Exposure to 10 Hz Pulsed Magnetic Field Induced Slight Apoptosis and Reactive Oxygen Species in Primary Human Gingival Fibroblasts
Zheng Huang, Cheng Ding contributed equally to this paper.
Conflicts of interest: None.
Grant sponsors: Key medical disciplines of Hangzhou, grant number: OO20200387; Health and Science Project of Hangzhou, grant numbers: A20210056, 20211231Y028; Medical Science and Technology Project of Zhejiang Province, grant number: 2021KY005; Guided Project of Science and Technology of Hangzhou, grant number: 20211231Y028; Hangzhou Biological Medicine and Health Industry Development Support Science and Technology Project, grant number: 2021WJCY131; National Science Foundation of China, grant number: 31700734.
Abstract
Extremely low frequency pulsed magnetic fields (MFs) have been increasingly used as an effective method in oral therapy, but its potential impact on health has not been clarified. In this study, we investigated the impact of 10 Hz pulsed MF exposure on primary human gingival fibroblasts (HGFs) derived from eight healthy persons (four males and four females). Cells were exposed to 10 Hz pulsed MFs at 1.0 mT for 24 h. Cell apoptosis, cell cycle progression, intracellular reactive oxygen species levels, DNA damage, and cell proliferation were determined after exposure. The results showed that 10 Hz pulsed MFs exposure have slight effects on cellular apoptosis, cell cycle progression, and DNA damage in primary HGFs from some but not all samples. In addition, no significant effect was found on cell proliferation. © 2022 Bioelectromagnetics Society.
INTRODUCTION
In recent years, electromagnetic fields (EMFs) stimulation had been increasingly used as an effective, noninvasive and economical method in clinical treatment, such as dysphagia, implant osseointegration, wound repair, and so on [Showkatbakhsh et al., 2010; Abdelrahim et al., 2011; Costantini et al., 2019; Mohajerani et al., 2019; Nayak et al., 2020; Nagashima et al., 2021; Nunes et al., 2021]. Although, many investigations had mentioned the beneficial effect of EMFs treatment, the potential hazards were paid little attention to [Saliev et al., 2019]. In 2011, extremely low frequency (ELF) EMF has been classified as possible carcinogens to human (2B) by International Agency for Cancer Research. Furthermore, some studies reported the toxic effect of EMF exposure on DNA damage and oxidative stress though the results were inconsistent in other studies [Bagheri Hosseinabadi et al., 2019; Schuermann and Mevissen, 2021]. Thus, it is necessary to investigate the potential impact of EMFs treatment on human health.
As an important part of body health, oral health has attracted more and more attention with the development of economy. The increasing use of EMFs in oral clinical treatment also raises concerns about the side-effects. As the most abundant cells in gingival connective tissues, human gingival fibroblasts (HGFs) have distinctive characteristics in extracellular matrix synthesis and remodeling, proliferative, migratory, immunological, immunomodulatory capacities [Bartold et al., 2000]. HGF damage would impair the integrity and function of gingival tissue [Palmer et al., 2005]. Thus, it is necessary to explore the impact of EMFs exposure on gingival fibroblasts.
There were a few studies on the damage effect of static MFs (SMFs) on gingival fibroblasts. Yagci and Kesim [2016] reported that SMFs might be genotoxic to HGFs, but Yamaguchi et al. [1993] and Shen et al. [2007] did not find any biological effect of SMF exposure on HGFs. The results indicated that MFs might be genotoxic to HGFs. However, research on the biological effects of MFs on HGFs is still limited, more studies on other types of EMFs used in clinical treatment are required. ELF (<100 Hz) pulsed MFs were potentially one of the most applied EMFs in future oral therapy. To determine the impact of ELF pulsed MFs on HGFs, we investigated the effect of 10 Hz pulsed MF exposure on cellular apoptosis, cell cycle progression, DNA damage, intracellular reactive oxygen species (ROS) levels and cell proliferation in primary HGFs in this study.
MATERIALS AND METHODS
Tissue Collection and Cell culture
Gingival tissues were obtained from 8 healthy human subjects (four males and four females, 18–35 years old), with good oral hygiene and without chronic systemic disease history (Table 1). All participants provided written informed consent for the donation of gingival tissue. Each sample was named to guarantee the anonymity of the donors, the information contained the patient's gender and age, such as M20y (20-year-old male) and F24y (24-year-old female). The study was approved on July 20, 2021 by the Institutional Ethics Committee of Affiliated Hospital of Hangzhou Normal University, Hangzhou, China (2021 (E2)-KS-068).
| Sample ID | Gender | Age | Occupation |
|---|---|---|---|
| F20y | Female | 20 | Student |
| F24y | Female | 24 | Student |
| M28y | Male | 28 | Worker |
| M20y | Male | 20 | Student |
| F18y | Female | 18 | Student |
| M33y | Male | 33 | Worker |
| M27y | Male | 27 | Worker |
| F23y | Female | 23 | Student |
After stripping, gingival tissues (about 5 × 3 × 1 mm3), were placed in a tube with fetal bovine serum (FBS) free high glucose Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY), and transported to the laboratory for cell culture in 30 min. Blood on tissues were washed with phosphate-buffered saline (PBS; Gibco), and then, the gingival epithelium was removed. The remainder were cut into pieces and cultured in a 25 cm2 T-25 flask (Corning, NY). The slim, spindle-shaped fibroblast cells can be seen around the edges of the gingival tissue after 5–10 days culture, coded as first passage cells. Then, the cells were cultured in high glucose DMEM with 10% FBS, 100 U/ml penicillin (Beyotime, Shanghai, China), and 100 mg/ml streptomycin (Beyotime) at 37°C with 5% CO2 in an incubator (Thermo Scientific, Shanghai, China).
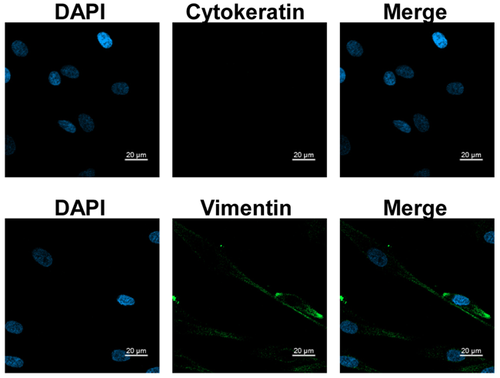
The cells were identified at passage 3 by immunofluorescence (Fig. 1). The Immunofluorescent stain assay was performed following the protocol as described [Liu et al., 2016] with some modification. Briefly, the coverslips carrying cells were blocked and stained with Vimentin and Cytokerain antibodies (1:100; Beijing Zhongshan Jinqiao Corporation, Beijing, China). After washing with PBS, the coverslips were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin G (IgG). For visualization of nuclei, DAPI (4,6-diamidino-2-phenylindole; Thermo Scientific) staining was performed. All photographs were taken by a confocal laser scanning microscope (Zeiss, Oberkochen, German). Cells were 1:2 or 1:4 subcultured when the culture confluence was almost 80%. Cells at passages 4 to 8 were used in this study.
Pulsed MFs Exposure System and Exposure Protocol
The MF generation device was designed and manufactured by CH_HAIL Electronic Devices company (Beijing, China), which had been previously described [Sun et al., 2021]. In brief, the device consists of a pair of Helmholtz coils, a signal generator, a power amplifier and a gauss meter. The Helmholtz coils were placed in a 5% CO2 incubator (Thermo Scientific). Cells cultured in the flasks or dishes were placed in a transparent holder between the coils (1 cm below the center of the coil) for MFs exposure. The direction of MFs is parallel to the bottom of the flask or dish. Before exposure, cells were subculture into 35 mm petri dish (1 × 104/dish) and cultured at 37 °C with 5% CO2 for 1 day. Cells were used for MF exposure experiment when cells were 50%–60% confluent. Cells in the exposure group were placed in the central region of the Helmholtz coils and exposed to 1.0 mT 10 Hz pulsed MFs for 24 h, while the control group were cultured in another same type of incubator at same conditions without MF exposure. The pulse period and width were 100 and 50 ms, respectively, the rise and fall time were both about 20 ns, and the calculated maximum induced electric field was about 27.6 V/m. During exposure, the temperature of the cell culture medium was monitored by a temperature logger (SSN-13E; Yuwexe, Shenzhen, China), and no significant difference was found between control and exposure group (Fig. 2).
Cell Viability Assay
After exposure, cells were trypsin (Gibco) digested and re-seeded into 96-well plates at a density of 2 × 103 cells per well, and then incubated at 37 °C in a humidified atmosphere containing 5% CO2, the medium was changed every other day. The cell proliferation was detected by cell counting kit-8 (CCK-8; Beyotime) at 1 to 4 days after exposure. The optical density (OD) was detected by a microplate photometer (Thermo Scientific) at the wavelength of 450 nm.
Cell Cycle Analysis
After exposure, cells were immediately trypsin digested and resuspended in 70% ethanol and stored at −20 °C for 24 h. Then, removed ethanol, and washed cells with PBS twice. The DNA content in cells were stained with 50 μg/ml propidium iodide (PI; Beyotime) diluted in PBS containing 50 mg/ml RNase A (Thermo Scientific) at room temperature for 30 min, and then detected by flow cytometry (Cytoflex, Beckman Coulter), 1 × 104 events per sample. The proportions of cells in the G0/G1, S, and G2/M phases were determined by Modfit 3.0 software (Verity Software House, Topsham, ME).
Apoptosis Assay
The Annexin V-FITC/PI Apoptosis Detection Kit (Beyotime) was used to determine the HGFs apoptotic ratio. According to the manufacture's induction, after exposure, cells were immediately harvested and washed with PBS, and then 195 μl of the reaction solution combined with Annexin V-FITC (5 μl) and PI (10 μl) for 30 min. Samples were then assessed via flow cytometry (Cytoflex; Beckman Coulter) and data procession was conducted by FlowJo vX.0.7 software (Treestar, San Carlos, CA). The percentage of Annexin V-FITC and PI positive cells were determined. Hydrogen peroxide solution (H2O2) treatment were applied as positive control.
Intracellular ROS Measurement
Intracellular ROS was detected by the Reactive Oxygen Species Assay Kit (Beyotime). As instructed, after exposure, cells were immediately washed with FBS free culture medium twice and then incubated with 2′,7′-dichlorodihydrofluorecein diacetate (DCFH-DA) diluted in FBS free culture medium at 37 °C for 20 min. Then, cells were harvested and washed. The signal of ROS was determined by flow cytometry.
Western Blotting
After exposure, cells were washed with ice-cold PBS and then lysed in cold radioimmunoprecipitation assay (RIPA; Beyotime) buffer with 1× protease inhibitors (Roche Diagnostics, Indianapolis, IN). Cell lysates were transferred into a precooled microfuge tube, protein extracts were then centrifuged at 4 °C for 20 min at 12,000 rpm. Then, equal amounts of each protein sample were separated by electrophoresis on 12% sodium dodecyl sulfate (SDS)-polyacrylamide gels, transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA), blocked with 5% skimmed milk in PBS with 0.05% Tween 20 for 2 h. The membranes were incubated with primary antibodies, i.e. anti-BAX, Bcl-2, Caspase-9, p-cdc2 (Tyr15), p53, p-Rb (Ser807) (1:1000; Beyotime) and anti-β-actin (1:1000; Cell Signaling Technology, Danvers, MA), at 4 °C overnight, followed incubation with Q20 HPR-conjugated goat antimouse (Cell Signaling Technology) or goat anti-rabbit IgG (Cell Signaling Technology) at room temperature for 1 h. Immunoreactive bands were detected by using enhanced chemiluminescence (ECL; Biosharp, Anhui, China). The gray values were determined and protein levels relative to β-actin were calculated.
Alkaline Comet Assay
Alkaline comet assay was performed as previously described [Sun et al., 2021]. Briefly, after exposure, cells were resuspended in culture medium, about 2 × 104 cells were mixed with 75 μl 0.65% low-melting agarose, and pipetted onto 0.65% normal-melting agarose precoated on slides. The slides were then placed on ice for approximately 2 min to promote agarose coagulation. The slides were placed in lysis buffer at 4 °C for 1 h and then in enzymatic hydrolysate at 37 °C for 2 h. Then, the DNA in cells were unwind in ice-cold alkaline electrophoresis solution for 20 min, and electrophoresis at 20 V for 20 min. After electrophoresis, cells were neutralized in Tris buffer (0.4 M, pH = 7.5) for 2 × 5 min. DNA “comets” were stained by Gel-red (Beyotime) and photographed by a fluorescence microscope (Zeiss). Images of 100 cells per slide were collected and DNA damage indexes were determined by the CASP 1.2.2 software (Krzysztof Konca, Wroclaw, Poland).
Cell Proliferation Rate Assay
After exposure, cells were cultured with culture medium containing 10 μΜ 5-ethybyl-2′-deoxyuridine (EdU; Beyotime) for 2 h. Then, cells were harvested and the EdU signal were detected by flow cytometer according to the instruction of EdU Cell Proliferation Kit (C0071S, Beyotime). The ratio of EdU positive (EdU+) cells was analyzed by FlowJo vX.0.7 software (Treestar).
Statistical Analysis
All the experiments in this study were at least triply repeated, and data were shown as mean ± standard error of the mean (SEM). Students' t test and Wilcoxon rank sum test were applied for the comparisons between two groups, and P < 0.05 was considered as statistically significant difference.
RESULTS
The Effect of 10 Hz Pulsed MF Exposure on Cellular Apoptosis of HGFs
Exposure to 10 Hz pulsed MF significantly increased the percentage of early apoptosis (Annexin V positive staining) in most HGFs from male group and all from female group, the percentage of late apoptosis (Annexin V and PI positive staining) were significantly increased in all HGFs from male group and two from female group (Fig. 3A). But the impact was weak, which the average increases of early and late apoptosis rate were approximately 1.35% and 0.59%, and the maximum increase of apoptosis rate in male and female group were 3.37% (sample M20y) and 2.22% (sample F24y), respectively.
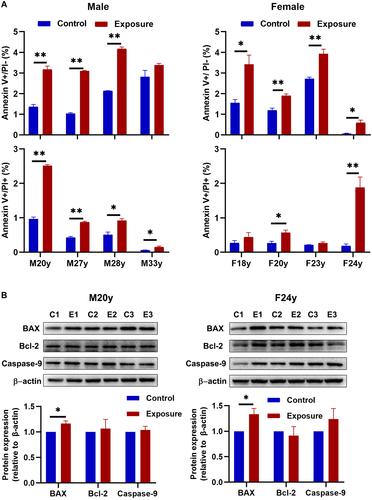
For further determination, we detected apoptosis-related proteins, i.e. BAX, Bcl-2 and Caspase-9 in sample M20y and F24y (Fig. 3B). The results showed that 10 Hz pulsed MF exposure elevated the expression of BAX (P < 0.05), but no significant change in Bcl-2 or Caspase-9 expression in HGFs of M20y and F24y (P > 0.05), indicated that 10 Hz MF exposure have effect on inducing apoptosis in HGFs.
The Effect of 10 Hz Pulsed MF Exposure on Cell Cycle Progression in HGFs
Cell cycle progression is a common index for cell function and state [Uzbekov and Prigent, 2022]. Our results showed that, in male HGFs, 10 Hz pulsed MF exposure significantly decreased the percentage of cells in G2/M phase in sample M20y, M27y and M33y, but no significant changes in sample M28y, compared to control group. In female group, 10 Hz pulsed MF exposure significantly decreased the percentage of cells in G0/G1 phase and increased the percentage of cells in S phase in sample F18y, F20y, and F24y, but no significant changes in sample F23y (Fig. 4A).
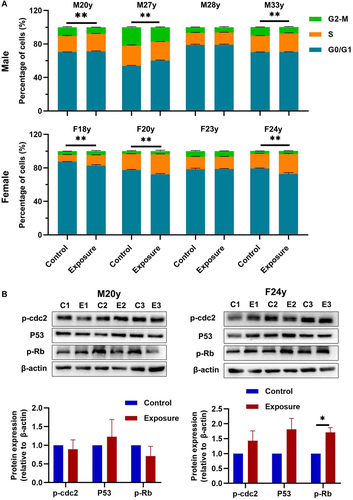
To give more information of the effect of 10 Hz pulsed MF exposure on cell cycle, we further detected the expression levels of cell cycle regulation proteins, phosphorylated cell division cycle protein 2 (p-cdc2) [Serpico and Grieco, 2020], p53, and phosphorylated Rb (p-Rb) [Engeland, 2022] in HGFs from sample M20y and F24y (Fig. 4B). The results showed that 10 Hz pulsed MF exposure significantly increased the p-Rb in HGFs from sample F24y but no significant effect on M20y. In addition, no significant changes of p-cdc2 or p53 was found in HGFs from both samples.
The Effect of 10 Hz Pulsed MF Exposure on DNA Damage in HGFs
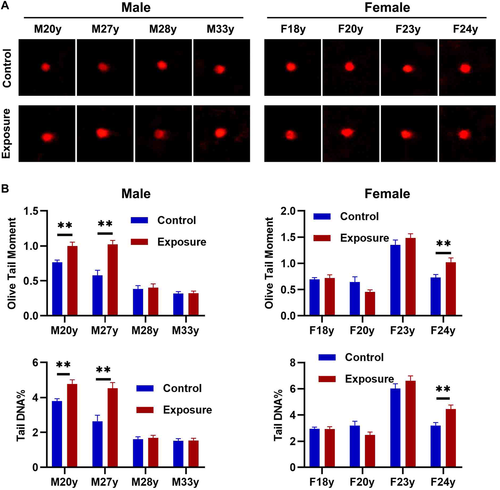
To determine the genotoxicity of 10 Hz pulsed MF, the DNA fragmentation in HGFs were detected by alkaline comet assay. The results showed that, in male HGFs, 10 Hz pulsed MF exposure significantly increased DNA fragmentation in sample M20y and M27y, but no significant change in sample M28y or M33y when compared to control group (Fig. 5A). In female HGFs, 10 Hz pulsed MF exposure significantly increased DNA fragmentation in sample F24y, but no significant change in sample F18y, F20y, or F23y, compared to control group (Fig. 5B). These results indicated that 10 Hz pulsed MF exposure has genotoxic effect in HGFs from some but not all samples.
The Effect of 10 Hz Pulsed MF Exposure on Intracellular ROS Levels in HGFs
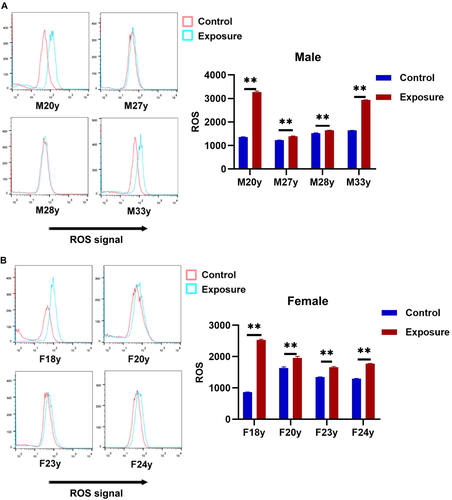
ROS had been suggested to be the main mechanism of the DNA damage induced by EMFs [Yuan et al., 2020]. Thus, the effect of 10 Hz pulsed MF on ROS were determined. The results showed that exposure to 10 Hz pulsed MFs for 24 h significantly increased intracellular ROS in HGFs from all samples but the effects were very slight in sample M27y and M28y in male group (Fig. 6), suggested that 10 Hz pulsed MF exposure has an effect on ROS generation in HGFs.
The Effect of 10 Hz Pulsed MF Exposure on Cell Proliferation
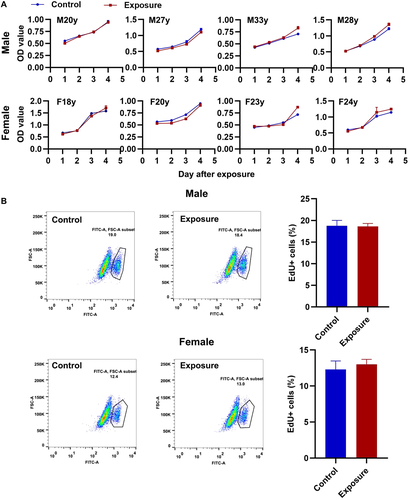
The cell viability of HGFs from each sample were determined from 1 to 4 days after exposure. The results showed that 10 Hz pulsed MF exposure does not induce significant changes on cell viability in HGFs from each sample compared to control group (Fig. 7A). For further confirmation, the cell proliferation rates were determined immediately after exposure, and no significantly changes were observed in 10 Hz pulsed MFs exposed HGFs compared to that in the control group (Fig. 7B). These results suggested that exposure to 10 Hz pulsed MF do not affect cell proliferation in HGFs.
DISCUSSION
LF pulsed EMFs were increasingly used in clinical practice as a noninvasive and effective method, especially in stomatology, but the potential impacts of LF pulsed MFs on oral health have not been fully investigated [Naskar et al., 2021]. Considering HGFs play an important role in gingival tissue function and oral health maintenance [Chiquet et al., 2015], we explored the impact of 1.0 mT 10 Hz pulsed MF exposure on primary HGFs from eight healthy persons, and the results showed that 10 Hz pulsed MF exposure have some effect on apoptosis, cell cycle progression, DNA damage and ROS generation, but not on cell proliferation.
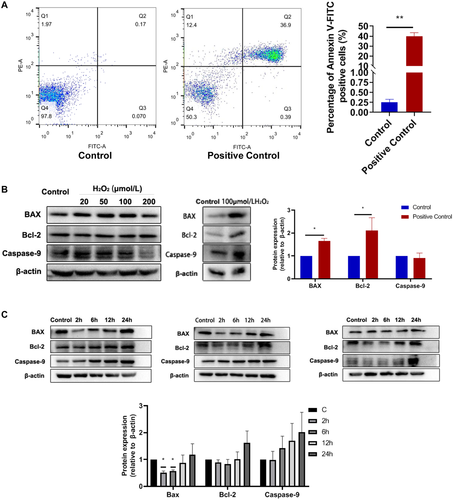
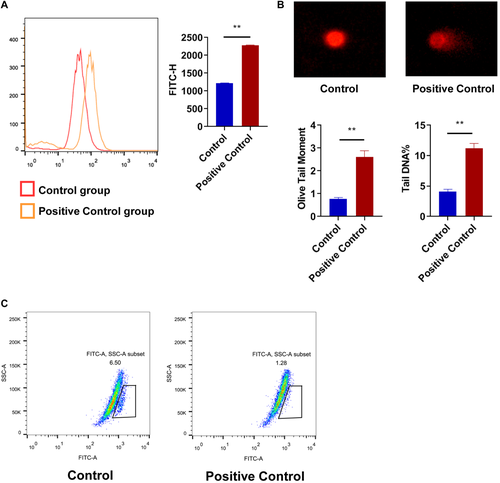
In this study, we found that 10 Hz pulsed MF exposure induced apoptosis in HGFs from most samples. Cellular apoptosis is a type of programmed cell death and is a common index of the status of cells [Obeng, 2021]. But the apoptosis rates were still very low, the maximum increase of apoptosis rate in male and female group were 3.37% (sample M20y) and 2.22% (sample F24y), respectively (Fig. 3A). Significant changes in apoptosis related proteins were observed only in BAX, but not in Bcl-2 or Caspase-9 (Fig. 3B). In the positive control, H2O2 treatment significant increased BAX and Bcl-2 but not Caspase-9 (Fig. 8A and B). Thus, these results indicated that 1.0 mT 10 Hz pulsed MF exposure has effect on apoptosis in HGFs but the effect might be weak or by a different mechanism compared to H2O2 treatment. Similar effect had been reported by Nie et al. [2013] that the cell apoptosis was slightly increased after 5 days exposure to LF-MF, but the expression of apoptosis associated genes, such as, Bcl-2 or Survivin, was not significantly affected. The effects of ELF-MFs on cell apoptosis have been investigated in other previous studies. Kim et al. [2014] reported that continuous exposure to a 60 Hz MF can induce apoptosis of testicular germ cells. Yang and Ye [2015] observed that exposure to 1 mT ELF‑EMF exposure can induced apoptosis of MG‑63 cells and decrease in viability of the cells. There were studies with different results. Brisdelli et al. [2014] found that ELF-MFs do not induce significant apoptosis. Thus, the effect of ELF-MF exposure on cell apoptosis were very complicated. The different results might due to the different experimental conditions, including EMF types, intensity, cellular models, or other factors.
Cell cycle progression is also an important index to view the effect of external stimulus on cells [Panagopoulos and Altmeyer, 2021]. Previous studies had shown that ELF-MFs have effect on cell cycle progression [Lekovic et al., 2020; Cios et al., 2021]. In this study, we also found that 10 Hz pulsed MF exposure have effect on cell cycle progression in HGFs from most samples, and what is even more interesting is that the effect was different between male and female group (Fig. 4A). In male group, 10 Hz pulsed MF exposure decreased the percentage of cells in the G2/M phase, while, in female group, 10 Hz pulsed MF exposure increase the percentage of cells in the S phase. And, 10 Hz pulsed MF exposure increased p-Rb in HGF from female sample (F24y) but no significant changes in HGFs from male sample (M20y) (Fig. 4B). To our knowledge, this is the first time that reported a sex-dependent effect of LF-pulsed-MFs exposure on cell cycle progression in human derived cells. Sex-dependent effect of ELF-MFs on biological process in vitro had been previously reported by Baranowska et al. [2018] that the effect of pulsed EMFs (7 Hz, 30 mT) on the release of proinflammatory cytokines from adipose-derived stem cells (ADSCs) of Wistar rats were different between male and female. And, more similar phenomenon in in vivo studies [Vallejo and Hidalgo, 2012; Acikgoz et al., 2022; Hanlon and McCalley, 2022]. But, none of these studies uncovered the mechanisms. We thought that the different gene background or hormone secretion might be the reason, however, we did not have any supporting data, and more investigation were required.
To further investigate the mechanism of apoptosis or cell cycle changes induced by 10 Hz MF exposure in HGFs, we detected DNA damage and ROS generation which are both factors that cause cell cycle arrest [Verbon et al., 2012; Chao et al., 2017; Matthews et al., 2022] and apoptosis [Boonstra and Post, 2004; Lee et al., 2014]. In present study, we found that 10 Hz pulsed MF exposure elevated DNA damage levels in HGFs from some but not all samples (Fig. 5). Moreover, when compared to H2O2 treatment (Fig. 9B), the increase of DNA damage induced by 10 Hz pulsed MF exposure was very weak, even hard to be visualized in images of alkaline comet assay (Fig. 5A). The genotoxic effect of EMFs had been widely investigated in previous studies with the inconsistent results [Lai, 2021; Lv et al., 2021]. In ROS generation, we observed increased ROS levels in all HGFs exposed to 10 Hz pulsed MF (Fig. 6). ROS was the most possible mechanism in EMF induced biological effects especially in apoptosis and DNA damage [Wolf et al., 2005; Lai, 2021]. However, there were also studies reported that EMFs increased ROS but did not induce DNA damage [Di Loreto et al., 2009], indicated the consequences of increased ROS induced by EMFs depends on the special conditions of research. Another hypothesis points out that the reason why ELF-EMFs can't directly damage DNA is that LF MFs can't transmit high enough energy to affect chemical bonds. The induced electric field may induce the movement of electrons in DNA. This may produce guanine free radicals, which maybe converted to oxidative DNA damage after reacting with water [Focke et al., 2010]. In this study, the DNA damage induced by 10 Hz MF exposure might be caused by ROS increase in HGFs form partial samples, and the different results among HGFs from different samples might due to the individual difference of each sample. Besides, considering there were no significant effect of 10 Hz pulsed MF exposure on cell proliferation (Fig. 7), and weak effects on cell apoptosis and DNA damage, we speculated that the effect of increased ROS and its impact on HGFs can be compensated under our experimental conditions.
Cell cycle progression is closely related to cell proliferation, but in this study, neither cell viability (Fig. 7A) nor cell proliferation rate assay (Fig. 7B) showed that 10 Hz pulsed MF induce significant change on cell proliferation in HGFs. Thus, we speculated that the effect of 10 Hz pulsed MF exposure on cell cycle progression in HGFs is temporary and limited.
In conclusion, we investigated the impact of extremely LF pulsed MF exposure on primary HGFs, and found that exposure to 10 Hz pulsed MFs at 1.0 mT for 24 h have an effect on cell apoptosis, cell cycle progression and DNA damage in HGFs from some but not all samples, but no significant effect on cell proliferation in primary HGFs. The results suggests that pulsed MF might have effect in inducing apoptosis and ROS in gingival tissue which need to be taken into account when using pulsed MFs in oral therapy.
ACKNOWLEDGMENTS
We would like to thank Prof. Genxiang Mao of Zhejiang Provincial Key Lab of Geriatrics & Geriatrics Institute of Zhejiang Province, Department of Geriatrics, Zhejiang Hospital, and his group for laboratory support and dedicated technical assistance. This study was supported by the Key medical disciplines of Hangzhou (No. OO20200387), Health and Science Project of Hangzhou (No. A20210056 & 20211231Y028), Medical Science and Technology Project of Zhejiang Province (No. 2021KY005), Guided Project of Science and Technology of Hangzhou (No. 20211231Y028); Hangzhou Biological Medicine and Health Industry Development Support Science and Technology Project (No. 2021WJCY131) and National Science Foundation of China (No. 31700734).
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Zheng Huang. Chuan Sun, Cheng Ding, Xinzhao Huang contributed to the data analysis. The first draft of the manuscript was written by Zheng Huang and Cheng Ding. Chuan Sun and Liangjun Zhong revised manuscript content. All authors commented on previous versions of the manuscript and approved the final manuscript.




