FOXO in aging: Did evolutionary diversification of FOXO function distract it from prolonging life?
Abstract
In this paper we contrast the simple role of FOXO in the seemingly non-aging Hydra with its more diversified function in multicellular eukaryotes that manifest aging and limited life spans. From this comparison we develop the concept that, whilst once devoted to life-prolonging cell-renewal (in Hydra), evolutionary accumulation of coupled functionality in FOXO has since ‘distracted’ it from this role. Seen in this light, aging may not be the direct cost of competing functions, such as reproduction or growth, but the result of a shift in emphasis in a protein, which is accompanied by advantages such as greater organismal complexity and adaptability, but also disadvantages such as reduced regeneration capacity. Studying the role of FOXO in non-aging organisms might, therefore, illuminate the path to extend life span in aging organisms.
Editor's suggested further reading in BioEssays Stem cells and aging from a quasi-immortal point of view Abstract
Introduction
Aging is obscure
According to Medawar 1, aging is defined as the collection of changes that render human beings progressively more likely to die. Whereas the causes of mortality are almost always evident, the causes of aging, in complete contrast, remain virtually obscure 2. The mutation accumulation theory 1 suggests that late in life, deleterious mutations start to accumulate because they evade the cutting blade of natural selection. The antagonistic pleiotropic theory 3 complements this by assuming that genes that are beneficial early in life, e.g. those that maximise reproductive success, are selected for then, only to prove detrimental later by facilitating a dialectic mechanism called aging. On the one hand, the aging process does prolong life; but on the other, it gradually increases the probability of us all confronting an unpredictable death. In nature, however, many organisms never reach old age 4, 5.
Aging-humans have been rapidly extending their life expectancies for the past century 6. 50% of children born after the year 2000 are currently expected to celebrate their 100th birthday 7. In simple language, this means that in the future most people will be spending most of their lives doing something they know almost nothing about: aging 8. This will have material, medical, social, mental and philosophical implications on the actual meaning and substance of life.
One of the problems in trying to understand aging is that it is a biological process with no definite target. It might therefore be either the positive or negative outcome of other processes 9 which we are doomed to find, or the stochastic outcome of many unrelated processes, which we might never know 10, 11.
Aging theories are biased by economic principles
Most aging theories have a subordinate economic factor in which time in a limited life span becomes a currency. Death is the price we pay for reproductive success at younger age (antagonistic pleiotropy), for wear and tear, for poor maintenance (mutation accumulation), or for making room for others (programmed/adaptive aging 12). Nevertheless, the main question seems to be far from being resolved: By aging, what are we actually paying for?
The cost of aging is assumed to be the result of various life history trade-offs between reproduction, growth and survival. Life history theory 13 predicts that change in one phenotypic trait, like life span, may occur together with change in another. Energy that is allocated to increase survival, thus extending life span, is thought to be excluded from being used for other processes, such as reproduction 14. Aging might therefore be the outcome of a complex balance among various and independent traits 15, 16. Consequently, interactions among genetic (intrinsic), environmental (extrinsic) and stochastic factors might play a role in driving the aging process. This complexity makes it unlikely to find a single cause for this deterioration process (a gene or a physiological attribute), and even more unlikely to find a single reason why this process accelerates with time in some organisms, but not in others. Although difficult to understand, the aging process may not rely entirely on the economic principles of resource limitation and cost. It might simply be the inability of a genetic network to function over time without upgrade (renewal), while performing rapidly evolving tasks.
A life with no aging
In order to better understand the process behind aging, it might be valuable to learn more about species that live a life without aging. One candidate is the freshwater polyp, Hydra 17. Hydra has been studied for over 300 years 18, 19, mainly for its profound ability to regenerate (for a review, see 20). It is a freshwater radial-symmetric predator (Phylum Cnidarian; Class Hydrozoa) which diverged from Anthozoans >540 million years ago, placing it at the basal root of animal life. In a pioneering study, Martínez 17 showed a constant low death rate in 145 Hydra vulgaris polyps kept for four years under laboratory conditions. This unprecedented finding forces us to reevaluate our aging theories 6. It joins many studies that demonstrate that life span is a plastic commodity.
This paper will attempt to evaluate the process of aging in light of a single playmaker, the transcription factor FOXO. Pioneering studies by Kenyon and others utilising the nematode C. elegans 21-25 (for a review, see 26) identified a single transcription factor, DAF-16, a forkhead box O (FOXO) homologue, whose activation downstream a mutated daf-2 [abnormal DAuer larva Formation-2; the C. elegans Insulin like Growth Factor-1 receptor (IGF-1)] was obligatory for extending life span.
FOXO mediates the extension of life span in aging organisms by enhanced maintenance of post-mitotic cells (for reviews, see 27-29). However, recent findings on the role of FOXO in non-senescent Hydra, underline its role in stem cell proliferation 30, 31, suggesting an evolutionary origin that mediates longevity by pathways committed to renewal and not to maintenance.
In this paper, we would like to present the idea that aging is the cost of coupling a ‘popular’ transcription factor (FOXO), originally committed to renewal (non-aging), to an increasing number of additional (maintenance) tasks that arose with the evolution of organisms with greater organisational, behavioural and life-history complexity.
Our argument is based on four observations that summarise two long decades of research into FOXO and its role as ‘the master regulator of longevity’ 32. The first is that DAF-16/FOXO transcription factors show a direct regulatory effect on hundreds of target genes 33-35, which suggests that FOXO has evolved a central role in many molecular pathways that are obligatorily coupled. The second observation is that, at least in some experiments, FOXO-mediated extension of life span proves to have either a zero or a negative cost. In such experiments, organisms live longer without compromising their reproductive output (zero cost) while enjoying enhanced health at a negative cost. The third observation is that in mutation-based experiments, the range and heterogeneity in life span within mutated cohorts also seems to expand. This phenomenon often occurs despite the fact that mutated individuals are genetic replicates maintained under the exact same laboratory conditions. It suggests that aging might be the result of a (stochastic) process that prevents FOXO from manifesting its full potential to elongate life. The fourth observation is just starting to emerge through the study of FOXO function in long-living and non-aging organisms 30, 31, 36-38. Such studies imply that FOXO-mediated cell renewal has evolved to perform other tasks related to maintenance, and that this shift results in aging.
FOXO in the aging model
FOXO has a direct effect on hundreds of coupled side- and downstream genes
In a study by Murphy et al. 33 that utilised a DNA microarray, 512 genes, mostly related to the ability of C. elegans to resist external stress, were found to be regulated by DAF-16 on a daf-2 mutation background. In a study by McCormick and colleagues 34, 230 target genes were found to be regulated by DAF-16 on the ablated germ cell line background. A Chip-Seq analysis of FOXO1 DNA binding sites in mouse liver 35 revealed 401 specific targets, of which over 100 were exclusive to promoter and flanking regions sites.
Another perspective, obtained by looking at all of the genes with a putative role in regulating life span, places FOXO at a regulatory crossroads 39. According to the GenAge database of aging-related genes (http://genomics.senescence.info/genes/), more than 1,000 genes regulate life span in model organisms 40-42. However, when groups of potential candidates are crossed 43, only a handful turn up, with FOXO topping these lists 39. In screens conducted on long-lived humans, single nucleotide polymorphic (SNPs) variants of FOXO link to longevity, however, with no direct evidence on their role in extending life 44-47.
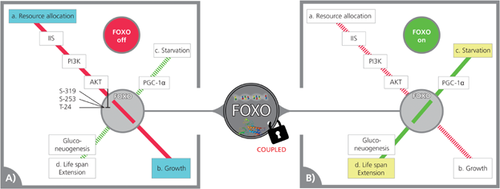
Like running multiple railway tracks through a grand junction, any genetic operating system might evolve junctions (nodes). Central junctions seem to evolve spontaneously. Their main advantages are control, efficiency and adaptability. Because two trains rarely pass through the same junction at the same time (control), you can use one junction for providing complex connections (efficiency) and for adding new connections (adaptability). But this is also a junction's greatest disadvantage – congestion. That is, many trains can be waiting, and any change made to one schedule naturally results in changes to all other coupled schedules. Another problem is that grand junctions rarely become smaller with time, but instead tend to get larger. FOXO's evolved multi-tasking might present a similar problem, as many connections passing through a central node may be coupled: i.e. functions cannot be executed simultaneously, thus resulting in congestion and delay. A good example of this principle is FOXO's role in mediating gluconeogenesis in mammalian hepatic cells following starvation 48, 49. Because this anabolic function contradicts insulin-like peptide response during resource allocation, FOXO is often silenced by triple phosphorylation in response to signals down the insulin/IGF1-like signalling (IIS) pathway 50 (for a simplified outline, see Fig. 1). However, in doing so, coupled FOXO-mediated extension of life span is also halted (Fig. 1A) and cannot be restored other than indirectly by starvation or IIS inhibition (Fig. 1B). This operational limitation induced by coupling has economic implications, which is the cost of running all pathways through a central node. In fact, however, this limitation has no economical foundation, since theoretically, many coupled pathways may function better independently of each other (i.e. on separate tracks). The coupling of a + b, resource allocation and growth through FOXO to c + d, and starvation and longevity (Fig. 1) does not necessarily constitute an obligatory ‘trade off’ between b and d (growth and longevity). This principle has been demonstrated by Kenyon et al. 21, who doubled C. elegans life span by uncoupling insulin signalling from life span extension in performing a brilliant, pioneering experiment. Nevertheless, in biological systems, coupling is often presented as trade off.
FOXO extends life at a negative cost
FOXO negative cost means that besides being involved in prolonging life, FOXO-mediated expression adds to good health regardless of whether this prolongs life directly or not. A good criterion for testing this hypothesis is the balance between reproduction and life span. At least in theory, aging is considered to be the price we pay for youthful agility 51, and the onset and progression of aging should correspond to decreased reproductive output 52. The first studies by Kenyon et al. 21, 26 demonstrated that brood size did not change in longer living nematodes, and that ablation of germ line precursor cells and somatic gonads in controls had no effect on life span 21. In D. melanogaster, the ablation of germ line cells in L3 larvae increased FOXO expression and insulin-like peptides, resulting in hypoglycemia and life span extension 53. Females with a mutation for differentiation of cytoblasts into oocytes lived shorter lives than controls, which suggests that the metabolic cost of oogenesis has no effect on life span 53. This uncoupling of life span extension from reproduction was also demonstrated in several other studies 54-56. In the fruit fly, the over-expression of FOXO in female pericerebral fat body prolongs life by suppressing a neuronal insulin-like peptide (DILP-2) at no reproductive cost 57, whereas its over-expression in the females fat body prolongs life with a negative effect on fertility. In long-living snell mice, the artificial restoration of reproductive output by injections of growth and thyroid hormones 58 did not reduce life span extension, and the deletion of insulin receptors in the adipose tissue of FIRKO mice showed an 18% extension of life span 54, with fertility unaffected. However, demographic studies that compared the fitness of C. elegans canonical longevity mutants age-1, daf-2 and clk-1 with the fitness of wild type worms 59-61, demonstrated that the lower fitness of mutants was due to their lower levels of reproductive output in early adulthood. A study by Dillin et al. 62 demonstrated that partial mutation of daf-2 resulted in the reported late onset of fertility, and that this late onset was due to elevated DAF-16 expression. Similarly, mutating daf-2 by RNA interference (RNAi) during worm adulthood demonstrated longer life span with no effect on reproductive output or timing. It resulted not only in a longer life span, but also in a higher tolerance for oxidative 63 and possibly thermal stress 64. According to Dillin et al. 62, life span extension in C. elegans is not aimed at generating longevity per se, but is instead a form of elevated fitness that allows L3 larvae to enter the dauer stage in response to starvation 65 and L4 larvae to postpone the onset of reproduction as they wait for food. A study by Anderson et al. 66 selected for early fecundity in a heterogeneous C. elegans population maintained over 47 generations. They found that early reproduction came at the cost of late reproduction, but not at the cost of life span, as predicted by antagonistic pleiotropy. The examples presented here support the assumption that life span-extension mechanisms mediated by FOXO are not necessarily traded-off for other functions, and may occur at zero or negative cost. Thus, accumulating damage or reproductive effort may not be the direct causes of aging, but merely its symptoms.
FOXO rarely manifests its full potential to extend life span
By utilising artificial mutations, FOXO decoupling becomes feasible. As a result, the life span of C. elegans is doubled on a daf-2 mutation background 21; it increases fourfold on a double daf-2/daf-12 mutation background 22, five- to sixfold by germ cell line ablation on a daf-2/daf-12 mutation background 67, and almost tenfold by an age-1 mutation/temperature combination 68. In these experiments, identically mutated individuals build up a ×50 life span range between those dying first and last, which demonstrates that FOXO's far-reaching life-extending powers are far from being equally distributed. One question remains: if life span extension occurs at a negative cost, why does FOXO so rarely manifest its full potential to elongate life?
FOXO functions to increase the heterogeneity in life span
FOXO genes are excellent prototypes to explain why it is that, even under constant conditions, heterogeneity in life span occurs. Post-transcriptional modifications (PTMs) support high variability of FOXO function 69, suggesting the existence of a FOXO code 70. For a review, see also 28. As the number of PTMs increases, the number of FOXO coupling patterns also increases. A good example for this principle is the interchange of FOXO-phosphorylations 71. It has been suggested that following oxidative stress, the FOXO-PI3K-AKT phosphorylation pathway is overridden by MST1 (mammalian Ste20-like-kinase)-mediated phosphorylation at Ser-207, which enables FOXO's re-entry into the nucleus to promote apoptosis 72. In contrast, phosphorylation of FOXO4 at Thr-447 and Thr-451 by Jun-N-terminal Kinase (JNK) activates it in response to oxidative stress, while overriding its deleterious apoptotic effects 73. It seems that PTMs function like ‘by-passes’ that are needed because FOXO is often inhibited by coupling, as shown above. The availability of four FOXO genes in mammals and their compartmentability to specific organs, including their autonomous and non-autonomous regulation, may even further enhance the variation in FOXO's life span-extending powers.
A study by Gems et al. 64 compared the maximum and median life span of C. elegans carrying 16 different daf-2 mutated alleles and incubated at 15 and 22.5 °C. Interestingly, a shift to higher temperatures resulted in a dramatic drop in median life span increase, which suggests that some temperature/mutation combinations may have deleterious effects on younger adults. For example, when the worms carrying the canonical daf-2 mutation (e1370) 21 were shifted from incubation at 15 °C to 22.5 °C, the average maximal life span increased from +150% at 15 °C to over +200% at 22.5 °C, compared to controls, while the median life span dropped from +140% at 15 °C to +110% at 22.5 °C, compared to controls. This implies that even within this cohort of isogenic long-lived worms, the life span of only half its members was changed through the introduction of the canonical mutation 64. An attempt to explain life span heterogeneity brings the concept of stochastics to the fore 4, 10, 11, 74, 75; i.e. that the accumulating sum of gene expression leading to life span extension is unpredictable. Following this concept, it has been shown that stochastic variability dominates muscle degeneration in aging isogenic C. elegans 76, 77. Another approach postulates that the evolutionary drive to extend life span by mutation increases the range of age at death among isogenic individuals, because it successfully affects only part of the population 78. It could also be true that life-ending mutations accumulate only in the short lived, while life span-extending mutations accumulate only in the very old, almost halting the process of aging 5. The concept of stochastic gene regulation 79 has recently been demonstrated in Saccharomyces cerevisiae. Similar to FOXO, the S. cerevisiae transcription factor MSN2 (Multicopy suppressor of SNF1 mutation 2) performs at a junction of pathways. Like FOXO, it is mostly repressed and cycles in and out of the nucleus in response to stress. According to Petrenko et al. 80, bursts of nucleic retention arise from signalling noise resulting in heterogeneous downstream transcription levels, which are observed in identical cells. Similarly, the contribution of FOXO to life span heterogeneity can be explained by its wiring plan; i.e. that identical stress signals result in stochastic bursts of activated genes downstream of foxo. The fact that equally complex organisms, such as rats and squirrels, evolved very different life spans (3 years for rats and 25 years for squirrels; 81), supports the hypothesis of a stochastic evolution of life span. Nevertheless, this heterogeneity in life span fails to explain why aging evolved. It also fails to explain why species, or even sub-populations, may display heterogeneous life spans, yet distinct aging patterns 82.
FOXO-mediated cell renewal has evolved to perform other tasks related to maintenance
The role of FOXO in prolonging life can be divided into two categories: maintenance of post-mitotic cells, tissue and organs to reduce wear and tear, and mediating soma to germ line transformation to promote renewal 37. In principle, the first category repairs damage that has already occurred, while the second prevents damage from occurring in the first place. It is often hard to determine which of these two mechanisms sustains life span extension and which results in aging. The functions of FOXO in mediating maintenance and stress response have been studied extensively 28, since they are linked to the treatment of age-related disease. Thus, we will not address these issues here. In contrast, very little is known about the mechanisms that mediate self-renewal. A pioneering study by Curran et al. 37 utilising IIS pathway longevity-mutants (daf-2 e1370; age-1 mg305) demonstrated a shift from soma to germ line function in C. elegans following uncoupled DAF-16 expression. In this study, two genes that are exclusively expressed in the immortal germ line (pgl-1 and its promoter pie-1) were expressed in hypodermal and intestinal somatic tissues of mutated dauers and late larval worms. This germ line-like activation was enabled by DAF-16 binding to sites located on the pie-1 promoter. This shift is unique because under constant feeding conditions the proliferation of C. elegans soma is completely extinguished.
Absence of aging in the Hydra model
Constant cell renewal
Hydra presents an interesting deviation from the life histories of human beings and from aging model organisms. Under laboratory conditions, it shows no signs of senescence in either reproduction or mortality 17. Hydra has a defined body plan and a simple nervous system. It consists of three active but separated stem cell communities 83: ectodermal epithelial stem cells, endodermal epithelial stem cells and interstitial stem cells. Hydra's epithelial and interstitial stem cells proliferate continuously and migrate either to the upper or lower body column, where they differentiate into foot and head soma. Alternatively, they also migrate into growing buds (= clonal reproduction). In addition, Hydra reproduces sexually. The multipotent interstitial stem cells differentiate not only into somatic cells (e.g. nematocysts and nerve cells), but also into gametes: eggs and sperm. Hydra, with its simple body plan of no more than 25 cell types, possesses a profound regenerative capacity. Hydra polyps fully regenerate even after being homogenised 19. It is hypothesised that maintaining continuous stem cell proliferation 84, 85 provides Hydra with a unique survival edge over competing organisms, while resulting in a lack of senescence 17. In contrast, growth in Hydra is limited to gametes and body extremities (head and foot) where differentiation occurs, which suggests that in Hydra, life span extension by renewal is spatially decoupled from terminal growth.
In Hydra FOXO is active exclusively in stem cells
Three recent studies on the role of FOXO in Hydra provided new insights into mechanisms that enable the extension of life span and non-aging. In a pioneering study by Bridge et al. 30, a single copy of the FOXO gene was identified in the non-senescent Hydra magnipapillata and its closely related species, H. vulgaris. FOXO expression was measured by whole mount in situ hybridisation and by utilising a H. vulgaris transgenic GFP-FOXO expression construct. This study found FOXO expression mainly in interstitial stem cells of the ectodermal body column. The results showed less expression in foot and head regions, in developing gametes and in epithelial stem cells. Additional findings verified that insulin signalling inhibited FOXO nuclear retention. FOXO expression also correlated positively with exposure to thermal stress and negatively with JNK-pathway inhibition, as was previously observed 86. These findings could not, however, verify the role of Hydra FOXO in sustaining non-senescence. Previous studies of Hydra identified an insulin receptor homologue 87, including three insulin-like peptides 88. A study by Lasi et al. 89 reported that ectopic expression of FOXO caused massive apoptosis in Hydra epithelial stem cells, and that this apoptotic wave could be inhibited by the co-expression of Hydra Proinsulin-1. In Hydra, apoptosis is a conserved function and may play a key role in maintaining body plan while partaking in cell recycling and defence against pathogens 90-92. The study by Bridge et al. 30 verified FOXO involvement mainly in cell maintenance, but not in inducing apoptosis.
A recent study by Hemmrich et al. 38 identified FOXO expression in all H. vulgaris stem cell lines based on 454-transcriptome sequencing. A subsequent study by Boehm et al. 31 confirmed these results, demonstrating a direct correlation between the over-expression of FOXO-GFP and elevated proliferation of interstitial stem cells and interstitial nematoblast precursors. The authors utilised confocal microscopy to identify FOXO-GFP in both the nucleus and the cytoplasm of interstitial and ectodermal stem cell lines. They found decreasing expression in the differentiated head, the basal disc, and in gametes, as previously demonstrated by Bridge et al. 30. As a result of FOXO over-expression, elevated cell numbers were observed. The study by Boehm et al. 31 clearly demonstrated FOXO's contribution to stem cell maintenance by showing that a gene associated with stem cell activity, cniwi, was expressed only in nematoblast precursors carrying the FOXO-GFP construct and in their terminally differentiated nematocysts (stenoteles and isorhizas). In contrast, a foxo knockout in epithelial stem cell lines (ecto and endodermal), resulted in enhanced growth of terminal head and foot and in impaired budding rates. This finding supports the results of a study by Chevalier and colleagues 36 on FOXO expression in the related hydrozoan species Clytia hemisphaerica. In this study, FOXO expression was detected at all stages of the polyp/medusa life cycle, although C. hemisphaerica polyps display negligent senescence and the more complex medusa did not 93. In their study on Hydra, Boehm et al. 31 demonstrated that genes associated with stem cell activity, cniwi and cnvas1, were also down regulated in epithelial stem cells carrying the foxo knockout. This study is the first to show Hydra as a model organism in which FOXO activity is constitutive, spatially confined, and exclusively associated with ongoing stem cell proliferation and renewal.
The role of FOXO in stem cell maintenance is conserved
In mice, FOXO-deficient haematopoietic stem cells (HSCs) show poor transplant potential 94, defective long-term populating, apoptosis and increased cell cycling 95, 96. A study by Paik et al. 97 reports high expression levels of FOXO1 and FOXO3 in the multipotent progenitors (Sox2+) of the mouse forebrain sub-ventricular zone (SVZ) and in the hippocampus sub-granular zone (SGZ). Deletion of foxo1 and foxo3 resulted in increased weight and brain size combined with an increase in cell cycle re-entry. Interestingly, in the young adult brain, FOXOs were found to constrain the proliferative activity of neural stem cells (NSCs) and/or of progenitor cells 98. This mechanism was found to be related to the effect of FOXO on Wnt signalling 97. However, as time went on, FOXO1, FOXO3 and FOXO4 null NSC cultures exhibited decreased proliferation and marginally increased apoptosis, which led to observed renewal defects and a progressive decline in total NSC number 94-97. The study of Hydra foxo mutants 31 did not identify a pleiotropic effect on stem cells, or the enhanced Wnt signalling observed in mammalian cells. Wnt signalling has been extensively studied in Hydra. Activated Wnt signalling in Hydra results in terminal differentiation of nematoblasts present in the gastric region 99. A similar phenotype was also observed in Hydra foxo mutants in which the differentiation of epithelial stem cells was observed 31, suggesting a potentially similar antagonistic role for Hydra FOXO in counterbalancing Wnt signalling. Interestingly, a study by Liu et al. 100 demonstrated accelerated aging due to cellular senescence in Klotho-protein-deficient mice, where Wnt signalling was not curtailed.
In Hydra FOXO renewal is naturally uncoupled
Having come this far, it would be highly intriguing to explore whether foxo-mutated Hydra not only demonstrate a morphological shift towards growth and proliferative retardation 31, but also the actual onset of senescence. The findings by Boehm et al. 31 suggest that FOXO has been preserved in non-aging Hydra since early metazoan life. Although crosstalk between FOXO and insulin signalling has been observed 30, this was not shown to impose any limitation on constitutive cell proliferation of laboratory polyps. These findings support the assumption that FOXO in Hydra is uncoupled from growth and sexual reproduction, and that Hydra continuously extends its life span by segregating FOXO expression away from head, foot and gametes, where no FOXO expression is detected.
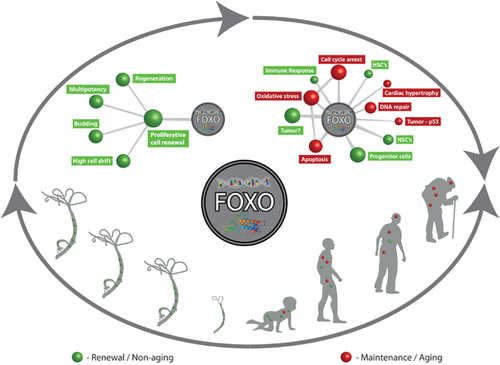
Figure 2 presents a simplified model of this view. In non-aging Hydra, FOXO is constitutively involved in mediating the proliferative renewal of stem cell lines (Fig. 2, left), which includes parallel regeneration, mutipotency, asexual reproduction and the maintenance of body plan by high cell drift. In contrast, in aging organisms (human; Fig. 2, right), FOXO position at a central axis initiates the coupling of many pathways. Some pathways cause its inhibition, while others (Fig. 2, right) distract it from its original role in mediating constitutive renewal. This is when aging begins. With time and increasing cellular complexity, we postulate that FOXO erodes its capacity to extend life by its commitment to increasing maintenance tasks (Fig. 2; red). Thus, a dichotomous shift towards maintenance becomes counter-productive with time. This is because the decreasing investment in renewal results in an escalating demand for maintenance and damage repair. In the Hydra model, in contrast, constant cell-renewal results in non-aging, longevity at no cost and very low variability in life span under optimised laboratory conditions.
Conclusions and outlook
The focus of this paper has been on understanding aging by examining its antagonist: FOXO – the master of longevity. In this paper, we assert the claim that during evolution, FOXO's life span-extending capacity (mediating renewal) diversified into mediating multiple other pathways related to maintenance 28 that are coupled but can no longer be executed constitutively and simultaneously. FOXO's place at a regulatory crossroads of possibly hundreds of target genes supports the idea that coupling might be the direct cause of aging.
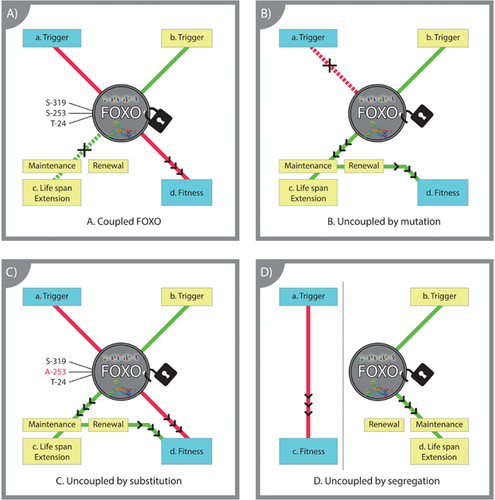
Figure 3 presents a simplified model of our view, in which FOXO is restrained from prolonging life by coupling to pathways involved in optimising reproductive fitness early in life (Fig. 3A). Mutating the IIS pathway to uncouple FOXO's life span-extending capacity has been a focus of most FOXO studies (Fig. 3B). Further uncoupling can also be achieved by mutating FOXO target sites for inhibition (Fig. 3C), such as by substituting its phosphorylation-prone Serine-253 with Alanine-253 101. Finally, uncoupling naturally occurs in Hydra, in which the constitutive life-extending mechanism is diverted away from growth and sexual reproduction (Fig. 3D), thus enabling FOXO to manifest its full potential to elongate life.
When we look back on two decades of FOXO research, it is encouraging to observe that numerous pioneers of aging studies have identified the concept of extending life span through uncoupling 26, 62, 102-104.
We suggest that aging is the cost that coupling exerts on a given genetic operating system. Coupling, interlocking, and junction overload are common in computational operating systems. Interestingly, in computational systems, hardware ages very fast when evolving tasks accumulate. Unless renewed, hardware often becomes redundant, even before physical damage occurs. Aging in biological systems may reflect some of these ‘wiring’ problems and aging organisms may utilize operating systems that carry distinct ‘built-in’ aging components.
While it is tempting to conclude that organismal complexity is the single driver behind the evolution of aging organisms, this may not be the case. Some types of genetic hardware perform complex maneuvers without aging, as shown for Hydra. In organisms, such as C. elegans, aging and non-aging could be alternated. Here, too, stochastic and environmental conditions could influence how aging patterns evolved. Future studies will definitely shed new light on the evolution of genetic hardware, organismal complexity and their influence on aging. In Hydra, e.g., gene deletion and the incorporation of transposable elements might have played a key role in shaping its genomic evolution 105.
In the future, uncoupling may become a key principle for extending human life span, and a tool for curing the ailments of the aged. Studying the role of FOXO in non-aging organisms might, therefore, help to illuminate this path.
Acknowledgements
We would like to thank Janek Pilzecker for his help creating the figures and Felix Ringelhan for his critical comments on figures. We thank the editor and two referees for constructive comments and suggestions. The project was funded by the Max Planck Institute for Demographic Research in Rostock, Germany.