Multiple actions of Lucilia sericata larvae in hard-to-heal wounds
Larval secretions contain molecules that accelerate wound healing, reduce chronic inflammation and inhibit bacterial infection
Abstract
In Europe ≈15,000 patients receive larval therapy for wound treatment annually. Over the past few years, clinical studies have demonstrated the success of larvae of Lucilia sericata as debridement agents. This is based on a combination of physical and biochemical actions. Laboratory investigations have advanced our understanding of the biochemical mechanisms underlying the beneficial effects of larval secretions, including removal of dead tissue, reduction of the bacterial burden, and promotion of tissue regeneration. The present article summarizes our current understanding of the microbiological, immunological, and wound healing actions of larval therapy, and the molecules involved in these beneficial effects. Future studies will focus on the isolation, identification, and (pre)clinical testing of the effective molecules of L. sericata larvae. These molecules may be candidates for the development of new agents for the treatment of several infectious and inflammatory diseases, including chronic wounds.
Abbreviations
-
- bFGF
-
- basic fibroblast growth factor
-
- fMLP
-
- N-formyl-methionine-leucine-phenylalanine
-
- IL
-
- interleukin
-
- LPS
-
- lipopolysaccharide
-
- LTA
-
- lipoteichoic acid
-
- MDT
-
- maggot debridement therapy
-
- MIF
-
- macrophage inhibitory factor
-
- MIP
-
- macrophage inflammatory protein
-
- PDGF
-
- platelet-derived growth factor
-
- TIME
-
- tissue, infection/inflammation, moisture imbalance, wound edge
-
- TNF
-
- tumor necrosis factor
-
- tPA
-
- tissue-type plasminogen activator
-
- uPA
-
- urokinase-type plasminogen activator
-
- VEGF
-
- vascular endothelial growth factor
Introduction
Larval therapy displays important benefits in the management of chronic, infected wounds, and is used in hundreds of clinics worldwide 1-3. Since ancient times, people were aware that larvae of certain Dipteran flies, e.g. the green-bottle fly Lucilia sericata, cleaned and disinfected wounds. Evidence for the use of larvae to heal wounds has been found on paintings from tribes of the Mayas in Central America and aboriginals in Australia and the first written description of beneficial effects of larvae was from Baron D.J. Larrey, inspector-general of the medical department of Napoleon's army 4, 5. Further observations of the beneficial effects of larvae were communicated by the Confederate Army surgeons J. Joseph and J.D. Zacharias, the latter intentionally introduced larvae into wounds for debridement 6. In 1929, William Baer, a consultant orthopaedic surgeon at the Johns Hopkins Hospital in Baltimore, reported that for the treatment of children with osteomyelitis, larvae of L. sericata could (1) rapidly debride, (2) reduce bacterial counts, and (3) decrease odor and alkalinization of the wound surface 7. However, the reports of Baer were only transiently noticed due to the discovery of penicillin by Alexander Fleming in 1928 8. Larval therapy became obsolete when industrially produced penicillin was introduced in clinics by the early 1940s. However, due to a rise in antibiotic resistance and failure of modern wound care to heal many chronic, infected wounds, medicinal larvae were reintroduced in the late 1980s 9. The US Food and Drug Administration approved Maggot Debridement Therapy (MDT) (510(k) #33391) in 2004 and described the indication for MDT as follows: “For debriding non-healing necrotic skin and soft tissue wounds, including pressure ulcers, venous stasis ulcers, neuropathic foot ulcers, and non-healing traumatic of post surgical wounds.” (http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/classification.cfm?ID=5372). The debridement efficacy of MDT has been proven in randomized clinical studies, although there is no evidence in these studies that larvae influence the time to wound closure 10, 11. However, many other beneficial effects of larvae and their secretions on wounds have been observed. While wound debridement by larvae has been extensively reviewed by others 12, 13, this review focuses on the potential other beneficial effects of larvae and their secretions, such as antibacterial effects, reduced inflammation, neo-angiogenesis and improved wound healing 14-20.
Larvae influence wound bed preparation
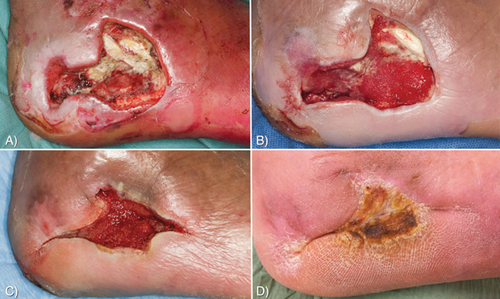
An example of a patient receiving larval therapy is presented in Fig. 1. The photographs show the foot of a 48-year-old male patient with diabetes, who suffered a burn wound caused by a heating stove. An X-ray also showed osteomyelitis of the calcaneus. The wound did not exhibit a tendency to heal despite intensive (antibiotic) therapy for two months. A characteristic debris, resulting from chronic inflammation, was also present on the wound bed (Fig. 1A). After two applications of larvae, all debris was removed (Fig. 1B) and after four applications the wound progressed (Fig. 1C) and healed completely within four months (Fig. 1D). An X-ray also showed that the osteomyelitis had been cleared.
Obviously, wound conditions in patients vary a lot and not a single case is identical or comparable. To describe the wound situation of individual patients in the context of their underlying diseases, and to provide the basis for the removal of barriers to healing, the holistic clinical concept of wound bed preparation was developed over ten years ago 21. The definition of wound bed preparation, described in the article by Schultz et al. is: “the management of the wound to accelerate endogenous healing or to facilitate the effectiveness of other therapeutic measures.” The TIME acronym was introduced to facilitate the understanding of wound bed preparation. It stands for Tissue (non-viable), Infection or inflammation, Moisture imbalance, and wound Edge (EWMA Position Document. 2004. Wound bed preparation in practice. http://ewma.org/fileadmin/user_upload/EWMA/pdf/Position_Documents/2004/pos_doc_English_final_04.pdf). Recently, the TIME concept was reviewed by Leaper et al. 22 who concluded that, although there are many new developments in the field of wound healing, the basic concept is still extremely relevant.
Larval therapy is thought to have an effect on at least three of these components: it removes non-viable tissue effectively, it helps combat infection by reducing the bioburden, and it may facilitate the remodeling processes. As a consequence of these effects, moisture balance might also be normalized, as excessive exudation is often caused by infection, slough, and dead tissue on the wound surface, and tissue fluid resulting from inflammation 21.
Larval therapy is considered a suitable treatment modality to remove barriers to healing and prepare chronic wounds either for spontaneous healing or further interventions. Medicinal larvae are available as free-range, which are suitable for cavity wounds with undermined edges, and as so-called bagged larvae where they are contained in a sealed net dressing. The netting allows for free flow of larval secretions and the physical removal of solubilized eschar and slough, on which they feed. Medicinal larvae can stay on the wound for up to four days and the application of fresh larvae is recommended, should one treatment cycle not result in a clean and well-granulated wound bed.
Larvae of L. sericata are widely applied to treat chronic, infected wounds 1-3, 23-25. In Europe, ≈15,000 patients are treated annually. Despite clinical observations of beneficial effects of larvae in wound treatment since ancient times, their mechanisms of action are only recently becoming better understood. Investigations into the biological mechanisms that underlie the clinical effects of larval therapy have led to the identification and isolation of several molecules that exhibit proteolytic, antimicrobial, and growth-promoting activities 26-30. The scope of this review is to obtain insight into our current understanding of the microbiological, immunological, and wound healing actions of larval therapy and the identification of the molecules involved in these actions.
Cellular and biochemical processes regulate physiological wound healing
The physiological process of wound healing consists of three stages: inflammation, tissue proliferation, and tissue remodeling 31. Wounding immediately initiates various processes including clot formation to stop loss of fluids, and an inflammatory response. During the inflammatory phase, which normally lasts five days, complement components, cytokines, chemokines, enzymes, extracellular matrix proteins and growth factors, as well as inflammatory cells, including polymorphonuclear neutrophils, arrive at the injury site 32. Production of growth factors and chemokines by macrophages stimulates angiogenesis and attracts cells that are necessary for the next phase of wound healing: the proliferative phase. This phase may start a day or two after wounding and can last for a maximum of three weeks, during which time new stromal tissue, called granulation tissue, is formed. This process involves the migration of endothelial cells to the injury site and the accumulation of fibroblasts 31. The fibroblasts contribute collagen on the wound bed, and secrete the principal components of the extracellular matrix, such as fibronectin and hyaluron. Simultaneously, neovascularization occurs, while part of the fibrin clots and extracellular matrix are degraded. Besides endothelial cells and fibroblasts, granulation tissue comprises blood vessels, inflammatory cells, and myofibroblasts that cover the wound bed during the inflammatory and proliferative phase 31, 33. Myofibroblasts cause wound contraction and reduce the size of the wounded area. At the end of the second phase, granulation tissue is fully replaced by collagen and epithelial cells migrating onto the wound area. The epithelial cells degrade the remaining fibrin clots and extracellular matrix and once the wound surface is covered by keratinocytes, they begin to secrete proteins that form a new basement membrane. During this remodeling phase of the wound, redundant cells die by apoptosis and collagen is remodeled and re-aligned 31, 32. The remodeling phase can last up to two years.
How is wound healing disturbed in chronic wounds?
Prolonged inflammation or infection, and imbalance of moisture and/or deleterious composition of wound fluid can lead to impaired healing 31. As a result of these disturbances, chronic wounds are often characterized by slough and necrosis (debris) on the wound bed. Persistent influx of neutrophils into the wound bed associated with an elevated level of proteolytic activity, and unbalanced oxidant/antioxidant levels cause damage of the surrounding tissue rather than repair 31. Effective debridement, e.g. by larvae and their secretions, is thus essential to facilitate and continue the process of wound healing 33. In addition, larvae and their secretions affect the inflammatory and the wound healing processes 14, 15, 19, 20, 30. These combined actions may establish an optimal wound bed preparation.
Which beneficial actions do Lucilia sericata larvae and their secretions show in vitro?
Larvae produce antibacterial factors
The presence of a potential antibacterial entity in the elimination products of larvae was first described in the 1930s 34. Numerous studies have since investigated the antibacterial activity in larval secretions against both Gram-positive and Gram-negative bacteria, with inconsistent findings. While some studies have revealed that larvae and their secretions are poorly effective against Staphylococcus aureus and even less effective against Gram-negative bacteria, such as Pseudomonas aeruginosa and Acinetobacter baumannii 35, 36, others have identified antimicrobial molecules within larvae and their secretions 16, 17, 27, 28, 37-40. The reasons for this apparent discrepancy are not entirely clear, but may include different methods to collect larval secretions 41, the type of assay 39, 41, and bacterial species 37 to detect the antimicrobial effects, as well as the use of different concentrations of secretions 42. For example, Bexfield et al. 39 collected secretions from sterile larvae and applied them in different types of antibacterial activity assays. The zone of inhibition assay did not allow for the detection of any antibacterial activity within larval secretions, whereas a turbidometric assay assessing bacterial growth demonstrated significant antibacterial activity against a number of bacterial species including S. aureus and Escherichia coli. However, no effect of larval secretions on S. aureus and P. aeruginosa survival has been seen using in vitro killing assays 36 as well as MIC assays 35. P. aeruginosa was even shown to be toxic to L. sericata 43. Of note, Mumcuoglu et al. 44 have shown that larvae can ingest fluorescent bacteria, and subsequently the amount of fluorescent bacteria was reduced in their gastrointestinal tract, suggesting that the bacteria were destroyed. In contrast, Daeschlein et al. 45 assessed the ability of larvae to ingest and excrete bacteria and found that within 48 hours viable bacteria could still be found in the gut of larvae. Furthermore, it has recently been reported that the depletion of bacterial quorum-sensing-controlled virulence genes results in an increased uptake of bacteria by larvae 43.
Several antimicrobial molecules have been isolated from L. sericata in the last few years 16, 17, 27, 28, 37-41. For example, a study on the structural characterization and antimicrobial activities of compounds released externally by L. sericata revealed the presence of a diversity of antimicrobial compounds 46. These compounds were categorized into two groups: polypeptides (between 6,466 and 9,025 Da) and small molecules (between 130 and 700 Da). Whilst some of these molecules corresponded well to known insect antimicrobial peptides, such as lucifensin 37, 38 and MAMP 40, other antibacterial molecules present in the secretions had no clear homology to existing analogues, and therefore warranted further investigation. Recently, a <500 Da fraction of larval secretions was shown to be active against many pathogenic strains of bacteria (Staphylococcus sp., Bacillus sp., E. coli, Pseudomonas sp., Proteus sp., Enterococcus sp., Enterobacter sp., etc.), and 12 out of 15 clinical isolates of MRSA 16. The mass and empirical formula of this active antibacterial agent has been accurately determined as C10H16N6O9, and the molecule is patented and registered as a novel antibiotic, Seraticin® 47. The molecular structure of Seraticin® is currently being investigated allowing for chemical synthesis. The mode of action, minimal inhibitory concentrations and determination of molecular target(s) are also currently being studied (Nigam Y. et al., unpublished). Thus, the presence of antibacterial molecules present in the secretions of L. sericata has now been universally accepted. For convenience, the antibacterial effects within larvae are summarized in Table 1.
| Microorganism | Molecule | Effect | Reference |
|---|---|---|---|
| Planktonic bacteria | |||
| Micrococcus luteus, Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Enterococcus spp. and Enterobacter spp. | Lucifencin, MAMP, and seraticin | Inhibits bacterial multiplication | Cerovsky et al. 38; Zhang et al. 40; Bexfield et al. 39 |
| Bacterial biofilm formation | |||
| Staphylococcus aureus, Pseudomonas aeruginosa | Not identified | Inhibition | Cazander et al. 35; Van der Plas et al. 36 |
| Staphylococcus aureus, Staphylococcus epidermidis | Chymotrypsin | Inhibition | Harris et al. 56 |
| Break down bacterial biofilm | |||
| Staphylococcus aureus, Pseudomonas aeruginosa | Not identified | Degradation | Cazander et al. 35; Van der Plas et al. 36 |
| Staphylococcus epidermidis | Chymotrypsin | Degradation | Harris et al. 56 |
| Pseudomonas aeruginosa | DNase | Degradation | Brown et al. 58 |
| Combination with antibiotics | |||
| Daptomycin against biofilm-derived Staphylococcus aureus | Not identified | Enhances antimicrobial effect | Van der Plas et al. 54 |
| Gentamicin and flucloxacilin against Staphylococcus aureus | Not identified | Enhances antimicrobial effect | Cazander et al. 55 |
Can antibacterial activity in larvae be induced?
Initial studies suggested that expression of antibacterial molecules present in larval secretions may be inducible rather than constitutive 17, 48. For example, a three- to six-fold increase in antibacterial activity was found with larvae removed from chronic wounds as compared to sterile larvae 17. In agreement, Kawabata et al. 48 showed that infected larvae had greater antibacterial capacities than sterile larvae. These researchers argued that the clinical wound situation would enable larvae in their infected environment to influence the production of their antibacterial activities. However, this hypothesis has not been confirmed in a clinical trial 10. The inducibility of the antibacterial activity in larvae needs to be further investigated in future research.
Larval secretions reduce bacterial biofilms
Adherent bacteria in wounds may form microcolonies that produce a tough and protective layer called a biofilm. Biofilm-associated infections are notoriously difficult to treat; many topical treatments are ineffective and antibiotics often fail to destroy bacteria in the biofilm 49. It has been hypothesized that bacterial biofilms play a major role in wound colonization and infection 50. In agreement, 60% of chronic wound specimens taken from 77 subjects were shown to contain a biofilm, as opposed to 6% of specimens taken from acute wounds 51. Nowadays, it is widely accepted that bacterial biofilms contribute to the chronicity of wounds 22, 49.
In this connection, several researchers have shown that larval secretions disrupt established biofilm 51-53. Moreover, it was found that larval secretions not only break down established biofilms, but also prevent the biofilm formation on abiotic surfaces (polyethylene, surgical stainless steel, and titanium) 52, 53 and biotic surfaces, e.g. dermal pig skin explants 49. Larval secretions degraded biofilms of P. aeruginosa at 10-fold higher concentrations than the effective concentration used to degrade S. aureus biofilms 54. Together, larval secretions display anti-biofilm activities (Table 1), resulting in the release of bacteria from the biofilm thus allowing the bacterial cells exposure to the actions of the immune systems and antibiotics. In this connection, it has been demonstrated that larval secretions do not affect the antibacterial action of many antibiotics and that at high concentrations secretions enhanced the action of several antibiotics, such as daptomycin, gentamicin, and flucloxacillin 54, 55.
Recently, chymotrypsin derived from larval secretions was found to be responsible for the disruption of protein-dependent bacterial biofilm formation mechanisms, and the greatest effect of recombinant chymotrypsin was seen on nascent and established biofilms of S. epidermidis 5179-R1 56. Furthermore, chymotrypsin degrades macromolecules in venous leg ulcer slough, and this activity persists in an environment with intrinsic gelatinase activity, such as a (chronic) wound 57. Besides, anti-biofilm activities may be mediated by a DNAse, which is contained in larval secretions. Of note, both DNA from slough/eschar of venous leg ulcers and bacterial DNA was digested by the purified DNAse 58.
To further verify the potential inducible nature of the expression of molecules with antibiofilm activities within L. sericata secretions, researchers have recently demonstrated that externalized secretions collected from larvae pre-treated with bacteria, dose-dependently prevented the formation, and initiated the breakdown of P. aeruginosa biofilm 59.
To conclude, it appears that larval secretions contain at least two different molecules, chymotrypsin and DNAse, that are able to prevent bacterial biofilm formation and break down established biofilms.
Larval secretions inhibit inflammatory processes
The complement system is part of the innate immune defense, and complement activation plays an important role in the activation of the inflammatory response to injury. Invading organisms or tissue injury activate the complement cascades via three pathways: the classical pathway, the alternative pathway and the lectin pathway 60, 61. The result of activation of any of these pathways is cleavage of central factor C3 into C3a and C3b by C3 convertase. Finally the terminal pathway of the complement system with factors C5b to C9 is reached. These factors form the membrane attack complexes, which form pores in the microbial wall resulting in cell lysis 62, 63.
While complement activation is needed for the tissue healing process, inappropriate complement activation can result in prolonged inflammation and can therefore cause injury and contribute to further tissue damage 60-63. Clinical studies show enhanced levels of complement activating factors in chronic wounds 64-67, and it has been shown that animals with a genetic complement deficiency, or individuals treated with a complement inhibitor, are protected from the symptoms resulting from chronic inflammatory processes 68-71.
Improved healing of chronic, inflamed, or infected wounds is observed when larval therapy is used in clinical practice. Based on the consideration that overactivation of the complement system impairs the wound healing process, it was hypothesized that larval secretions interfered with complement components. An in vitro study investigated the effect of larval secretions on final complement activation in healthy and post-operatively obtained donor sera 15. The principal finding was that all tested secretions clearly reduced complement activation in healthy and immune-activated sera. A dose-dependent correlation between the protein concentrations of secretions and their complement activation reducing effect was shown 15.
The mechanism underlying this observation most probably involves breakdown of individual complement components 15. These findings may explain part of the improved wound healing during larval application and, furthermore, could provide new insights into the role of complement activation in the development and maintenance of chronic wounds. Ultimately, the complement-inhibitor(s) present in larval secretions, that are currently being isolated, could provide a novel treatment modality for diseases, resulting from an (over)active complement system, e.g. (chronic) infections, ischemic-reperfusion injury, and severe inflammatory response syndrome.
Regarding the effect of larvae and their secretions on the cells of the inflammatory response, such as neutrophils, monocytes, and macrophages, Van der Plas et al. focused on the production and release of enzymes, i.e. elastase, and reactive oxygen intermediates, such as hydrogen peroxide, generated by human neutrophils. These products induce tissue damage and thus enhance the inflammatory response 14. The results revealed that larval secretions dose-dependently reduced the production and release of elastase and hydrogen peroxide by neutrophils in response to chemotactic and activating stimuli 14. Moreover, secretions also inhibited the fMLP-induced chemotaxis of neutrophils, indicating that larval secretions may reduce the influx of these inflammatory cells into the sites of infections 14. It was found that secretions did not affect the phagocytosis and intracellular killing of S. aureus and Candida albicans by human neutrophils and monocytes 14, 30. In addition to clearing micro-organisms from infected sites, monocytes and macrophages produce an array of factors mediating the inflammatory response as well as healing the wound. Larval secretions dose-dependently reduced the production of pro-inflammatory cytokines, such as IL-12, TNF-alpha, and MIF, by monocytes and pro-inflammatory macrophages, while enhancing the production of anti-inflammatory cytokines, such as IL-10 14, 30.
In summary, larval secretions break down several complement components which result in a decrease of complement activation 15. Furthermore, secretions reduce multiple neutrophil pro-inflammatory responses 14. The various immunomodulatory effects of larval secretions are summarized in Table 2.
| Inflammatory component | Molecule | Effect | Reference |
|---|---|---|---|
| Complement system | |||
| Classical, alternative, and lectin pathways | Not identified | Inhibition | Cazander et al. 15 |
| Neutrophils | |||
| FMLP-induced chemotaxis, elastase release, and hydrogen peroxide production | Not identified | Inhibition | Van der Plas et al. 14 |
| Monocytes | |||
| LPS cytokine production | Not identified | Increase in IL-8, IL-10, MCP; no effect on IL-1β, Il-6; decrease in IL-12, TNFα, MIF | Van der Plas et al. 29 |
| LTA cytokine production | Not identified | Decrease in IL-12, TNFα, increase in IL-10 | Van der Plas et al. 29 |
| Monocyte-macrophage differentiation | Not identified | Decrease in LPS-stimulated IL-12, TNFα, MIF, MIP-1β, and RANTES; increase in IL-6, IL-8, MCP-1; no effect on IL-10 by type 1 and 2 macrophages; increase in bFGF and VEGF, but not PDGF, by type 2, but not type 1, macrophages | Van der Plas et al.30 |
| Keratinocytes, fibroblasts | Serine proteinase | Enhances fibroblast migration | Horobin et al. 20, 72 |
| Angiogenesis | Small organic compounds, e.g L-histidine, L-valinol, and 3-guanidino-propionic acid | Enhances vascular endothelial cell migration | Bexfield et al. 18 |
| Eschar | Chymotrypsin | Degrades wound eschar | Telford et al. 26 and Pritchard et al. 57 |
| DNase | Degrades DNA associated with eschar | Brown et al. 58 | |
Larval secretions affect fibroblast migration, angiogenesis, and growth factor production
Fibroblasts migrate from the edge of the wound and from the dermis to the wound bed to promote healthy tissue formation. Serine protease activity within larval secretions has a significant effect on the motogenesis of fibroblasts in two-dimensional and three-dimensional cultures, and on keratinocytes 20, 72-75. In addition, it has recently been shown that larval secretions may exert pro-angiogenic effects by activating a key signaling pathway involving PI3K and AKT1 76. It is possible that this signal pathway is also linked to the induction of motogenesis of fibroblasts, given the observation of increased phosphotyrosine expression at the migrating cell front during ex vivo wound healing. Wang et al. 76 showed that human microvascular epidermal cells, which were exposed to larval secretions, significantly increased their migration via the PI3K:AKT1 protein kinase pathway, and that P13K itself was activated by many pro-angiogenic factors. The authors suggest that these observed positive wound healing effects, clinically demonstrable in a wound treated with larvae, may be due, in part, to their role in the activation of this pathway.
In addition, amino acids L-histidine, 3-guanidinopropionic acid, and L-valinol, were recently identified within larval secretions, each exhibiting significant pro-angiogenic effects on a human endothelial cell line 18. Valinol in particular, caused an increase in cell density by 25% after a 48-hour exposure. None of the three amino acids stimulated fibroplasia, confirming that these components did not affect fibroblast growth or motility, but were specifically acting to promote the growth of blood cells 18. Other researchers have also reported the pro-angiogenic activity of larval secretions. Zhang et al. 19 used dried extracts of L. sericata larvae on dermal excision wounds, and showed a significant increase in the up-regulation of VEGF expression (VEGFA mRNA and VEGFA protein expression) after three days of treatment. In agreement, Van der Plas et al. 30 found that larval secretions enhanced the production of growth factors, such as VEGF and bFGF, by monocytes and macrophages. Other researchers too, report that larval secretions enhance the expression of bFGF in ulcers 77. Growth factors in combination with low levels of pro-inflammatory cytokines such as TNF-alpha, are involved in endothelial cell migration and proliferation, which are essential for angiogenesis 78. It appears, therefore, that larval secretions stimulate fibroblast and keratinocyte migration 20, 72, exert pro-angiogenic effects 18, 78, and increase the production of growth factors 19, 30, 78.
Larval secretions influence matrix composition and turn-over
Immediately after injury the coagulation system is activated to stop hemorrhage. Subsequently the fibrinolytic system is triggered to break down clots and other extracellular matrices. Experiments have revealed that blood clot formation is not affected by larval secretions, whereas the secretions enhance the conversion of plasminogen by urokinase-type plasminogen activator (uPA) and tissue-type plasminogen activator (tPA) into plasmin, which degrades fibrin deposits (Van der Plas MJA, Andersen AS, Nazir S, van Tilburg NH, et al. 2013, unpublished).
In wounds, migrating fibroblasts create a provisional matrix on the wound bed, comprising fibronectin and hyaluron as principal components 31, 32. This provisional matrix is essential for cell migration over the wound bed in a balanced wound. However, in a chronic wound, this matrix may be partially degraded by proteolytic enzymes derived from inflammatory cells such as neutrophils and macrophages 31. Unfortunately, the remaining fibrin deposits (slough) no longer support cell migration and granulation tissue formation and even inhibit these cellular responses. Moreover, slough is a rich source for bacteria. Obviously, molecules within larval secretions such as chymotrypsin 26, 57 together with the enhancing effect of sericase on fibrinolytic system in chronic wounds may promote wound healing by removal of slough.
Bioactive molecules from larvae are being isolated for future therapy
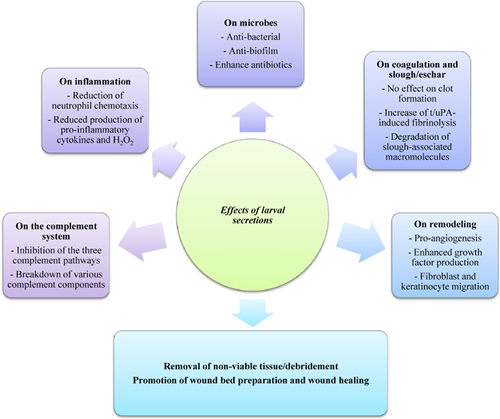
Larvae are successfully used to treat necrotic, sloughly, and infected wounds, either as free-range larvae or larvae contained in a biobag (success ranges from 80% to over 95% in wounds that failed to heal with conventional treatments) 79, 80. Unfortunately, therapy with live larvae is sometimes hampered by the so-called “yuk factor” 81 and the acceptance could be improved by the use of recombinant or synthetically produced bioactive molecules from the larvae and/or larval secretions 82. Of course, possible systemic effects of therapy with such larval derived molecules should be investigated in order to establish whether adverse effects occur. Importantly, no severe side effects, especially no immune reactions, have been reported in relation to larval therapy so far 83. The beneficial effects of larval therapy beyond debridement, in particular the antiseptic, anti-inflammatory and wound healing effects (Fig. 2), should be investigated in clinical trials. Since larval secretions contain many components that may affect the molecules and cells responsible for the poor healing of chronic wounds, isolation, and characterization of the various bio-active molecules is complex. Nevertheless several active components have been identified by others and ourselves (Tables 1 and 2), but many other molecules with beneficial activities within larval secretions still need to be identified and characterized 14, 15, 18, 52, 53, 84. For this purpose, various chromatographic techniques and mass spectrometry, recombinant technology, and synthetic chemistry combined with functional assays have been successful. These strategies should also be instrumental in the identification of other bioactive components within larval secretions. In this connection, we are currently identifying the molecules in larval secretions with antibiofilm activity and those inhibiting the complement pathways. To aid in the identification and production of these potentially therapeutical agents within larval secretions, the transcriptome and genome of L. sericata should be available. Next generation sequencing could produce these data for medicinal larvae. Recently, researchers have reported the transcriptome of L. sericata 85 and this may be helpful in the identification of bioactive proteins within larval secretions. In addition, performing BLAST between the transcriptome and the reads and contigs of the whole body genome of L. sericata should show that all genes expressed in the transcriptome align with a match in the genome. This information assists in de novo assembly of L. sericata's genome.
Conclusions
Clinical studies (RCTs) have proven the value of larvae as debridement agents 10, 11, while several other beneficial effects of larvae on wounds, including anti-infection, immunomodulation, angiogenesis, and tissue remodeling and regeneration, have been widely reported clinically and supported by numerous in vitro studies 14-20. Several molecules have been isolated from L. sericata larval secretions, such as lucifensin 37, MAMP 40, Seraticin® 47, DNAse 58, chymotrypsin 26, 78, 86, sericase (Van der Plas MJA, Andersen AS, Nazir S, van Tilburg NH, et al., 2013, unpublished) and other bioactive compounds 18, 75. Furthermore, larval secretions contain at least two different molecules that are able to prevent bacterial biofilm formation and break down established biofilms 56, 58. In addition, several molecules within larval secretions decrease complement activation by breaking down individual complement components 15. In terms of wound healing abilities, larval secretions enhance the production of growth factors by monocytes and macrophages in the wound 30. We conclude that together, the actions of these molecules and most likely several others in larval secretions are responsible for the beneficial effects of larval therapy (Fig. 2).
Why do larvae produce molecules that promote healing of hard-to-heal wounds? Larvae secrete an array of enzymes to digest dead tissue and it may well be that these enzymes also break down various host molecules within the wound. In addition, larvae may produce antibacterial molecules to survive in an environment heavily contaminated by bacteria that compete with the larvae for their nutritional source. Moreover, the bacteria may be harmful for larvae 43. By reducing the bacterial load, larvae may not only control the process of decay, but also protect themselves against pathogenic bacteria. In addition, larval survival in wounds may depend on their mechanisms to suppress the host's immune responses, which may itself be detrimental to larvae.
In order to promote larval therapy, to produce therapeutic agents from larval secretions for medical purposes, and to stimulate research into the mechanisms underlying the beneficial actions of larval therapy, the authors joined forces during the first meeting of the European Larval Therapy Research Group earlier this year. This review is the first product from this initiative. Currently, the European Larval Therapy Research Group is focusing on the effective molecules of L. sericata larvae that could be used for treatment of several infectious and inflammatory diseases, including chronic wounds.
Conflict of interest
One of the authors, Dr. W. Jung, works for Biomonde GmbH, Barsbüttel, Germany, a production company of larval products. None of the other authors has any potential conflict of interest related to this manuscript.