Collagen scaffolds for tissue engineering
Abstract
There are two major approaches to tissue engineering for regeneration of tissues and organs. One involves cell-free materials and/or factors and one involves delivering cells to contribute to the regeneraion process. Of the many scaffold materials being investigated, collagen type I, with selective removal of its telopeptides, has been shown to have many advantageous features for both of these approaches. Highly porous collagen lattice sponges have been used to support in vitro growth of many types of tissues. Use of bioreactors to control in vitro perfusion of medium and to apply hydrostatic fluid pressure has been shown to enhance histogenesis in collagen scaffolds. Collagen sponges have also been developed to contain differentiating-inducing materials like demineralized bone to stimulate differentiation of cartilage tissue both in vitro and in vivo. © 2007 Wiley Periodicals, Inc. Biopolymers 89: 338–344, 2008.
This article was originally published online as an accepted preprint. The “Published Online” date corresponds to the preprint version. You can request a copy of the preprint by emailing the Biopolymers editorial office at [email protected]
CELL-BASED AND CELL-FREE APPROACHES TO TISSUE ENGINEERING
Traditionally, two-dimensional (2D) monolayer culture systems have been used to investigate the regulation of cellular differentiation and cellular production of extracellular matrix (ECM). Micro-mass tissue culture methods aim to deposit and maintain droplets of cells at very high density to more suitably model the 3D microenvironment of living tissues. Three-dimensional (3D) scaffolds have become more routine for in vitro investigations of histogenesis and have been evaluated for tissue engineering applications.
Tissue engineering concerns ways to regenerate tissues and organs. Many tissue engineering approaches involve 3D scaffolds.1 Substances frequently used for scaffolds include natural polymeric materials, synthetic polymers or ceramics, biodegradable polymers, or polymers with adsorbed proteins or immobilized functional groups. Naturally occurring matrices or their components have advantages because of their outstanding biocompatibility properties. Popular ECM molecules of connective tissues are collagen, hyaluronan, and glycosaminoglycans. Collagens have been used in native form or as denatured gelatin. Because of its abundance, ubiquity, and biocompatibility,2 collagen type I is the most commonly used (Table I). It can be used intact or after proteolytic removal of the small nonhelical telopeptides, which reduces possible antigenicity. Further, there are two forms that native collagen can have, as swollen hydrogels or as sparse fibers in a lattice-like organization. Fibers can be crosslinked by various methods. Dehydrative crosslinking occurs when water content falls below approximately 1 wt% and allows for formation of interchain peptide bonds.3 Glutaraldehyde crosslinking is favored by some, especially in copolymers of collagen type I and chondroitin 6-sulfate.4 Concerns have been raised about cytotoxicity5, 6 and bioincompatibility7 of some glutaraldehyde crosslinked collagens. Cytotoxicity can be minimized by neutralization,8 by using lower concentrations of glutaraldehyde,9 or by substitution with other tanning agents, such as hexamethylene diisocyanate.10 In our studies, we expose native collagen to UV-irradiation,11 which is believed to stabilize collagen fibrillar organization and decrease degradation and antigenicity.12, 13 Porosity and pore size are other features that can be changed depending upon design demand.
| Advantages | Limitations |
|---|---|
| Biocompatible | No inherent rigidity |
| Osteocompatible Minimal potential for antigenicity after removal of telopeptides | Potential for antigenicity through telopeptides |
| Adhesive | |
| Fibrous, cohesive, and nonfriable | |
| Suturable | |
| High porosity gives space for neohistogenesis | |
| Fibers appear to become incorporated into the new tissue matrix | |
| Can be combined with other materials (e.g., DBP) | |
| Medium can be perfused | |
| Fluid pressure can be transduced |
One tissue engineering approach entails cell-free scaffolds; another includes cells or tissues generated in vitro. In the former, the scaffold is used to define the space for new tissue in-growth and to enhance the maturation and function of tissue that has been directed to grow into the scaffold. To enhance the process, the scaffolds can be designed to attract specific cells by their composition (called “smart” or biomimetic materials) or by delivery of specific factors or genes.
Cell-based tissue engineering approaches entail seeding of a scaffold or carrier with cells that will be delivered to and retained within the lesion site. In some cases, the cell/scaffold construct will be precultured to generate the needed tissue and later transplanted to the needed location. These are expected to be tissue equivalents, living structures that will be incorporated and function normally, but there are concerns about the survival and endurance of many cell types.
TISSUE ENGINEERING FOR MUSCULOSKELETAL DEFICIENCIES
The motivation for tissue engineering is to promote biological repair or regeneration. The conventional treatments for musculoskeletal deficiencies, both congenital and acquired, are surgical reconstructions with fresh bone autogenous grafts, banked allogeneic tissue, or prosthetic devices, such as metallic hip and knee implants. On the one hand, bone tissue has remarkable regenerative capacity. Even in elders, fractures can heal with true regeneration of the bone. On the other hand, cartilage has very poor capacity for healing. The wear and tear of articular cartilage surfaces accounts for the aging of joints in osteoarthritis.
Articular cartilage lesions have shown the earliest applications for cell-based therapy. Other potential applications are for replacement of damaged intervertebral discs, knee menisci, anatomical disorders of the temporomandibular joint, and severe deformities of the midface and ears.14, 15 Cartilage is well-suited for tissue engineering because it lacks a blood supply, is nourished by diffusion, and has a low cell-to-matrix ratio. Although there is some organization of zones, it is composed of a single cell type, the chondrocyte. In 1997, FDA approval was issued for a process to repair focal cartilage lesions; in a first procedure, a biopsy from nonweight-bearing normal cartilage is obtained arthroscopically from the patient. The sample is sent to a commercial processing laboratory where cells are isolated, expanded, and returned to the surgeon for the second procedure, in which the lesion is covered with a fresh periosteal flap beneath which the suspension of cells is injected. When properly sealed, the periosteal flap contains the cells and they generate new cartilage.16, 17 No scaffold was involved in this procedure. Use of a scaffold could simplify the second surgery and enhance retention of the cells in the lesion.18
In spite of its regenerative capacity, bone tissue may need augmentation in cases of congenital deformities, of very large defects, or in individuals with conditions that limit healing, such as diabetes or poor nutrition. Bone tissue engineering seeks to augment or accelerate bone formation to meet functional demands.15, 19 To do so, there are additional demands beyond those for cartilage because bone is a more rigid tissue and is vascularized.
POROUS COLLAGEN SCAFFOLDS FOR HISTOGENESIS
In Vitro Histogenesis
Collagen has a number of advantages as a scaffold for cell-free and cell-based tissue repair (Table I). Porous collagen sponges made from pepsin-digested (i.e., telopeptide-free) bovine skin collagen were found to be biocompatible when implanted into subcutaneous pockets in normal rats, in contrast to sponges made in an identical manner from acid-soluble bovine skin collagen.11 Because sponges made from the telopeptide-containing collagens provoked an inflammatory reaction, albeit mild, they were not used in subsequent in vitro and in vivo studies to avoid concerns about compatibility with cells and regenerative processes.
Collagen sponges made from 0.5 wt% solutions, lyophilized to maintain a porous surface, and crosslinked by ultraviolet irradiation have interconnected pores suitable for cellular in-growth and histogenesis (Figure 1).11 They are highly porous (>95%) with pore diameters in the range of 120–200 μm. Without crosslinking, they became amorphous hydrogels when rehydrated, and cells could not migrate into them.11 The porous collagen sponges were first used to generate cartilage in vitro. Bovine articular chondrocytes (bACs) were used as an abundant cell source for assessment of the effects of other matrix, mechanical, and growth factors on chondrogenesis.20 It is likely that different tissue types require different conditions for optimal histogenesis in vitro. We have determined a number of parameters that enhance chondrogenesis by bACs (Table II). Most important, our studies showed the biocompatibility of bACs within the 3D sponges, their viability, and matrix accumulation, as assessed by cartilage-specific ELISAs.20, 21 Addition of a small amount of hyaluronan to the 3D scaffold was shown to enhance the amount of tissue formed and the expression of two cartilage-specific genes, aggrecan core protein and collagen II.22 For eventual clinical application, it would be desirable to avoid fetal bovine serum, which is commonly used for cell and tissue culture. We reported that chondrogenesis with the serum substitute, Nutridoma, was equivalent to control sponges in serum; addition of growth factors like TGF-β or IL-1α to the serum substitute significantly stimulated cartilage matrix.23 It is well known that in monolayer 2D culture, chondrocytes dedifferentiate to a less specialized fibroblastic phenotype and produce less cartilage ECM. When grown in 3D porous collagen sponges, however, production of cartilage-specific ECM20 and expression of chondrocyte genes (aggrecan core protein and collagen type II)24 were maintained with time, in contrast to the decreases seen in monolayer.

Porosity of collagen lattice sponges. Scanning electron micrograph; bar indicates 100 μm.
| Category | Example |
|---|---|
| Matrix additive | Hyaluronan |
| Serum substitute | Nutridoma |
| Growth factors | TGF-β, IL-1α |
| Incubator gas | Low 02 (2–5%) |
| Mechanical | Hydrostatic fluid pressure |
Porous collagen sponges were used for 3D culture of other cell types, including human marrow cells,25, 26 human oral keratinocytes,27 human fibroblasts,11 human osteoblastic cells,28 murine marrow,29 and murine osteosarcoma cells.30 Because porous collagen sponges lack rigidity that could be required for weight-bearing applications, composites can be developed with additives such as a bone-like calcium phosphate.31
One of the known limiting factors of traditional high-density 3D culture is that cells in the center of the mass do not survive because of poor exchange of nutrients and dissolved gases. To optimize conditions for engineering of different tissues, we developed a system to perfuse a medium through collagen sponges seeded with different cell types. Low flow (1.3 ml/min) perfusion of the medium through collagen sponges seeded with adherent (stromal) and nonadherent (hematopoietic) cells from mouse bone marrow resulted in greater cell viability and more hematopoiesis, in comparison with static cultures29 (Figure 2). Likewise, perfusion enhanced viability and osteogenesis by murine osteosarcoma cells30 and mucosa formation by oral keratinocytes.27 In sharp contrast to the positive effects on other cell types, perfusion of medium inhibited chondrocyte gene expression and matrix synthesis and accumulation,32 probably due to chondrocytes' preference for low pH and oxygen concentration. We tested that hypothesis by lowering oxygen tension in the perfused medium; there was significantly more cartilage matrix in sponges perfused with the medium at 5% O2 than with the medium at standard atmospheric 19% O2.33
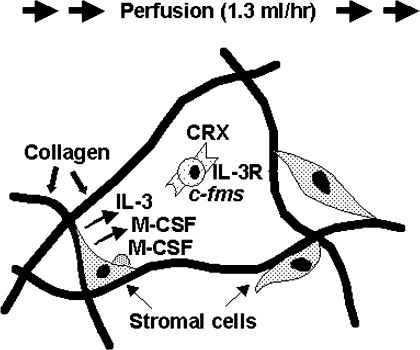
Schematic of the effect of perfusion on coculture of adherent murine marrow stroma and nonadherent hematopoietic progenitor cell, CRX, in collagen sponges. Increased viability of stromal cells increases their production of secreted IL-3 and M-CSF and surface expression of M-CSF. Hematopoietic progenitor CRX is dependent on IL-3 for its proliferation. CRX cells respond to IL-3 and M-CSF through their receptors, IL-3R and c-fms, respectively. Perfusion of medium increases hematopoiesis, i.e., proliferation of CRX blood cells in porous collagen sponges.
We also examined the effects of mechanical loading on chondrocytes in 3D collagen sponges at levels comparable to physiological loading of the knee.34 A bioreactor was designed to apply hydrostatic fluid pressure without deformation of the cells.34 Although perfusion of the medium inhibited chondrocyte gene expression and matrix synthesis and accumulation,32 simultaneous application of perfusion and hydrostatic fluid pressure at 2.8 MPa, either continuous or cyclic at 0.015 Hz tripled matrix accumulation.34 The stimulation by fluid pressure is evidence that the bioreactor transduces the pressure uniformly through the collagen sponge and the cells. With our validated automated, computer-controlled bioreactor,35 we also showed that cyclic (0.5 Hz) hydrostatic fluid pressure (0.5 MPa) enhanced proliferation of human dermal fibroblasts.36 In addition, constant hydrostatic fluid pressure (3.0 MPa) stimulated human osteoblastic cells to produce matrix and to increase expression of an osteoblast regulatory gene, IGF-I.28 Other evidence of mechanisms by which fluid pressure stimulates is provided by measurement of intracellular fluxes of calcium in a pressure-proof optical chamber.37 Application of 0.5 MPa increased intracellular calcium levels to a peak after 73 s; this was inhibited by drugs that blocked either stretch-activated membrane channels or cytosolic calcium storage release.
In Vivo Fate of Chondrocyte/Collagen Sponges
We have evaluated the in vivo fate of some tissues engineered with porous collagen sponges. As a model for bioengineered joint, we used porous collagen sponges as a scaffold for bovine articular chondrocytes to generate articular cartilage in a chimeric construct with a devitalized chick knee.38 Two sponges were used to resurface the femoral and tibial joint surfaces (Figure 3). First, the construct was maintained in vitro and evaluated at intervals. At 18 h after seeding, the bACs were evenly distributed throughout the collagen sponge, with some pericellular ECM accumulation. With time, cartilage matrix increased and began to invade the devitalized chick structures. Chondrogenesis was so robust in the collagen sponges that there was fusion across them, obliterating the joint space. In fact, in controls in which the collagen sponges did not contain chondrocytes, there was fusion of the empty collagen sponges; there was excellent adhesion of collagen to itself and to the underlying chick structures. To maintain the joint space, membranes of expanded polytetrafluoroethylene (ePTFE) were inserted between the opposing pairs of sponges. In a second study, chimeric constructs were transplanted into immunodeficient SCID mice.39 The bACs invaded the chick knees and obliterated the interface between the collagen sponge and the devitalized chick cartilage. The bovine neocartilage expanded and replaced the chick matrix. The devitalized chick knee provided a framework for supporting chondrogenesis. The porous collagen sponges were shown to be an effective delivery system for the viable chondrocytes. Because the host mouse was immunodeficient, this study does not address the issue of immunological acceptance of a chimeric construct, but these results support the clinical potential we imagine. A clinical example would be the use of devitalized, deantigenized porcine knee as a biological scaffold for seeding with an infant's chondrocytes for construction of a congenitally missing knee. It would be necessary to ensure that the porcine scaffold be completely cleansed of immunogenic or inflammatory material and that the construct could grow with the child.
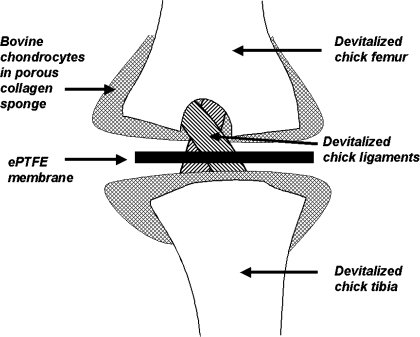
Composite chimeric joint. Two porous collagen sponges containing bovine chondrocytes are placed against shaved articular surfaces of femur and tibia of a devitalized embryonic chick knee. An ePTFE (expanded polytetrafluoroethylene) membrane is positioned to maintain the joint space.
Experimental studies with fluorochrome-labeled cells confirm that autogenous chondrocytes survive transfer even in scaffolds.40 This is likely because chondrocytes are inherently less affected by deprivation of oxygen and nutrients than are other cell types. Progenitor cells do not survive transfer as well as chondrocytes, but there are concerns about the fate of cells after transfer.40, 41 Even so, evidence suggests that recipient cells may populate the scaffold after transplanted cells produce neomatrix and/or secrete chemokines.42 This raises the possibility of identifying the factors to which local or circulating cells are attracted and applying that information for design of cell-free smart scaffolds. A proof of this principle is demonstrated by animal studies that indicate osteoclast recruitment to implants containing osteocalcin and hydroxyapatite.43, 44
POROUS COLLAGEN/DBP DEVICES FOR CHONDRO/OSTEOINDUCTION
Human Fibroblasts Differentiate to Chondrocytes When Cultured in Collagen/DBP Sponges
Demineralized bone was reported by Urist to have a remarkable ability to induce connective tissue cells within nonskeletal sites to form normal bone tissue.45 Reddi and Huggins described the sequential induction by demineralized bone, a process beginning with the development of cartilage-producing cells, followed by matrix calcification, vascular invasion, and replacement of the induced cartilage by normal bone with hematopoietic marrow.46 Clinically, decalcified autogenous implants have been used for spinal fusions,47 and surface-decalcified allogeneic cortical bone was used for intertransverse process fusion.48 Demineralized bone has been used in osseous reconstructive procedures by plastic and reconstructive, oral, periodontal, craniofacial, hand, and orthopedic surgeons [reviewed in 49]. Allogeneic demineralized bone has become widely available through regional tissue banks. On the basis of clinical investigations and experimental work in a number of animal models,49 we sought to establish an in vitro system that would model the mechanism, whereby demineralized bone powders (DBPs) stimulate connective tissue to form normal endochondral bone, a process termed osteoinduction. Because it was inefficient to sprinkle DBPs onto monolayers of target fibroblasts, we first tested candidate materials for compatibility with in vivo osteoinduction by DBP. Many materials inhibited bone formation in the subcutaneous test, but collagen was compatible (Table III). Lyophilization of a simple admixture of DBP and collagen resulted in uniform distribution of the powder, but the density of DBP was insufficient for a strong effect in vitro.50 A device with a packet of DBP deposited between two layers of porous collagen was more efficient to show chondroinduction with human dermal fibroblasts.11, 21, 51 Critical design elements include the porosity and compatibility of the collagen layers through which fibroblast migrate within hours. Fibroblasts that border on the DBP produce cartilage-specific collagen type II (Figure 4) and upregulate collagen type II and other cartilage-specific genes.11, 21, 51, 52 In contrast, fibroblasts cultured in plain collagen sponges did not produce collagen type II ECM (Figure 4). Finding that lowered oxygen (2–5% compared with the usual 19%) increased chondrogenesis by articular chondrocytes in plain collagen sponges,33 we also showed that low oxygen enhanced chondroinduction with fibroblasts.53 The culture device has also been used to study chondroinduction in other post-natal cell types, such as human gingival fibroblasts from elders and to identify the early gene regulatory events in chondroinduction.54, 55
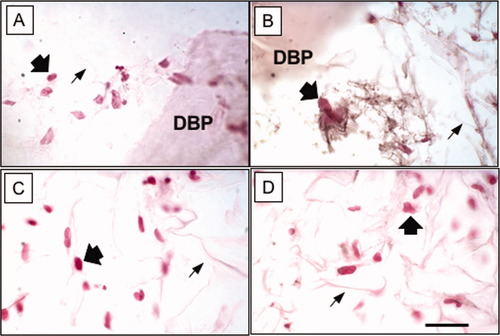
Immunohistochemistry of porous collagen sponges 10 days after seeding human dermal fibroblasts. Wide arrows indicate contra-red counter-stained nuclei. Thin arrows indicate lattice of the collagen type I sponge. DBP indicates a particle of demineralized bone powder. A) Staining control for a collagen/DBP sponge with no primary antibody. Cells were distributed in the lattice near the DBP. B) Immunolocalization of collagen type II appears as black fibrillar ECM around cells near the DBP. C) Staining control for a plain collagen sponge with no primary antibody. Cells were distributed in the lattice. D) Staining with antibody against collagen II showed no immunoreactivity around cells in plain collagen sponge. Polyclonal antibody against human collagen type II was obtained from Chemicon International. Immunoreactivity was detected with 3,3-diaminobenzidine and nickel as black precipitates. μm thick. The bar indicates 20 μm.
| Inhibition by | Osteogenesis with |
|---|---|
| Gelatin | Collagen fibers |
| Polymethylmethacrylate | Ceramic hydroxyapatite |
| Polylactic acid | Bioactive glass |
| Polysebacic acid | Bone particles (BPs) |
| Polycarboxyphenoxypropane | |
| Resorbable tricalcium phosphate |
In Vivo Responses to Collagen/DBP Sponges
An early design criteria for a DBP/polymer composite device was that it be osteoinductive in vivo. Evidence showed that the mild inflammation that occurred with the implantation of DBP devices containing intact collagen completely blocked induction, whereas pepsin-digested telopeptide-free collagen-containing devices were inductive.11 We concluded that infiltration of macrophages and granulocytes irreversibly blocked the migration of target connective tissues to the DBPs. Further, preclinical development of collagen/DBP implants entails scale-up to useful sizes and shapes, with the addition of radio-opaque and rigidifying components, and evaluation of cell-free and cell-seeded systems for cartilage or bone reconstruction.
FUTURE
One area of research focuses on cells with the potential to expand and differentiate to the needed lineage. Embryonic stem cells have those properties but may be too plastic or teratogenic for some applications. Various sources of autogenous cells from adults have some potential to expand in numbers and can be differentiated into different phenotypes; these sources include bone marrow, adipose tissue, dental pulp, and others. There are critical hurdles that need to be overcome for these approaches to become adopted for clinical indications. Sources of autogenous cells are limited and require a harvesting operation with enrichment and control of phenotype. FDA regulations and associated expenses are involved because of safety issues. Use of xenogeneic or allogeneic cells would require selective shattering of the immunogenicity barrier in transplantation. Tissues and organs with multiple cell types pose complexity on micro- and macro-levels of organization. Viability of transplanted cells and tissues requires that they become nourished by the host vascular system.
There remains great interest and potential for developing materials or composites that could be used without cells as implants to stimulate endogenous regeneration. Because of its many favorable properties, collagen may become useful alone, in combinations, or with modifications for promoting tissue and organ regeneration.
CONCLUSION
Many materials have been investigated and tested for use in cell-free and cell-seeded devices for stimulating tissue repair and regeneration. Collagen, as used in highly porous lattice sponges, has been shown to have high compatibility for supporting growth and function of many cell types. It can be manufactured into devices that are adhesive and can be sutured. Further, the high porosity provides volume for matrix accumulation. It appears that the fine collagen fibers can become incorporated into the expanding new tissue matrix. Evidence shows that bioreactors that apply medium perfusion or hydrostatic fluid pressure enhance histogenesis by cells in porous collagen sponges. Collagen sponges were used in combination with a chondro/osteo-inductive material.




