at 20 mM for the reaction to go to completion, giving a mixture of the E and Z isomers. No dissociation of the oxime into free aldehyde and methylhydroxylamine was observed by proton NMR, indicating that the aldehyde was fully converted under our experimental conditions.
The exchange reaction (Figure 2) between the methyl oxime 2 and the tert-butylhydroxylamine 13 was performed in buffered D2O (200 mM, d3-acetate buffer). Addition of one equivalent of tert-butylhydroxylamine 13 to a solution of model oxime 2 induced the formation of the oxime 14. The proportion of the different species can be determined by integration of the CH signal of the E imines of 2 and 14 in the 1H NMR spectrum of the mixture. The kinetics of the exchange are represented on Figure 3.

Model exchange reaction between 2 and tert-butylhydroxylamine 13.
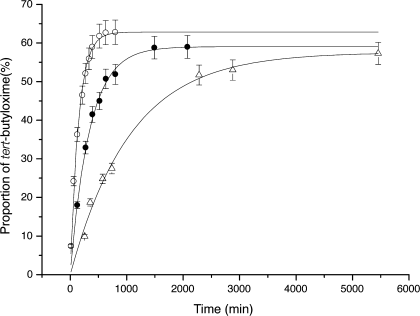
pD dependence of the rate of the exchange reaction between 2 and 13. ○ pD = 4; • pD = 5; ▴ pD = 6. The proportion of tert-butyloxime was obtained from the ratio of the integration of the CH E imine signals of 2 and 14. Systematic error bar ±5%.
The half-life for exchange at 20 mM, that is half of the time needed to reach equilibrium, was 98 min at pD = 4, 242 min at pD = 5, and 777 min at pD = 6. So mild acidic conditions (pD = 4) are suitable to attain reasonable exchange rates, and exchanges can be slowed down by increasing the pD.
After demonstration of the conditions of reversibility of the oxime junction, we designed monomers for the preparation of oligofuranosides analogs.
Synthesis of the Monomers
Mycobacterial arabinans were chosen as model. These polysaccharides are present in the cell wall of some mycobacteria like Mycobacterium tuberculosis and Mycobacterium leprae. α-(1→5)-D-Oligoarabinofuranosides constitute the core of arabinogalactan and lipoarabinomannan that are major components of the mycobacteria cell wall.51 The replacement of the α-(1→5)-glycosidic bond of arabiogalactans by a covalent reversibility cassette, such as the RCHNOR unit, can give access to an analog were monomers are linked in a reversible manner (Scheme 1).
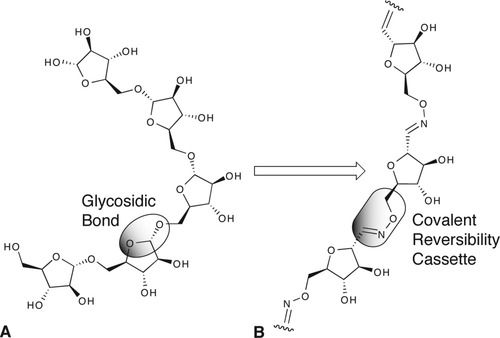
A: Natural α-(1→5)-D-oligoarabinofuranosides; B: dynamic analog obtained by replacement of the glycosidic bond by an oxime linkage.
Two different approaches were chosen to access either of the polymer P1 or the alternative copolymer P2 (Figure 4). The monomer 5 was prepared in two steps from the known tosylate 3. It consists in a furanose moiety bearing one masked aldehyde function on C1 and an aminooxy group at the C6 position; therefore, 5 can be polymerized by in situ deprotection-polycondensation. The generation of the copolymer P2 requires the protected dialdehyde monomer 12, prepared in two steps from the known diol 10, and the bis-alkoxylamine 9, obtained in two steps from the known bis-tosylate 7 (Figure 1).
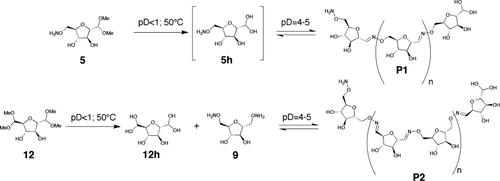
Generation of the dynamic polymers P1 and P2.
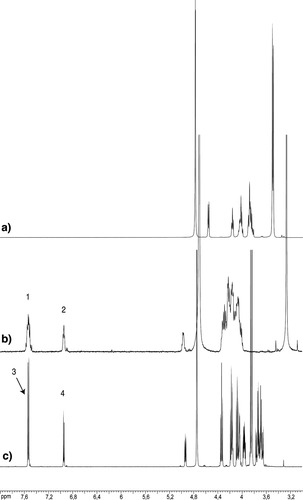
1H NMR spectrum of: (a) starting monomer 5; (b) dynamic polymer P1, signals 1 and 2 correspond to E and Z oximes, respectively; (c) model methyl oxime 2, signals 3 and 4 correspond to E and Z oxime, respectively.
Preparation of the Polymers P1 and P2
The deprotection of 5 and 12 was achieved in situ by acid hydrolysis at pD < 1 at 50°C for 24 h giving the hydrates 5h and 12h. In the case of 5, polycondensation occurs during deprotection and even under the highly acidic conditions required for acetal cleavage oxime condensation is observable. After complete deprotection the pD of the solution is adjusted to pD = 4, 5, or 6 yielding P1 (Figure 5). P2 was obtained after addition of one equivalent of 9 to a neutralized solution of 12h. The polycondensation reactions were conducted at an initial concentration of 20 mM of the starting monomers.
Characterization of the Dynamic Polymers P1 and P2
The resulting polymers P1 and P2 were characterized by Diffusion Ordered NMR SpectroscopY (DOSY). The pulsed-field gradient stimulated echo NMR sequence was used to measure the translational diffusion coefficient (D) of the polymers, which is a function of the molecular size and shape. In our laboratory, this technique has already been used for the analysis of DCLs of helicoidal and grid types metallic complexes.52, 53 It is also suitable for the characterization of dynamic polymers as it can be used to determine their diffusion coefficient in solution under conditions were they are still reversible.
This 2D NMR technique has been proposed as an alternative to GPC for polysaccharides, as the diffusion coefficient can be correlated to the molecular weight of polymers.54, 55 In these studies a calibration curve was established by plotting the diffusion coefficients of oligosaccharide standards as a function of molecular weight. This calibration curve was used to estimate the molecular weight of synthetic or natural samples of oligo- and polysaccharides. The translational diffusion coefficient D allows the determination the spherical hydrodynamic radius rsph of a particle, using the Stokes–Einstein equation relation D = kBT/(6πηrsph) were kB is the Boltzmann constant, T is the absolute temperature, and η the viscosity of the medium.
Using commercial arabinans we established the calibration curve log D = f(log Mw) shown in Figure 6 (Table I). As the structures of the polymers P1 and P2 are close to α-(1→5)-arabinofuranoses, this calibration curve enables us to estimate the molecular weight of the main species in solution. The main species have a molecular weight between 800 and 1100 g mol−1 for P1 and between 1600 and 2200 g mol−1 for P2, corresponding to a degree of polymerization lying between 5 and 7 for P1, and between 10 and 14 for P2, for initial concentrations of 20 mM of the starting monomers. One expects of course an increase in molecular weight with concentration.
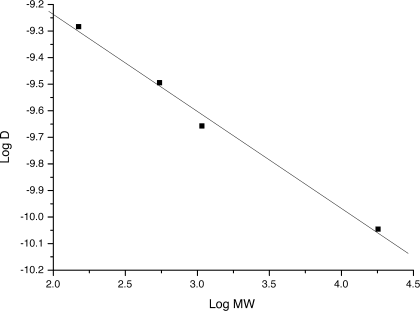
Plot of the diffusion constant as log D versus the molecular weight as log Mw for a series of standard arabinan compounds: D-arabinofuranose, α-(1→5)-L-arabinotetraose, α-(1→5)-L-arabinoaoctaose, debranched α-(1→5)-L-arabinan (Mw = 18,000 g mol−1). These data were fitted to a straight line: log D(m2 s−1) = −(0.365)log Mw (g mol−1)−8.506, R = −0.99584.
| Degree of Polymerization of Arabinan Standards | Mw (g mol−1) | D (μm2 s−1) | rSPH (Å) |
|---|---|---|---|
| 1 | 150 | 520a | 3.8 |
| 4 | 546 | 320a | 6.2 |
| 8 | 1,075 | 220a | 9 |
| 120 | 18,000 | 90a | 22.1 |
- a D (D2O) = 1690 μm2 s−1.
These results were further supported by MALDI mass spectrometry, which showed polycondensation products having a degree of polymerization n up to 12 for P1 and up to 8 for P2 (Figures 7 and 8). The majority of the signals observed corresponded to cyclic species. As the oxime bond is reversible, in solution these macrocyclic species are in equilibrium with linear oligomers.
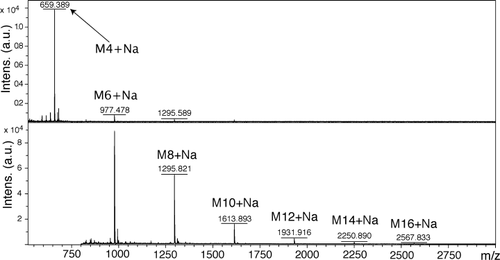
MALDI mass spectrum of a sample of polymer P2. The M4-16+Na peaks correspond respectively to the Na+ adducts signals of macrocycles containing four to sixteen sugar units.
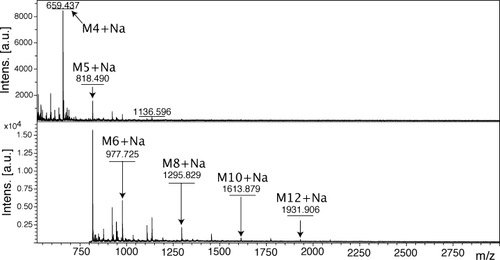
MALDI mass spectrum of a sample of polymer P1. M4-12+Na correspond respectively to the Na+ adducts of the macrocyclic tetramer-dodecamers.
Demonstration of the Dynamic Character of the Polymers P1 and P2
As the structure of the model oxime 2 is very close to the monomers, we can expect a good correlation between its properties and those of our polymers. To demonstrate the reversible character of polymers P1 and P2, we chose to add a termination agent to the polymers after equilibration. If polymerization is reversible, then this termination agent should be incorporated into the polymer and consequently reduce its length by chain termination (Figure 9).
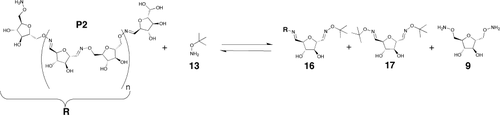
Dynamic chain termination: equilibration on addition of a termination agent to dynamic polymer P2.
The incorporation of one equivalent of the termination agent 13 was followed by 1H NMR. Integration of the signal corresponding to the tert-butyloxime unit gives the proportion of incorporated termination agent (Figures 10 and 11).
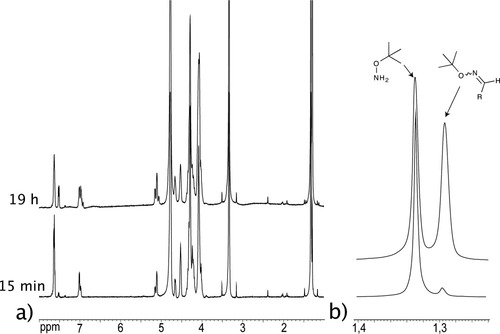
(a) 1H NMR spectrum of the exchange mixture obtained after addition of tert-butylhydroxylamine to P2 at pD = 4 after 15 min and 19 h at 20 mM concentration. (b) Partial zoom on the tert-butylhydroxylamine and tert-butyloxime signals.
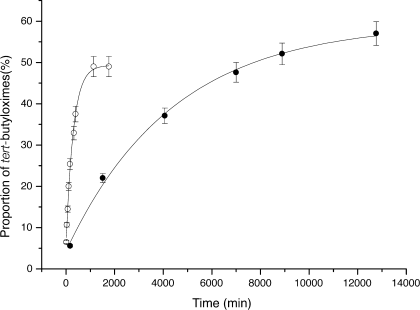
pD dependence of the rate of exchange between P2 and 13. ○ pD = 4; • pD = 6. The proportion of tert-butyloximes was calculated from the ratio between tert-butyl 1H NMR signals of the hydroxylamine and tert-butyloximes. Initial monomers concentration of 20 mM. Systematic error bar ±5%.
The half-life for exchange was 244 min at pD = 4 and 5440 min at pD = 6 for P1 with an initial monomer concentration of 20 mM. For P2 it was 165 min at pD = 4 and 2410 min at pD = 6 with an initial monomers concentration of 20 mM. For P2 at pD = 5 it was not possible to follow the exchange with 13 due to tert-butylhydroxylamine and tert-butyloxime signals overlap.
When this exchange was followed by NMR DOSY, we could clearly observe incorporation of the tert-butylhydroxylamine (D = 640 μm2 s−1) into the polymer by measuring the diffusion coefficient of the terminal tert-butyloxime signals of the oligomer 16 (250 μm2 s−1 < D < 315 μm2s−1). The increase of the overall diffusion coefficient of the polymeric species can also be associated with shortening of the polymer as a consequence of the incorporation of the termination agent. After equilibration of P2 with one equivalent of tert-butylhydroxylamine at pD = 4 we determined the diffusion coefficient of the polymer and calculated a spherical hydrodynamic radius comprised between 6.3 and 8 Å. These values have to be compared with polymer P2 alone which has a rSPH comprised between 9.5 and 11 Å. We can conclude that addition of a termination agent indeed reduced the average size of P2.
CONCLUSION
The methyl-oxime 2 was used as a model to demonstrate the applicability of the oxime junction to reversibly connect saccharide derivatives with other components in mild acidic aqueous media. This junction was then applied to the generation, by polycondensation, of carbohydrates based dynamers P1 and P2, where the functionally complementary polymerizable groups were either on the same monomer or on two different sugars units. The polymeric nature of this new kind of biomimetic material was demonstrated by NMR DOSY and MALDI mass spectrometry, which showed the formation of oligomers in solution. These polycondensation products can be considered as dynamic analogs of oligosaccharides. Their reversibility was demonstrated by dynamic chain termination. As exchange is associated with introduction of a new moiety into the polymer, the constitution of these analogs of polysaccharide is dynamic and may be modified by component incorporation and decorporation. Exchanges are slower at pD = 6 whereas a marked increase in rate was observed at slightly acidic pD = 4 and 5.
The present approach is an efficient tool for the chemical synthesis of oligoarabinofuranoside analogs and is therefore of interest for the preparation of potential arabinan biosynthesis inhibitors, immunomodulator analogs, or as synthetic ligands for cellular interactions.56-59 As the 2,5-anhydromannose unit can be introduced into various glycoaminoglycans,60-63 it is likely that this methodology can be extended to the preparation of other dynamic polymers. Of major interest is the fact that such glycodynamers combine constitutional dynamics with biomimetic character, giving thus access more generally to CDC of glycans.
MATERIALS AND METHODS
Materials
α-(1→5)-D-Oligoarabinofuranosides standards were obtained from Megazyme International Ireland. Amberlite IR-120 H+ form resin was obtained from Fluka and washed three times with water before use. Other reagents were obtained commercially (Sigma–Aldrich) unless stated otherwise and used without further purification.
Spectroscopic Measurements
NMR spectra were recorded on a Bruker 400 MHz spectrometer. The chemical shifts are reported in parts per million downfield from solvent signal in CDCl3, and tBuOH in D2O; coupling constants are in Hertz. Electrospray ionization mass spectrometry (ESI-MS) was carried out on a Bruker Micro-TOF mass spectrometer coupled with liquid chromatography. MALDI mass spectrometry (MALDI-MS) was carried out on a Brucker MALDI-TOF mass spectrometer.
DOSY NMR Experiments
The spectra were recorded on a Bruker Avance500 spectrometer, at 11.7 tesla, at the resonating frequency of 500.13 MHz for 1H, using a BBI Bruker 5 mm gradient probe. The temperature was regulated at 298 K and no spinning was applied to the NMR tube. The diffusion NMR experiments were performed with a Pulsed-Field Gradient STimulated Echo (PFGSTE) sequence, using bipolar gradients. For the α-(1→5)-D-oligoarabinofuranoside standards, the bipolar gradient duration and the diffusion time were optimized for each sample, and were in the range of 2.2–4 ms and 100–500 ms, respectively. For the molecular weight determination of polymers the bipolar gradient duration was 2 for P1, 2.2 for P2, and the diffusion time 200 ms for both polymers. The evolution of the pulsed-field gradient during the NMR diffusion experiments was established in 30 steps, applied linearly between 1 and 50 G cm−1. The duration of each NMR diffusion experiment was adjusted to finally obtain a signal-to-noise ratio higher than 20. DOSY spectra were generated by using the program GIFA 5.2 (DOSY module), developed by the NMRTec company, using adapted algorithms, such as the inverse Laplace transform and maximum entropy, to build the diffusion dimension.
Deuterated buffer solutions for NMR measurements were prepared in D2O using a desired concentration of deuterated [D4]acetic acid (for pD 4–6) and then the p2H (pD) was adjusted by using a solution of NaOD in D2O. The pD of the solution was monitored with a pH meter and the pD of the buffer solution is equal to the pH meter reading + 0.4.
Synthesis of 2,5-Anhydro-D-mannose methyl oxime (2)
1 (4 g, 18.5 mmol) was dissolved in water (200 ml) at room temperature. After 4 h, this solution was cooled down to 0°C. NaNO2 (3.27 g, 3 eq) was then added as one portion followed by careful drop-by-drop addition of AcOH (3.5 ml, 55.5 mmol) at a rate keeping the temperature of the reaction mixture below 5°C. After complete addition of the acid, the reaction was stirred 4 h at 0°C and then allowed to warm to room temperature and degassed by argon bubbling. After neutralization of the solution to pH 7 the CH3ONH2·HCl (2.32 g, 37.1 mmol) was added followed by stirring other night at room temperature. After removal of the solvent under reduced pressure the crude product was purified by silica gel chromatography (CHCl3/MeOH 9/1→8/2) to afford 2 g of pure 2 as a colorless syrup (56%). 1H NMR (400 MHz, D2O, assignation based on a 1H NMR COSY experiment): δ 7.54 (d, 0.70 H, J = 6.5, HC(1), E isomer), 6.95 (d, 0.30 H, J = 5, HC(1), Z isomer), 4.94 (dd, 0.30 H, J = 5, 3.8, HC(2), Z isomer), 4.34 (t, 0.70 H, J = 6.5, HC(2), E isomer), 4.20–4.14 (m, 1 H, HC(3) E and Z isomers), 4.08 (t, 0.70 H, J = 6.5, HC(4), E isomer), 4.06–4.02 (m, 0.70 H, HC(6), 3.99–3.94 (m, 1 H, HC(5), E and Z isomers), 3.86 (s, 0.90 H, OCH3, Z isomer), 3.84 (s, 0.21 H, OCH3, E isomer), 3.79–3.64 (m, 1.3 H, HC(6), E and Z isomers). 13C (200 MHz, D2O): δ 152.51 (C(1), Z isomer), 150.55 (C(1), E isomer), 85.69 (C(5), Z isomer), 83.94 (C(5), E isomer), 80.84 (C(2), Z isomer), 79.99 (C(2), E isomer), 79.36 (C(4), E isomer), 79.24 (C(4), Z isomer), 77.30 (C(3), Z isomer), 76.59 (C(3), E isomer), 62.58 (C(6), Z isomer), 62.23 (C(6), E isomer), 61.87 (NOCH3, Z isomer), 61.72 (NOCH3, E isomer). ESI-MS(pos.): m/z: 214.05 [M + Na]+; elemental analysis calcd (%) for C7H13NO5: C 43.98, H 6.85, N 7.33; found: C 43.53, H 7.34, N 7.27.
Synthesis of 3,4-Di-O-acetyl-2,5-anhydro-6-O-phtalimido-D-mannose dimethyl acetal (4)
The tosylate 3 was prepared using known procedures.63, 64 3 (10.95 g, 24,5 mmol) and N-hydroxyphtalimide (6 g, 36.7 mmol, 1.5 eq) were dissolved in dry DMF (95 ml) under nitrogen atmosphere. DBU (5.5 ml, 36.7 mmol) was added resulting in a deep red color. After stirring at 90°C for 2 h the reaction was allowed to cool down to room temperature and diluted with AcOEt, washed two times with water, brine, and then dried over MgSO4. After filtration the organic phase was concentrated under reduced pressure to give the crude product which was purified by silica gel chromatography (CH2Cl2/AcOEt 9/1→8/2) to give 7 g of pure 4 as a light yellow oil (65%). 1H NMR (400 MHz, CDCl3, assignation based on a 1H NMR COSY experiment): δ 7.86–7.62 (m, 4H, arom. H)), 5.37 (t, J = 3.4, HC(4)), 5.28 (dd, J = 4.5, 3.4, HC(5)), 4.47–4.26 (m, HC(1,2,H6a,H6b)), 4.07 (dd, J = 5.7, 3.4, HC(3)), 3.37 (s, OCH3), 3.31 (s, OCH3), 2.06 (s, OOCH3), 2.04 (s, OOCH3). 13C (200 MHz, CDCl3): δ 169.98, 169.85, 163.14, 134.47, 128.91, 123.53, 103.35 (C(1)), 82.12 (C(2)), 81.08 (C(4)), 78.23 (C(5)), 77.69 (C(6)), 76.97 (C(3)), 55.50 (OCH3), 54.00 (OCH3), 20.91 (OOCCH3), 20.82 (OO CCH3). MALDI-MS(pos.): m/z: 460.11 [M + Na]+, 438.14 [M + H]+; elemental analysis calcd (%) for C20H23NO10: C 54.92, H 5.30, N 3.20; found: C 54.7, H 5.47, N 3.34.
Synthesis of 2,5-Anhydro-6-O-amino-D-mannose dimethyl acetal (5)
A solution of 4 (805 mg, 1.84 mmol) in 1/1 butylamine/MeOH (20 ml) was stirred at room temperature for 3 h. The reaction was then concentrated under reduced pressure, co-evaporated three times with EtOH, and dried under high vacuum for 3 h. The resulting crude product was purified by silica gel chromatography (CH2Cl2/MeOH 9/1) to give 200 mg of 5 as a colorless syrup (49%).1H NMR (400 MHz, D2O): δ 4.56 (d, J = 6, HC(1)), 4.16 (t, J = 6, HC(2)), 4.10–3.96 (m, 2H), 3.94–3.79 (m, 3H), 3.50 (s, 3H, OCH3), 3.48 (s, 3H, OCH3). 13C (200 MHz, D2O): δ 105.05 (C(1)), 82.25(C(2)), 81.41, 77.91, 77.65 (C(4)), 75.66 (C(3)), 56.57 (OCH3), 55.67 (OCH3). HRMS m/z calculated for C8H17LiNO6 [M + Li]+ = 230.1211; found 230.1225.
Synthesis of 3,4-Di-O-acetyl-2,5-anhydro-1,6-di-O-phtalimido-D-mannitol (8)
The synthesis of 665 and 764 is based on procedures described in the literature. A solution of 6 (1 g, 6 mmol) in dry pyridine (40 ml) under nitrogen atmosphere was cooled down to 0°C. The tosyl chloride (2.44 g, 12.6 mmol) was then added in small portions spread over 30 min. The reaction was then allowed to warm to room temperature. After stirring one night at this temperature, Ac2O (5 ml) was added to the reaction. The reaction was stirred 4 h at room temperature. Solvents were removed under reduced pressure and the residue dissolved in ethyl acetate, washed with saturated NaHCO3, 1N HCl solution, and brine. The organic phase was dried over MgSO4, and filtered. The filtrate was concentrated under reduced pressure to give the crude ditosylate 7 (2.52 g, 4.52 mmol) that was used directly without further purification. The crude 7 was dissolved in dry DMF (15 ml) under nitrogen atmosphere. N-Hydroxyphtalimide (1.47 g, 9.05 mmol), tetrabutylammonium iodide (50 mg, 0.13 mmol), and DBU (1.38 g, 1.35 ml, 9.05 mmol) were added to the reaction mixture. After 4 h at 90°C, the reaction was allowed to cool down to room temperature, diluted with AcOEt, washed with water and brine. The organic phase was dried over MgSO4, filtered, and the filtrate concentrated under reduced pressure. The crude product was purified by silica gel chromatography (CHCl3/AcOEt 9/1) to give 950 mg of pure 8 (40%) as a light yellow oil which crystallize after few days at room temperature. 1H NMR (400 MHz, CDCl3): δ 7.85–7.70 (m, 8H, arom. H), 5.47–5.43 (m, 2H, HC(1, 6)), 4.47–4.31 (m, 6H), 2.11 (s, 6H, 2–OOCCH3). 13C (200 MHz, CDCl3): δ 170.22 (quaternary C acetates), 163.36 (quaternary C phtalimide), 134.70, 129.10, 123.81, 81.24 (C(3, 4)), 78.07 (C(2, 5), 77.09 (C(1, 6)), 21.05 (OOCCH3). HRMS m/z calculated for C26H22LiN2O11 [M + Li]+ = 545.1379; found 545.1366.
Synthesis of 2,5-Anhydro-1,6-di-O-amino-D-mannitol (9)
A solution of 8 (433 mg, 0.8 mmol) in 1/1 butylamine/MeOH (30 ml) was stirred at room temperature for 24 h. The reaction was then concentrated under reduced pressure and co-evaporated three times with EtOH. The crude product was dried under high vacuum for 3 h and purified on silica gel chromatography (CH2Cl2/MeOH 4/1) to give 118 mg of pure 9 as a white solid (76%).1H NMR (400 MHz, D2O): δ 4.08–3.98 (m, 4H), 3.94–3.80 (m, 4H). 13C (200 MHz, D2O): δ 80.83 (C(1, 6)), 77.40(C(2, 5)), 75.83 (C(3, 4)). ESI-MS(pos.): m/z: 217.0776 [M + Na]+; elemental analysis calcd (%) for C6H14N2O5: C 37.11, H 7.27, N 14.43; found: C 36.56, H 7.17, N 14.48.
Synthesis of 2,5-Anhydro-3,4-O-dibenzoyl-1,6-dimethylacetal-D-manno-hexodialdose (11)
10 was prepared using known procedures.66 A solution of 10 (3.09 g, 8.29 mmol) in dry CH2Cl2 (50 ml) was cooled down to 0°C. Trichloroisocyanuric acid (4.24 g, 18.24 mmol) was added under nitrogen atmosphere. TEMPO (2,2,6,6-tetramethylpiperidine 1-oxyl) was then added in catalytic amount (5 mg). The reaction mixture turned rapidly to deep red and was then allowed to warm to room temperature. After 15 min, the color of the reaction turned to light yellow. The resulting suspension was filtered. The filtrate was washed successively with saturated NaHCO3 solution, 1N HCl solution, brine, and dried over MgSO4. After filtration, the organic phase was concentrated to half of its initial volume and diluted with dry MeOH (50 ml). CH(OMe)3 (25 ml) was added followed by a catalytic amount of p-toluene sulfonic acid monohydrate (100 mg). After stirring for one night at room temperature under nitrogen the reaction mixture was concentrated to a quarter of its initial volume and diluted with AcOEt. This solution was washed successively with saturated aqueous NaHCO3, brine, and dried over MgSO4. After filtration and concentration under reduced pressure the crude product was purified by silica gel chromatography (CH2Cl2/MeOH 98/2) to give 2.02 g of pure 11 as a colorless oil (53%).1H NMR (400 MHz, (CD3)2CO): δ 8.14–8.04 (m, 4H, arom.), 7.74–7.65 (m, 2H, arom.), 7.61–7.53 (m, 4H, arom.), 5.78 (dd, 2H, J = 3, 1.2, HC(3, 4)), 4.65 (d, 2H, J = 5.4, H-C(1, 6)), 4.41–4.36 (m, 2H, HC(2, 5)), 3.46 (s, 3H, OCH3), 3.45 (s, 3H, OCH3). 13C (200 MHz, (CD3)2CO): δ 165.80 (quaternary C benzoyl), 134.39 (arom. benzoyl), 130.91 (arom. benzoyl), 130.57 (arom. benzoyl), 129.66 (arom. benzoyl), 104.95 (C(1, 6)), 83.77 (C(2, 5)), 79.84 (C(3, 4)), 55.90 (OCH3 acetal), 54.87 (OCH3 acetal). MALDI-MS(pos.): m/z: 483.1338 [M + Na]+; elemental analysis calcd (%) for C24H28NO9: C 62.60, H 6.13; found: C 62.70, H 5.95.
Synthesis of 2,5-Anhydro-1,6-dimethylacetal-D-manno-hexodialdose (12)
11 (2.02 g, 4.38 mmol) was dissolved in MeOH (100 ml). NaOMe (100 mg) was then added under nitrogen atmosphere. After stirring at room temperature overnight the reaction was neutralized by ion exchange resin Amberlite IR-120 H+ form and filtered. The filtrate was concentrated under reduced pressure to afford the crude product that was purified by silica gel chromatography (AcOEt) to give 875 mg of pure 12 as a colorless liquid (80%).1H NMR (400 MHz, D2O): δ 4.46 (d, J = 6.1 Hz, HC(1, 6)), 4.09–4.07 (m, HC(2, 5)), 3.85–3.79 (m, HC(3, 4)), 3.416 (s, OCH3), 3.402 (s, OCH3). 13C (200 MHz, D2O): δ 104.806 (C(OMe)2), 82.982 (C(2, 5)), 78.150 (C(3, 4)), 56.450 (2–OCH3), 55.571 (2–OCH3). ESI-MS(pos.): m/z: 275.1123 [M + Na]+, 259.132 [M + Li]+; elemental analysis calcd (%) for C10H20O7: C 47.61, H 7.99; found: C 47.71, H 7.98.




