Effect of 40 Hz Magnetic Field Application in Posttraumatic Muscular Atrophy Development on Muscle Mass and Contractions in Rats
Conflict of interest: None.
Abstract
Muscle atrophy refers to the deterioration of muscle tissue due to a long-term decrease in muscle function. In the present study, we simulated rectus femoris muscle atrophy experimentally and investigated the effect of pulsed electromagnetic field (PEMF) application on the atrophy development through muscle mass, maximal contraction force, and contraction–relaxation time. A quadriceps tendon rupture with a total tenotomy was created on the rats’ hind limbs, inhibiting knee extension for 6 weeks, and this restriction of the movement led to the development of disuse atrophy, while the control group underwent no surgery. The operated and control groups were divided into subgroups according to PEMF application (1.5 mT for 45 days) or no PEMF. All groups were sacrificed after 6 weeks and had their entire rectus femoris removed. To measure the contraction force, the muscles were placed in an organ bath connected to a transducer. As a result of the atrophy, muscle mass and strength were reduced in the operated group, while no muscle mass loss was observed in the operated PEMF group. Furthermore, measurements of single, incomplete and full tetanic contraction force and contraction time (CT) did not change significantly in the operated group that received the PEMF application. The PEMF application prevented atrophy resulting from 6 weeks of immobility, according to the contraction parameters. The effects of PEMF on contraction force and CT provide a basis for further studies in which PEMF is investigated as a noninvasive therapy for disuse atrophy development. © 2022 Bioelectromagnetics Society.
INTRODUCTION
Skeletal muscle atrophy refers to the deterioration in muscle size due to a loss of organelles or proteins in cells [Bonaldo and Sandri, 2013]. Muscle atrophy may develop after a trauma or due to such diseases as Diabetes mellitus, cancer, nonhereditary immunological disorder syndrome, sepsis, and chronic preventive pulmonary disease [Herridge et al., 2011; Bonaldo and Sandri, 2013; Cornwell et al., 2014; Yuan et al., 2015; Perry et al., 2016]. Literature has identified four broad areas of muscle-wasting disease, including atrophy caused by denervation, atrophy from disuse due to immobilization, atrophy from low mechanical stress associated with prolonged bed rest and space travel, and cachexia caused by chronic disease. Under all of these different conditions, atrophy is characterized by reduced muscle mass, narrowed muscle fiber cross-sectional area, and decreased strength with increased activity of various protein degradation pathways. It has also been hypothesized that atrophy can result typically from a state of inactivity resulting from diminished autogenous reflex activation [McLachlan, 1981; Bialek et al., 2011; Morales et al., 2016].
Previous studies have shown pulsed electromagnetic fields (PEMF) to have benefits in muscle disease recovery, e.g. inducing muscle regeneration in crush injuries [Cheon et al., 2012], promoting tendon-bone healing [Tucker et al., 2017], and increasing the rate of nerve regeneration [Mert et al., 2006; Beck-Broichsitter et al., 2014]. Although the contribution of PEMF to recovery from musculoskeletal disorders has been shown, its effect on immobilization-induced atrophy formation is unknown. The aim of the present study, therefore, is to investigate the effects of in vivo PEMF application on the development of disuse atrophy in rats. To this end, we experimentally modeled a quadriceps tendon rupture (QTR) with a total tenotomy operation (full-fold cut of the quadriceps tendons) on the rats, thereby blocking the use of the quadriceps and stimulating disuse atrophy. We then assessed whether the application of PEMF during recovery could prevent the development of atrophy, and examined any changes in the structural and functional parameters of the rectus femoris muscle to assess the effects of PEMF application on disuse atrophy as a non-invasive treatment approach.
MATERIALS AND METHODS
Animals (Wistar albino rats) were kept in a controlled room (stable climate and light/dark conditions) with free access to food and water during the experimental procedures. Tenotomy and PEMF application procedures were approved and performed in accordance with Cukurova University Local Ethics Committee on Animal Experiments standards (Approval date/no: 2018/4-09).
Total Tenotomy Operation
Included in the study were 48 rats (200–250 g body mass, 3 months old), all of whom were anesthetized prior to surgery with an intraperitoneal injection of pentobarbital sodium (1 ml/kg). A single subcutaneous dose of cefazolin (Iespor; Ulugay, Istanbul, Turkey) was administered (15 mg/kg) for surgical infection prophylaxis. For postoperative analgesia, acetylsalicylic acid (Coraspin; Bayer, İstanbul, Turkey) (100 mg/kg) was administered orally for 3 days.
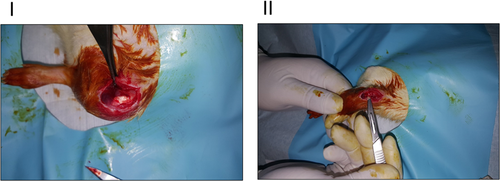
After the rats had received the appropriate infection prophylaxis and anesthesia, their right hind limb knees (surgery site) were cleaned and disinfected with povidone-iodine and the necessary sterile dressing was applied by using sterile disposable surgical drapes. The skins of the rats were passed through an approximately 1 cm long anterior long incision from the anterior of the right knee (Fig. 1). After reaching the quadriceps tendon, a dissection and full-thickness tenotomy were performed, and the connection of the quadriceps tendon to the medial and lateral retinaculum of the knee was thus terminated. The surgical field was cleaned with sterile saline solution, and subcutaneous and skin incisions were sutured with 4.0 Vicryl (Ethicon; J&J, Istanbul, Turkey). The surgical wound area was cleaned and the wound was closed with a bandage. After the operation, the animals were given free access to food and used their forelimbs for locomotion.
PEMF System and PEMF Application Protocol
After the total tenotomy operation, the rats were randomly divided into two groups with equal numbers, and PEMF was applied to one of the groups (PEMF group). The PEMF system and application protocol have been previously described [Mert et al., 2006]. Briefly, the PEMF system (ILFA Electronics, Adana, Turkey) comprises a power supply and Helmholtz coils (HCs) (coils are 60 cm in diameter and 30 cm apart, generating a 1.5 mT magnetic field), which were placed inside the Faraday cage (Fig. 2). The PEMF application protocol was set according to those adopted in previous studies in our laboratory [Mert et al., 2010; Gunay and Mert, 2011]. Briefly, magnetic field pulses were applied in three sequences, and each sequence consisted of four consecutive pulse trains of 240 s duration with a time interval between each pulse train of 60 s. The rats in the PEMF group were placed in 3 mm thick, 30 cm × 30 cm x 20 cm dimension transparent plexiglass cages and a magnetic field was applied per day for 1 h (40 Hz) for 6 weeks. The cage temperature (23–25 °C) and room humidity (40%–60%) were frequently monitored and kept stable during applications. The control group was also placed in the Faraday cage for the indicated time, but no PEMF was administered.

Tissue Preparations
At the end of the 6-week experimental period, the rats were fully anesthetized with pentobarbital sodium (50 mg/kg body mass, IP) and sacrificed by draining blood from the heart. The rectus femoris muscles were isolated by rapid and continuous treatment with a Krebs-Henseleit solution. The isolated skeletal muscle preparations were placed in the organ bath and fastened with a black braided silk suture from the distal and proximal parts. To achieve the isometric contraction parameters, the distal parts of the muscles were fixed downwards while the proximal parts were attached to the hook at the end of the transducer, allowing the tension to be adjusted using a micrometer in combination with a transducer [Struck et al., 2011].
Biomechanical Measurements
Each skeletal muscle was held under normal tension and placed vertically in an organ bath in contact with two platinum electrodes. The skeletal muscles were kept in the organ bath for 30 min. until the system had achieved thermoregulation and equilibrium. Subsequently, the muscles were simultaneously stimulated supramaximally with 0.5 ms duration and 0.05 Hz square frequencies (15–20 V) in order to obtain single (twitch) isometric contractions. Summation, incomplete tetanic, and full tetanic contraction forces were recorded upon stimulation with stimuli of 0.5 ms (15–20 V) and frequencies of 20, 50, and 100 Hz, respectively. After each recording, the organ bath solution was changed to remove contraction residue and create a fresh medium. The muscles were then kept in the organ bath for 15–20 min. until equilibrium was regained. For the stimulation and recording of the isometric contractions, a force-displacement transducer (FDT 10-A 500 g; Commat, Ankara, Turkey), stimulator (STPT02-A; Commat), a research organ bath, circulator (WBC 3044; Commat), and data acquisition system (Mp30; Biopac Systems, Goleta, CA) were used in the functional experiments. The specific muscle isometric contraction force (gram force unit), contraction time (CT), 80% relaxation time (RT), and tetanic contraction force were defined as the contraction parameters [Tastekin et al., 2018]. Since the force-generating potential of a muscle also depends on muscle mass, force data were expressed in terms of muscle mass (Force [g]/mass [g]) [Cameron et al., 1990].
Statistics
Data were presented as mean ± SEM. A one-way analysis of variance (multiple comparisons performed using Tukey's post hoc test) on GraphPad Prism 5.0 (San Diego, CA, USA) was used to identify statistical significance (at P < 0.05).
RESULTS
In the present study, the rats were subjected to a total tenotomy on the quadratus femoris tendon to inhibit knee extension and to block hind limb mobility, and the resulting muscle disuse during the recovery period led to the development of atrophy. To examine the effects of PEMF on the experimentally formed disuse atrophy, the rectus femoris was removed from the animals and muscle parameters and functions were analyzed at the end of the 6-week immobility period.
To this end, the muscle morphologies of the excised muscles (Fig. 3) were compared according to their size (surface area) and dry mass (Table 1), with a significant loss of muscle mass and surface area in the operated group indicating the development of muscle atrophy. However, in the operated group treated with PEMF (operated + PEMF), no difference in muscle mass and muscle size was measured compared to the control group. According to these two parameters, operated + PEMF groups showed no morphological signs of atrophy development.
| Control | Control + PMF | Operated | Operated + PEMF | |
|---|---|---|---|---|
| Muscle mass (g) | 0.62 ± 0.04 | 0.65** ± 0.03 | 0.43* ± 0.01 | 0.56** ± 0.02 |
| Surface area (mm2) | 631.3 ± 9 | 641.7** ± 22.1 | 536.5* ± 15.1 | 601.7** ± 18.5 |
- Note: Each group data presented as mean ± SEM, n = 6–8 animals/group. *demonstrates the difference as compared to the Control group (*P < 0.05), and **demonstrates significant differences as compared to the Operated group (**P < 0.05) by using ANOVA with Tukey test.
- Abbreviations: ANOVA = analysis of variance; PEMF = pulsed electromagnetic fields.
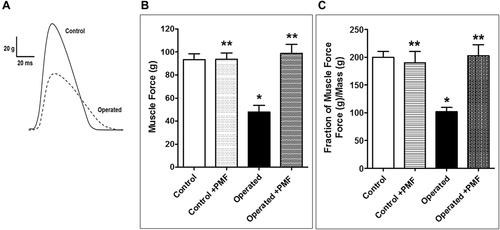
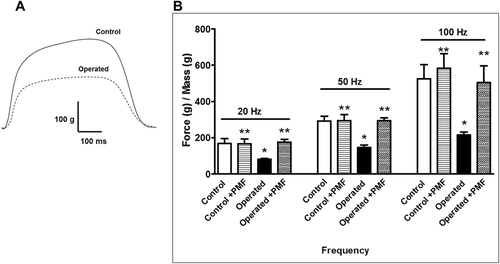
Isometric contractions were measured to test whether the functional responses reflected atrophy (Fig. 4B). In the operated group, reduced twitch tensions (47.9 ± 5.8 g) were recorded when compared to the control group (93.5 ± 5.1 g), while the operated + PEMF group showed increased tension (98.8 ± 7.8 g) according to the operated group. The application of PEMF to healthy rats (control + PEMF) revealed no significant difference in twitch force (93.8 ± 5.5 g). As shown in Figure 4C, the twitch tensions of each muscle were normalized by its mass (contraction force per gram of muscle mass). These findings suggest that PEMF application hinders immobility-induced weakness in both muscle fiber density and contraction force.
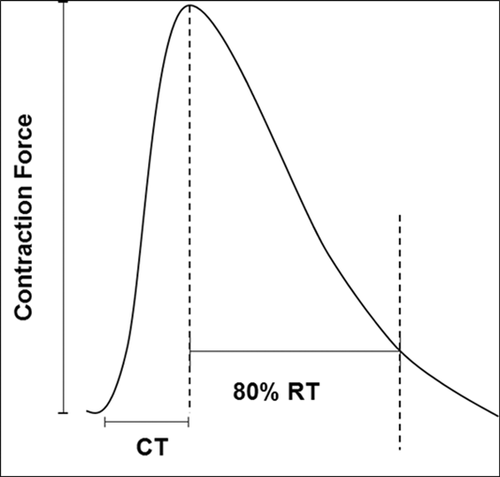
The twitch parameters for each group were examined in terms of contraction time (CT) and 80% relaxation time (RT), and the results are presented in Table 2 (the representative trace in Fig. 5 shows CT and RT). There was no significant variation in RT between the groups, although the operated group had an increased CT when compared to the control group, while the CT of the operated + PEMF group did not differ from that of the control group.
| Control | Control + PEMF | Operated | Operated + PEMF | |
|---|---|---|---|---|
| CT (ms) | 24.2 ± 0.6 | 24.4** ± 0.5 | 27* ± 0.2 | 25.5** ± 0.2 |
| RT (ms) | 62.6 ± 1.9 | 56.8 ± 5.9 | 65 ± 2.1 | 63.8 ± 0.7 |
- Note: Each group data presented as mean ± SEM, n = 6–8 animals/group. *demonstrates the difference as compared to the Control group (*P < 0.05), and **demonstrates significant differences as compared to the Operated group (**P < 0.05) by using ANOVA with Tukey test.
- Abbreviations: ANOVA = analysis of variance; CT = contraction time; PEMF = pulsed electromagnetic fields; RT = relaxation time.
For the study, the rectus femoris was stimulated at frequencies of 20, 50, and 100 Hz, in summation (at 20 Hz), incomplete tetanic (at 50 Hz), and full tetanic contractions (at 100 Hz) were examined. In addition, each tension was normalized with the mass of the muscle (Fig. 6). The muscular atrophy (operated) group recorded decreased responses to all stimuli when compared to the control group, as follows: At 20 Hz, control: 167.6 ± 26.7 g, operated: 81.2 ± 5 g, operated + PEMF: 176.1 ± 1 g, control + PEMF: 167 ± 26.6 g; at 50 Hz, control 293 ± 25.5 g, operated: 146.3 ± 1 g, operated + PEMF: 293.6 ± 1 g, control + PEMF: 294.6 ± 3 g, and t 100 Hz stimulation, control: 525.8 ± 7 g, operated: 214.8 ± 16.7 g, operated + PEMF: 504.6 ± 9 g, control + PEMF: 585.5 ± 8 g. The summation, incomplete tetanic, and full tetanic contractions of the operated + PEMF group showed no statistically significant difference from those of the control group, suggesting that the application of PEMF eliminated the effect of disuse atrophy at increased stimulation frequencies.
DISCUSSION
In this study, we simulated the development of atrophy in rats via QTR. The rats underwent total-tenotomy operations and were then maintained for 6 weeks for convalescence. During this recovery period, the animals were unable to use their right hind limb, and throughout this period, PEMF was applied to the assigned group and the effect of PEMF on the development of posttraumatic quadriceps muscular atrophy was examined.
QTR is a rare but potentially debilitating injury that affects two distinct patient populations. Most ruptures occur in older individuals over the age of 60, often after a prediagnosed medical condition such as obesity, diabetes, gout, or chronic renal failure [Aprin and Broukhim, 1985; Kaneko et al., 1994; Kannus and Natri, 1997]. In this population, age-related reduced elasticity, impairments of collagen fibers and inadequate vascular supply might play an important role in the condition [Kannus and Natri, 1997; Kelly et al., 1984]. In the second group, QTR usually develops in the final stage of chronic inflammation of the extension mechanism and tends to occur in young athletes [De Baere et al., 2002].
Immobilization due to QTR is an important cause of disuse atrophy [Ciriello et al., 2012], and long-term unloading and disuse are the most common causes. Muscle loses 30% of its volume after 90–120 days of non-use, and muscular atrophy begins within a few days of non-use, and muscle will lose 3% of its volume over the next 7 days. With the resulting atrophy, the reduction in contraction forces is more pronounced than the loss in muscle volume [Alkner and Tesch, 2004]. In a previous study, the knee extensor torque of a patient had decreased by 14.8% after 14 days, and by 21.0% after 23 days. The average rate of loss of strength was 1.06% over the first 14 days and 0.68% over the following 9 days [de Boer et al., 2007].
Although the development time of atrophy and rates of strength loss depends on the type of organism, muscle mass loss can be considered an important indicator of atrophy [Baek et al., 2020]. In the present study, the disuse atrophy model was formed on the hind limbs of the rats and the effect of PEMF on disuse atrophy development was examined by comparing the mass and the force production of the muscles.
PEMF therapy is a commonly used non-invasive treatment [Rawe et al., 2012] for the inducement of wound healing and enhanced bone repair [Guo et al., 2012]. A clinical study reported that the PEMF applications could prevent such postoperative symptoms as pain [Stocchero et al., 2015]. PEMF applications have been incorporated into the treatment protocols of many diseases in different clinical fields, including fracture healing, degenerative diseases of the musculoskeletal system, muscle spasms, denervated muscles, neurological diseases, and circulatory disorders [Canapp, 2007; Sutbeyaz et al., 2009].
The effect of postoperative PEMF application on disuse atrophy in skeletal muscle, however, is unknown. One of the main findings of the present study is that PEMF applications significantly reduce the loss of muscle mass due to muscle immobility, and it is demonstrated for the first time herein that PEMF reduces the development of disuse muscle atrophy. While atrophy is mainly due to an imbalance in protein production and the degradation of muscle, the inhibition of mass loss through PEMF could be attributed to the interaction of the magnetic field with protein synthesis [Blank, 2008]. PEMF has also been shown previously to support myoblast differentiation and proliferation [Xu et al., 2016; Liu et al., 2017] and to reduce muscle atrophy in diabetes mellitus by increasing protein synthesis and decreasing its degradation [Yang et al., 2018]. Chang and Lien [1994] reported atrophy formation in denervated muscles to be delayed by magnetic stimulation and demonstrated that magnetic field applications for 30 days delayed the decrease in muscle mass depending on the fiber type. The study revealed further that magnetic fields delayed atrophy, particularly in type II fibers (fast-twitch). It is known that the rectus femoris contains a higher percentage of type II fibers than the other muscle groups in the quadriceps femoris [Johnson et al., 1973; Heinonen et al., 2012]. In addition to the other metabolic differences between these fiber types, type II fibers have previously been shown to contain an abundance of dihydropyridine receptors (DHPR) [Manttari and Jarvilehto, 2005], which play a key role in skeletal muscle contractions while acting as inducers of Ca2+ release from the sarcoplasmic reticulum for excitation-contraction coupling (ECC) [Rios and Brum, 1987].
In the present study, a decrease in the amplitudes of both single (twitches) and multi-frequency stimulations (summation, incomplete tetanic, and full tetanic) and an increase in CT was observed that paralleled the development of atrophy.
The amplitude and CT parameters of contractions are related to the regulation of the contractile apparatus, and thus to calcium [Berridge et al., 2003]. Although the mechanism behind changes in the twitch durations associated with atrophy is unknown, it has been suggested that atrophy may be involved in the altering of Ca2+ signaling [Hyatt and Powers, 2020] or the handling of Ca2+ by Ca2+ binding proteins [Fitts et al., 1986; Weiss et al., 2010].
No change in the force or duration of contractions was observed in the operated + PEMF group when compared to the control group. The differences in CT between the operated and operated + PEMF groups suggest that PEMF may act through the contractile apparatus and thus through Ca2+ regulatory pathways.
Coletti et al. [2007] reported that a static magnetic field (SMF) promoted hypertrophy through the increased formation of actin and myosin in the myogenic cell line L6. Although has to date been no detailed study on the effect of PEMF on the contractile apparatus in literature, there is a broad knowledge of its effect on Ca2+. Wu et al. [2018] showed that a 50 Hz magnetic field increased the phosphorylation of CaV1.2, a subunit of the L-type Ca2+ channel (LTCC) in PC12 cells. Ullrich and Apell, on the other hand, claimed that PEMF targets the voltage-gated anion channel (VDAC), being a pore-forming protein that is also localized in the mitochondria [Massa et al., 2000], and that releases Ca2+ through the cytoplasm [Ullrich and Apell, 2021]. It has also been speculated that extremely low frequency (ELF) magnetic fields (50 Hz, 0.8 mT) can induce cytoplasmic Ca2+ uptake [Zhang et al., 2010]. Walleczek and Budinger examined the effect of PEMF on 45Ca2+ uptake in lymphocytes and found that although no effect of PEMF was observed in resting control cells, the 45Ca2+uptake in mitogen-treated cells was affected by PEMF. Their results suggest that the effect of PEMF depends on the cellular activation conditions of the cells [Walleczek and Budinger, 1992]. The impact of magnetic fields on Ca2+ dynamics was also demonstrated in experiments performed in a study of rat skeletal muscle fibers [Carroll et al., 1999], revealing that the magnetic field increased the resting cytosolic Ca2+ concentrations that were attributed to reduced Ca2+-ATPase activity.
In summary, PEMF significantly inhibited the development of posttraumatic muscular atrophy relative to muscle mass but also muscle strength and CT, suggesting its effect on cellular signaling pathways upon contraction. Further molecular studies are needed to provide a better understanding of the action mechanism of PEMF in skeletal muscle.
CONCLUSION
In the present study, the preventive effect of the application of PEMF on the development of disuse atrophy was demonstrated through analyses of muscle strength and the duration of contractions. These results support the use of PEMF as a noninvasive treatment for clinical pathologies causing disuse muscle atrophy, which is an area that requires further research.




