Comparison of membrane electroporation and protein denature in response to pulsed electric field with different durations
Abstract
In this paper, we compared the minimum potential differences in the electroporation of membrane lipid bilayers and the denaturation of membrane proteins in response to an intensive pulsed electric field with various pulse durations. Single skeletal muscle fibers were exposed to a pulsed external electric field. The field-induced changes in the membrane integrity (leakage current) and the Na channel currents were monitored to identify the minimum electric field needed to damage the membrane lipid bilayer and the membrane proteins, respectively. We found that in response to a relatively long pulsed electric shock (longer than the membrane intrinsic time constant), a lower membrane potential was needed to electroporate the cell membrane than for denaturing the membrane proteins, while for a short pulse a higher membrane potential was needed. In other words, phospholipid bilayers are more sensitive to the electric field than the membrane proteins for a long pulsed shock, while for a short pulse the proteins become more vulnerable. We can predict that for a short or ultrashort pulsed electric shock, the minimum membrane potential required to start to denature the protein functions in the cell plasma membrane is lower than that which starts to reduce the membrane integrity. Bioelectromagnetics 34:253–263, 2013. © 2012 Wiley Periodicals, Inc.
INTRODUCTION
Electrical injury has become more and more prevalent in civilized countries. When cells or tissues are shocked, even briefly, by an intensive electric field the electric field may cause severe damage to the cells and tissues. Because the cell's membrane resistance is many orders of magnitude higher than that of electrolytes in both intracellular and extracellular fluids [Goldman, 1943], the electric field mainly falls on the cell membrane, resulting in an extremely high field strength inside the membrane, which may break down the structure of the membrane phospholipid bilayer and the proteins embedded in the membrane.
A large body of evidence has shown that an electrical shock can generate pore or pore-like structures in cell membranes (electroporation), resulting in the leakage of intracellular ions and other metabolic materials including adenosine triphosphate (ATP) molecules. As a result, the cell may suffer osmalarity and volume change, loss of electrical activity, edema, necrosis, and eventually death. In the past, significant efforts have been made to study membrane electroporation [Lee and Kolodney, 1987; Tsong, 1991; Chen and Lee, 1994a; Weaver, 1995; Vasilkoski et al., 2006]. The minimum membrane potential that can electroporate the cell membrane has been investigated by using voltage-clamp techniques on cardiomycytes [O'Neil and Tung, 1991] and skeletal muscle fibers [Chen and Lee, 1994a]. For a brief 4 ms duration (to mimic the power line by using the RMS duration of the half cycle) pulsed electric shock, the minimum membrane potential that can electroporate the cell membrane resulting in a leakage current ranges from 200 to 300 mV. Weaver [1995, 2000] and Esser et al. [2010b] theoretically explored the possible mechanisms involved in membrane electroporation and showed that different kinds of pores can be generated by the electric shock in terms of the recovery time.
In addition to membrane electroporation generating pore or pore-like structures on the membrane phospholipid bilayer, intensive electric shock can also affect the membrane proteins, especially the functions of those sensitive to the membrane potential. Previously, we studied various membrane proteins including voltage-gated Na [Chen and Lee, 1994b; Chen et al., 2006] and K channels [Chen et al., 1998; Chen, 2004a], and L-type Ca channels (dihydropuridine receptor) [Chen, 2004b] in skeletal muscle fibers in response to a brief intensive electric shock. The results showed that a 4 ms pulsed membrane potential with a magnitude from 400 to 500 mV can damage or denature the membrane proteins, resulting in protein functional reductions.
We monitored various parameters of membrane proteins including the channel conductance, channel voltage sensitivity, and channel gating systems and found that the most vulnerable domain in the channel proteins is their gating systems. At a little over 400 mV, a 4 ms pulsed electric shock can damage the Na and K channel gating system, resulting in a reduction in the channel current and channel conductance [Chen and Lee, 1994b]. Interestingly, this value is higher than that which electroporates the cell membrane.
In a real situation, however, such as lightning shock or some transient electrocution, patients often show noticeable reduction in limb functions without obvious changes in the appearance of the limb. It seems that the membrane proteins that are directly related to the functions of cells, tissues, and limbs may suffer some changes while the cell integrity remains intact. This phenomenon is inconsistent with our previous voltage-clamp studies. One of the explanations is that in our studies, a voltage clamp was used to deliver the shock pulse to the cell membrane where the effect of membrane capacitance was compensated by the negative feedback of the voltage clamp, while in a real situation, the membrane capacitance inevitably affects the buildup of the field-induced membrane potential. A question remains as to whether the membrane phospholipid bilayer or the membrane proteins are more vulnerable to an external electric field.
In addition, ultrashort nanosecond pulsed electric fields (nsPEFs) have recently been demonstrated to affect intracellular organelles in addition to affecting the cell integrity [Stacey et al., 2003; Schoenbach et al., 2004; Beebe and Schoenbach, 2005; Nuccitelli et al., 2006; Vasilkoski et al., 2006; Esser et al., 2010a]. Scarlett et al. [2009] have shown that by monitoring a change in the concentration of intracellular Ca2+, a 60 ns shock pulse of 50 and 100 kV/cm will damage the membrane of the endoplasmic reticulum (the organelle that stores Ca2+), and the 100 kV/cm shock may also damage the cell membrane. The discrepancy may be because the field-induced membrane potential difference across the plasma membrane differs from that on the subcellular membrane. The size and thus the membrane capacitance of plasma membranes are much larger than those of subcellular membranes. The duration of an nsPEF may not be long enough to fully charge the plasma membrane, while the subcellular membrane can be quickly charged. Another question is how the ultrashort PEFs affect proteins in the cell plasma membrane.
We conducted investigations of the membrane electroporation and protein denaturation of skeletal muscle fibers in response to various pulsed electric shocks. Single-twitch skeletal muscle fibers were exposed to an external electric field without using the voltage clamp. The integrity of the cell membrane and functions of the membrane protein were monitored right after each electric shock. The results showed that in response to a relatively long PEF (longer than 1 ms), the minimum magnitude of the electric field that denatures the membrane proteins was higher than that which electroporates the cell membrane, which is consistent with previous studies. However, this was not true for short pulses (short than 1 ms), where the membrane proteins become more vulnerable. Based on these results, we can predict that in response to a short or ultrashort nsPEF, the proteins in the cell plasma membrane are more susceptible than the membrane phospholipid bilayer.
MATERIALS AND METHODS
Sample Preparation and Measurement Configuration
The experiments were conducted on single fibers from frog twitch skeletal muscles. The American frog, Renopipins, was used. The detail of muscle dissection and fiber preparation is similar to that described previously [Irving et al., 1987; Chen and Lee, 1994a]. The single fibers were dissected and then mounted in a double Vaseline gap voltage-clamp chamber [Hille and Campbell, 1976; Chen and Lee, 1994b]. The voltage-clamp technique was used to identify the changes in membrane integrity and protein function after each electric shock. In terms of the membrane integrity, we monitored the changes in the leakage of the cell membrane by measuring the holding current. Electric field-mediated pores or pore-like structures in the cell membrane decreased the membrane resistance and therefore increased the membrane holding currents.
In terms of the membrane protein, we selected the voltage-gated Na channel as the target. Because the channel protein is voltage gated, its electrical sensitivity is higher than other membrane proteins. The channel voltage sensitivity was monitored as a marker to represent the protein functions while the channel gating system has been shown to be most vulnerable to an electric shock.
Among the two kinds of membrane protein denaturations, reversible and irreversible, we selected reversible denaturation because we needed to screen various pulse durations. The study of reversible damages that can be quickly recovered in a range from seconds to sub-seconds allowed us to conduct multiple measurements on the same fiber. The cost is not only reduced but also the errors induced by using different fibers and preparations.
In contrast, the shock electric field was generated by a computer and delivered to a pair of external platinum electrodes (1 mm diameter) placed 1 cm apart in the external compartment on each side of the muscle fiber. The electric field was perpendicular to the axis of muscle fibers. Figure 1 shows the sketch of the external compartment. Due to the large size of the electrodes compared with the diameter of single muscle fibers (about 50 µm), and the long distance (1 cm) between the two electrodes, the electric field can be considered as a uniform field.
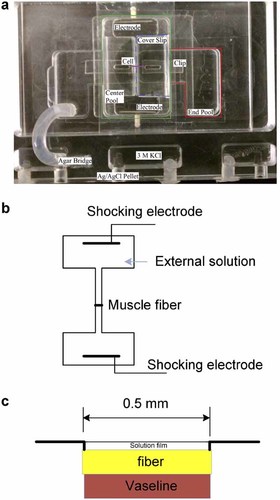
Configuration of the experimental setup. a: Photo of the experiment chamber; b: Top view of the external compartments and pair of electrodes. In the middle of the external compartment is a segment (500 µm long) of muscle fiber exposed to the external solution. Two bars represent the pair of electrodes that deliver the shock electric field; c: Cross section of the middle in (b).
There are two considerations in terms of using external electric fields to shock the fibers. First, for a short pulse, a large magnitude is expected to be needed and the commercially available voltage clamp can only clamp the membrane potential up to 200 mV, which is not nearly high enough. Secondly, the voltage-clamp technique overrides the effects of membrane capacitance while the use of an external electric field will mimic the real situation of electrical injury.
When a potential difference is applied to the pair of electrodes emerged in the solution, the field-induced thermal effects and electrochemical toxicity will inevitably affect the muscle fibers. In order to reduce these side effects, a Vaseline partition wall was built underneath the muscle fiber along the fiber axis to seal the gap between the bottom of chamber and the fiber. The bathing solution was also carefully removed until the solution surface was low enough, just submerging the muscle fibers. Special caution was taken because when the surface of the solution becomes curved along the curvature of the profile of the muscle fiber, meaning only a thin solution film above the fiber, any further removal of solution may suddenly expose the fiber to air due to the surface tension effect. Because the solutions have a much higher electrical conductivity than the cell membrane, the electric field falls mainly on two hemispheres of the cell membrane. The field-induced membrane potential of each hemisphere should be V/A, where V is the potential difference applied on the two electrodes, and the non-dimensional factor A, a factor that represents the sealing effect, equals 2 if the fiber is perfectly sealed. Otherwise, the field-induced membrane potential becomes smaller when the factor A is larger than, but close to, 2. This way, the required potential difference applied on the pair of electrodes will be significantly reduced and therefore decrease the side effects (thermal and toxicity).
The experimental solutions used in this study are the same as those in study of Na channel currents. The external solution contains 60 mM NaCl, 60 mM tetraethylammonium chloride (TEACl), 5.4 mM KCl, 4 mM 3-(N-morpholino) propanesulfonic acid (MOPS), 1.8 M MgCl2, 2 mM BaCl2, 0.2 mM CdCl2, and 1 mM CsCl, pH 7.1. The internal solution contains 45.5 mM Cs-glutamate, 5 mM Cs2-piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), 20 mM Cs2-ethylene glycol tetraacetic acid (EGTA), 6.8 mM MgSO4, 5 mM glucose, 5.5 mM adenosine-5′-triphosphate disodium (Na2-ATP), and 10 mM phosphocreatine di(tris), pH 7.1.
Experimental Protocol
The experimental protocol can be briefly described as follow: First, the voltage clamp was set in the Clamp mode to hold the membrane potential at −80 mV. Then, a 0.1 ms, 70 mV stimulation pulse was used to open the Na channels, and the channel currents were measured once per minute until reaching a stable state; this takes about 10 min. The stable transmembrane currents or holding currents, and the channel currents are recorded as controls. Then, the voltage clamp is switched to the record mode and a computer-generated pulsed intensive electric field is delivered to the electrode pair to shock the muscle fibers. This way, the patch clamp equipment was protected from electrical shock.
Twenty milliseconds after the electric shock, the voltage clamp was switched back to the Clamp mode to again hold the membrane potential at −80 mV. After 200 ms, the holding current was recorded and the Na channel current was re-measured using the same stimulation pulse (0.1 ms, 70 mV). The 200 ms delay after switching back to Clamp mode was to ensure that the equipment reached a stable state. If neither the membrane holding currents nor the channel currents changed, we gradually increased the pulse magnitude until the cell was injured.
For comparison, we set a standard criterion to clarify the membrane integrity change and channel protein denaturation. For channel proteins, as long as the peak channel current was reduced 5% from the control value measured before the electric shock, the channel proteins were considered denatured. Similarly, if the holding current increased by 1 nA stably for at least 10 s after the electric shock, the cell membrane was considered electroporated. Generally, both the channel currents and membrane holding currents were fully recovered within tens of seconds. After multiple shocks, the muscle fibers took a longer time to be fully recovered. Once the recovery time was longer than 3 min, the experiment was stopped.
Figure 2 shows the measuring system and the experimental protocol. The system includes a custom-built sample chamber, a TEV-200 voltage clamp (Dagan, Minneapolis, MN), and two data acquisition systems, DAQ1 and DAQ2. DAQ1 consists of a DIGIDATA 1322A digitizer (Axon Instruments, Union City, CA) controlled by Clampex 9.2 software (Axon Instruments). DAQ1 is used to control the voltage clamp, which is connected to the Ag/AgCl electrodes in the chamber to conduct voltage-clamp measurements. DAQ2 consists of a BNC-2110 DAQ box connected to a PCI-6221 board (National Instruments, Austin, TX) to generate a pulsed intensive electric field to electrically shock the muscle fiber connected to a pair of external platinum electrodes emerged in the chamber's external solution. DAQ2 is controlled by two custom-written LabVIEW programs (National Instruments). The LabVIEW programs control the timing of the experiments in three steps. Initially, the voltage clamp is in Clamp mode to measure the Na channel currents and transmembrane currents stored in the computer by Clampex using DAQ1. Then, DAQ2 sends a command pulse of 50 µs to switch the equipment to Record mode. A PEF is delivered to the platinum electrodes to shock the muscle fibers. Finally, another command pulse is sent by DAQ2 to return the equipment back to the Clamp mode and trigger DAQ1 to control the voltage clamp to re-measure the channel and transmembrane currents. The 50 µs command pulse that switches the mode is much shorter than the channel current measurement pulse (10 ms) and as such should not affect the measurement of the channel currents.

Experimental protocol. a: Schematic diagram of the measuring system; b: Arrangement of the electrical signals in terms of measurements of the transmembrane and voltage-gated Na channel currents by the voltage clamp and shock electric field applied by the external electrodes. The time scale is approximated.
RESULTS
Figure 3 shows the stimulation pulse and corresponding Na channel currents before the electric shock. The inward or negative current is the Na channel current, which has been blocked with 1 µM tetrodotoxin. After each shock, the channel current is re-measured (not shown because the difference cannot be identified visually). The peak value is measured with the computer. As long as the peak value is reduced by 5%, the channel proteins are considered denatured.
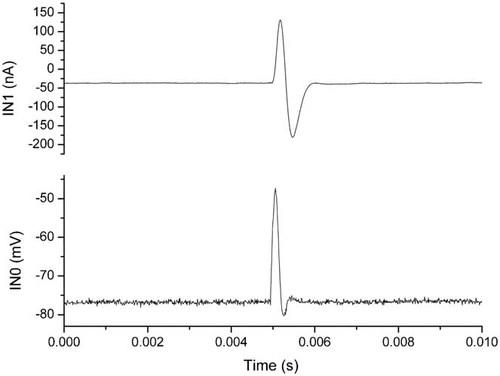
Na channel currents and stimulation pulse. Lower panel: Short (100 µs) 30 mV stimulation pulse. Upper panel: Elicited transmembrane current. The inward or negative current is the Na channel current.
In terms of the electric shock, we screened the shock pulse duration from 4 ms to 50 µs with a magnitude of 200 mV. For each shock pulse duration, the minimum potential difference required to either increase the membrane holding currents or decrease the channel currents are identified. In Figure 4, dots representing 35 measurements are plotted to show the minimum potential differences needed to electroporate the cell membrane as a function of shock pulse duration. Initially, the minimum potential difference across the pair of electrodes is in the range of 600–800 mV, which remains relatively unchanged as long as the pulse duration is longer than the membrane time constant of about 1 ms. When the pulse duration became less than 1 ms, the potential difference needed starts to increase. For pulse durations less than 100 µs, the minimum potential difference became a few volts.
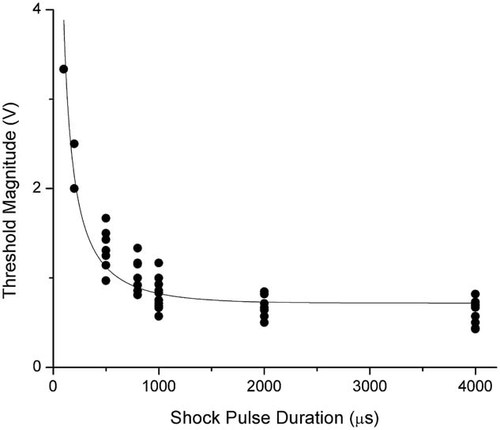
Minimum membrane potentials electroporating the cell membrane in response to various shock pulse durations. Fitted values of the data (value ± standard error) are: AVm,th = 0.718702 ± 0.0389495 V and τm = 488.619 ± 43.0204 µs. These values give an R2 value of 0.96. The 95% confidence interval for AVm,th is 0.640579–0.796824 V and the 95% confidence interval for τm is 402.331–574.907 µs.
Similarly, the minimum potential differences that denatured the Na channel proteins or reduced the channel currents are plotted as a function of pulse duration in Figure 5. Again, when the pulse duration is relative long, the required potential differences remain constant (about 1 V) until the pulse duration is reduced to about 200 µs. Then, the potential difference significantly increases with a much sharper pattern than the curve shown in Figure 4 for membrane electroporation.
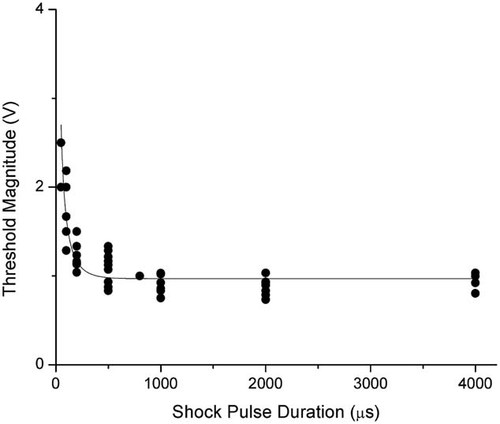
Minimum membrane potential denaturing the voltage-gated Na channel proteins in response to various shock pulse durations. Fitted values of the data (value ± standard error) are: AVp,th = 0.992253 ± 0.0278676 V and τp = 99.1823 ± 7.04425 µs. These values give an R2 value of 0.97. The 95% confidence interval for AVp,th is 0.93647–1.04804 V and the 95% confidence interval for τp is 85.0817–113.283 µs.
It is necessary to point out that the potential differences shown in Figures 4 and 5 are those applied to the electrodes and are not the potential differences of the cell membrane. Due to membrane capacitance, the field-induced membrane potential cannot be transiently changed in response to a PEF. It takes time for the electric field to charge the membrane capacitance.
In the whole-cell voltage-clamp experiment, the entire cell membrane is clamped to the same potential difference. Here, the field-induced membrane potential on different regions of the muscle fiber differs significantly, depending on the orientation of the region with respect to the electric field. The regions where the cell membrane is perpendicular to the electric field suffer the highest potential difference while those parallel to the electric field show no effect. Therefore, for a short pulse duration that is comparable to or shorter than the time constant in charging the cell membrane, only the regions or patches perpendicular to the electric field can be charged to the highest membrane potential while the potential difference on all other regions will be lower. If we clarify the membrane electroporation by observing the leakage currents, the pores must mainly occur at those regions perpendicular to the electric field. Similarly, if we identify the channel protein denaturation by observing the reduction in the channel currents, the denatured channel proteins must also be located mainly on these membrane regions.
Because of the fact that the conductivity of the phospholipid bilayer is eight orders of magnitude lower than that of electrolyte fluid [Goldman, 1943], very small pores or pore-like structures on the cell membrane will significantly increase membrane conductivity and leakage currents. When the leakage currents start to become observable, both the size and density of the electropores should be very small. If the cell membrane damage is reversible, the pore diameter is likely to be smaller than the thickness of the cell membrane, on the order of nanometer or subnanometer. It has been shown that the density of Na channel proteins in skeletal muscle fibers is only a few to the lower tens per square micrometer (or 106 square nanometers), depending on location [Hille, 2001]. Therefore, both the distance between the electropores, and the distance between the electropores and channel proteins are much larger (10–100 times) than the pore diameter. Even though lateral fields exist on the electropores present along the cell membrane, the crosstalk between the electropores and the channel proteins is not significant. These allow us to separately study the membrane lipid bilayer and membrane proteins.
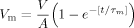 (1)
(1)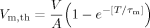 (2)
(2)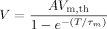 (3)
(3)Equation (3) gives the relation of the minimum applied potential difference, V, and the shock pulse duration, T, in terms of the critical membrane potential and the time constant in charging the cell membrane. In other words, as long as we conduct a large number of measurements of the minimum potential difference in response to various pulse durations, we can use Equation (3) to find the critical membrane potential Vm,th and the time constant τm. The best fitting results from 35 measurements using Equation (3) is the line shown in Figure 4. The fitting parameters are AVm,th = 0.7 V, and τm = 0.5 ms, and considering an unknown value of A, we only keep one significant digit. The software uses the nonlinear least squares fitting method based on the Levenberg–Marquardt algorithm.
From the viewpoint of statistics, the creation of pores in the cell membrane should not have a threshold potential. In this study, we are not monitoring the number of pores. Instead, we are measuring the membrane leakage currents due to the pore formation. The creation of pores in the cell membrane and the resultant leakage current are not necessarily linearly related. Due to the competition of different ions in the solution passing through the tiny pores with different driving forces, the interactions among these ions and pore structures may result in one membrane potential being more favorable than others in generating the leakage current, or exhibiting a critical membrane potential in the measurement of leakage currents. Similar things happen in other situations. For example, statistically, the voltage-gated Na channel open should not have a threshold while the channel currents often exhibit threshold phenomena.
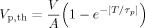 (4)
(4)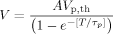 (5)
(5)In Figure 5, dots represent the measured minimum potential differences that reduce the channel current in response to various shock pulses durations. Based on these data points, the best fitting results from Equation (5) are shown as a line. The best fitting parameters are AVp,th = 1 V, and τp = 0.1 ms. The time constant (τp) is significantly smaller than that of the membrane electroporation (τm). This result is consistent with our prediction.
DISCUSSION
Comparison of the Critical Membrane Potentials in Damaging the Cell Membrane and Membrane Proteins When Exposed to an External Electric Field With Those From Previous Voltage-Clamp Studies
As discussed above, if the Vaseline wall is not built up perfectly or the solution is not removed enough, the field-induced membrane potential becomes smaller than V/2. In our previous studies using a voltage clamp to deliver a 4 ms shock pulse, we found that the minimum membrane potential for electroporation of the cell membrane is 250–300 mV [O'Neil and Tung, 1991; Chen and Lee, 1994a]. Based on these results and the best fitting results in Figure 4 (AVm,th = 0.7 V), we can estimate the value of A = 2.5, and the resultant minimum potential difference in electroporation of the cell membrane is Vm,th = 287 mV.
Based on the best fitting results in Figure 5 (AVp,th = 1 V) and the estimated value of A = 2.5, we can calculate the minimum membrane potential that denatures the Na channel protein as Vp,th = 400 mV. This result is consistent with our previous voltage-clamp studies, which showed that a 4 ms PEF in the range of 400–450 mV may denature Na channel proteins [Chen and Lee, 1994b].
Indeed, we are unable to accurately determine the value of A. The estimated value of A = 2.5 is reasonable because both the minimum potentials in electroporating the lipid bilayer and denaturing the membrane proteins are consistent with those we obtained from voltage-clamp studies. In addition, our goal was to compare the minimum potentials. The inaccuracy in the estimation of the A value does not affect our comparison. The results obtained here and in our previous voltage-clamp experiments consistently show that the minimum membrane potential Vp,th denaturing the membrane proteins is higher than the Vm,th electroporating the cell membrane.
This consistency has significant importance. First, it shows the validity of the method used in this study for field application. Therefore, the results we measured in response to other pulse durations are reliable. Secondly, in contrast to previous voltage-clamp studies where the shock pulse duration was fixed (4 ms) and Vp,th and Vm,th were directly measured, in this study, multiple pulse durations are involved and Vp,th and Vm,th were obtained by curve fitting. The results indicated that Vp,th and Vm,th are independent on the pulse duration and the field application. In other words, the results indicate that the critical or minimum membrane potentials in damaging the membrane phospholipid bilayer or denaturing the membrane protein are characteristics of the cell membrane, regardless of the shock pulse duration.
It is understandable that the value of Vp,th is larger than Vm,th, or the membrane phospholipid bilayer is more vulnerable to the electric shock than membrane proteins based on their structures. It is the hydrophobic force that limits the possible orientations of individual phospholipid molecules so that they can only form a stable bilayer configuration to remain in a minimal energy state. There is no chemical bond involved whatsoever in holding the lipid bilayer together. In contrast, many chemical bonds are involved in the membrane protein—not only the peptide bonds among the amino acid sequence but also many other chemical bonds in forming the three-dimensional protein structures. Because higher energy is needed to break down the chemical bonds than disrupting the orientation of lipid molecules, it is reasonable that the membrane lipid bilayer is more vulnerable to electric shock than the membrane protein.
Time Constant in Charging the Membrane Lipid Bilayer, τm,(0.5 ms) Is Much Longer Than in Charging the Potential Difference Across the Membrane Protein τp (0.1 ms)
Structures of the phospholipid bilayer and membrane protein differ significantly. The former consists of a large capacitance that needs a long time to be charged up while the latter has a much smaller capacitance. Even though the surrounding membrane capacitance may have some shunting effects, the relative large size of the membrane proteins compared to the thickness of the lipid bilayer makes the effects not significant. Therefore, the potential difference across the membrane lipid bilayer is built up more slowly than that across the membrane proteins.
The results showed that the time constant τm (0.5 ms) in charging the cell membrane is smaller than the intrinsic time constant of the cell membrane (about 1 ms), which is reasonable. Considering the series resistance in charging the cell membrane, the resultant charging time constant should become smaller than the membrane intrinsic time constant. The ratio of the time constants (τm/τp = 0.5/0.1 ≈ 5) mainly represents a ratio of the capacitance in the membrane lipid bilayer and those in the membrane proteins. This ratio may be an intrinsic characteristic of cell electrical injury because it is mainly a function of the membrane structure and independent on the experimental configuration.
Is the Cell Membrane or Membrane Protein More Vulnerable to a Pulsed External Electric Field?
On one hand, the critical membrane potential damaging the membrane proteins, Vp,th, is larger than that electroporating the lipid bilayer, Vm,th. On the other hand, the speed or time course in building up the membrane potential across the lipid bilayer is slower than that across the membrane protein. For a long PEF, which can fully charge the membrane capacitance, the critical membrane potentials dominate the results, while for a short PEF the highest potential difference that can be charged up throughout the pulse duration plays a significant role.
In order to compare the results for membrane electroporation and membrane protein denaturation, we plotted the two fitted curves superimposed in Figure 6. One can see that the two curves cross at the point between 0.5 and 1 ms. If the pulse duration is longer than this cross point, the required potential difference for membrane electroporation is lower than that for protein denaturation, or the membrane lipid bilayer is more sensitive to the electric shock. This result is consistent with our previous studies employing voltage-clamp techniques. However, when the pulse duration is shorter than the cross point, the potential difference needed for membrane electroporation is higher than that which damages the membrane protein, or the membrane proteins become more vulnerable to the electric shock.
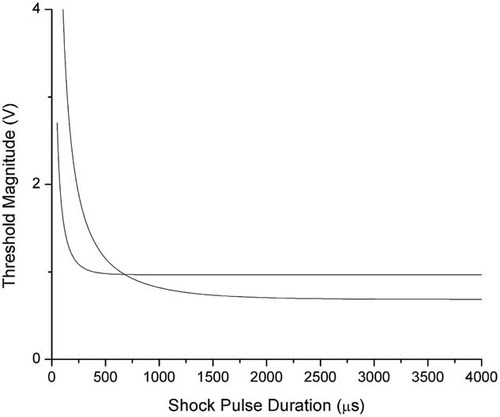
Comparison of the threshold magnitudes versus the shock pulse durations. Line closer to the y-axis: thresholds that denature the Na channel proteins; line away from y-axis: thresholds that electroporate the cell membrane.
How Does an Ultrashort PEF Affect Proteins in the Plasma Membrane?
Here, we studied the shock pulse duration down to tens of microseconds. An ultrashort pulse (down to tens of nanoseconds) has a frequency range much higher than the pulse duration of microseconds and milliseconds. In the frequency domain, the impedance of the cell membrane differs significantly in response to the different frequency contents of the electric field and the field-induced membrane potential due to membrane capacitance. This capacitance effect presented in the time domain is that the membrane potential is gradually (exponentially) charged up until the end of the pulse. It is necessary to point out that the measurements conducted in this paper are the thresholds and minimum potential differences in electroporating the lipid bilayer and denaturing the membrane proteins when the field starts to break down the corresponding dielectric constants, respectively. They are intrinsic characteristics of the cell membrane. As long as the membrane potential is charged up to these values, the lipid bilayer or membrane proteins will be damaged. In response to different pulse durations (milliseconds, microseconds, or nanoseconds), the pulse duration determines the highest membrane potential that can be charged up. Hence, this study has fully considered the effects of pulse duration. Therefore, the study of thresholds in response to millisecond and microsecond PEFs can be extrapolated to much narrower pulse durations. Figure 7 shows the extrapolation of the two curves in Figure 6 to the domain of microseconds and nanoseconds.
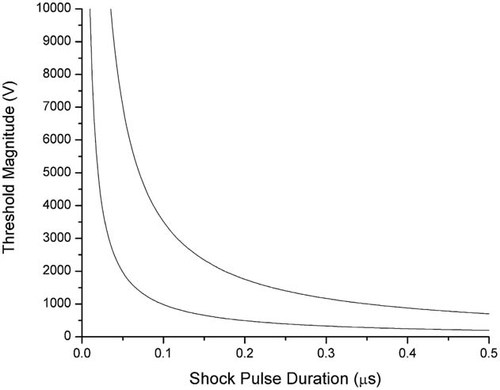
Prediction of the minimum magnitudes of the pulsed electric field needed to electroporate the cell membrane (line away from y-axis) or denature the membrane proteins (line closer to the y-axis) for ultrashort (nanosecond) shock pulse durations.
Based on the curve extrapolated from Figure 4, we can approximately estimate the necessary magnitude of the ultrashort pulses that electroporate the cell plasma membrane. For a nanosecond pulse, T = 100 ns, based on the value of AVm,th = 0.7 V and τm = 0.5 ms (from Equation 3), we need 3500 V applied to the pair of electrodes to charge the membrane potential within 100 ns up to the minimum membrane potential that can electroporate the phospholipid bilayer. If T is reduced to 50 ns, the minimum potential difference increases to 7000 V.
Similarly, we can estimate the potential difference needed to denature membrane proteins in the cell plasma membrane. For T = 100 ns, based on our results of AVp,th = 1 V and τp = 0.1 ms (from Equation 4), we can predict that if the pulse magnitude is higher than 1000 V, the Na channel proteins will be denatured. When T is reduced to 50 ns, the minimum pulse magnitude is 2000 V. For an intensive PEF with pulse duration in nanoseconds, the required or minimum potential difference to denature the membrane proteins is much smaller than that which electroporates the cell membrane.
Bio-responses of the cell membrane to nsPEFs depend on many factors including the duration, magnitude and polarity of the pulse, repetition rate, cell size, and orientation with respect to the electric field, and the way the electric fields are applied [Schoenbach et al., 2009; Joshi and Schoenbach, 2010]. The absolute value of the pulsed nanosecond electric field needed to damage the cell membrane or membrane proteins may not be the same for different experimental setups and sample preparations. However, the minimum potential differences that electroporate the lipid bilayer or damage the membrane proteins as well as their relationship are characteristics of the cell membrane. That is, for a long PEF (longer than the membrane intrinsic time constant) the membrane lipid bilayer is more sensitive to the electric field than the membrane proteins, while for a shorter pulse the proteins become more vulnerable.
From Figure 7, the results indicate that when the cells are exposed to an ultrashort pulsed electrical shock, which is often over tens of thousands of volts, the membrane capacitance may prevent the phospholipid bilayer in the plasma membrane from electrical injury, but the membrane proteins cannot.
In summary, we compared the vulnerability of the membrane phospholipid bilayer and membrane proteins in response to a PEF by studying their membrane thresholds. We experimentally measured the thresholds that either electroporate the cell membrane or denature the membrane proteins in response to different, relatively long pulse durations (from milliseconds to tens of microseconds), and then extrapolated the results to short or ultrashort pulsed electric shocks. By definition, the threshold is the minimum membrane potential that starts to damage the cell membrane or membrane proteins. Characteristics of the cell membrane, including conductance and the dielectric constant, remain unchanged immediately before the membrane loses its integrity. In order to compare the vulnerability, we selected a model where the rise and fall times of the PEF are much shorter than the pulse duration. When studying the process of membrane poration in response to a specific PEF, the pulse rising and falling phase [Vasilkoski et al., 2006] and the membrane conductance variation during the pore formation [DeBruin and Krassowska, 1999] have to be considered.
In addition, we studied the nsPEF in a range from tens to hundreds of nanoseconds, where the capacitance across the cell membrane and proteins are considered as constants. If the shock pulse duration becomes even shorter (<10 ns), the microdosimetric models have shown that the high frequency effects of ultrashort nanosecond pulses make the membrane capacitance frequency dependent [Merla et al., 2011, 2012] since the permittivity of the membrane and the cellular compartments become a function of frequency.
Acknowledgements
We thank James Weaver of MIT for his constructive discussions for the project and the manuscript.




