Combined effect of X-ray radiation and static magnetic fields on reactive oxygen species in rat lymphocytes in vitro
Abstract
The aim of this study was to investigate the effect of static magnetic fields (SMF) on reactive oxygen species induced by X-ray radiation. The experiments were performed on lymphocytes from male albino Wistar rats. After exposure to 3 Gy X-ray radiation (with a dose rate of 560 mGy/min) the measurement of intracellular reactive oxygen species in lymphocytes, using a fluorescent probe, was done before exposure to the SMF, and after 15 min, 1 and 2 h of exposure to the SMF or a corresponding incubation time. For SMF exposure, 0 mT (50 µT magnetic field induction opposite to the geomagnetic field) and 5 mT fields were chosen. The trend of SMF effects for 0 mT was always opposite that of 5 mT. The first one decreased the rate of fluorescence change, while the latter one increased it. Bioelectromagnetics 34:333–336, 2013. © 2012 Wiley Periodicals, Inc.
The influence of electromagnetic fields on the rate of radical pair recombination is one of the most frequently analysed biophysical mechanisms of electromagnetic fields (EMF) affecting living matter. This model was first proposed by Brocklehurst [1969], and since that time it has been improved [Timmel et al., 1998] and verified in many experimental studies [Molin et al., 1979; McLauchlan and Steiner, 1991]. This mechanism produces changes in the number of free radicals formed as a result of physiological processes and various external chemical and physical agents. Oxidative processes including, for example, peroxidation of lipids that serve as the main material of biological membranes, represent one of the biological effects of free radical activity. In our laboratory, we have already investigated the effects of various EMF frequencies on oxidative processes in different cells exposed to various factors that initiate the formation of reactive oxygen species (ROS) [Jajte et al., 2002; Zmyślony et al., 1998, 2004].
One of the physical agents that initiate processes resulting in the formation of radical pairs include ionising radiation. The combined activities of EMF and ionising radiation seem particularly interesting, as the latter has been broadly used in medical diagnostics and therapy, while exposure to man-made EMF is practically inevitable.
The aim of this study was to investigate the effects of static magnetic field (SMF) on ROS produced by X-ray radiation. The experiments were performed on lymphocytes from male albino Wistar rats (outbred stock Imp:WIST) aged 3–4 months and weighing 320–380 g. The method of collection of lymphocytes was described earlier [Zmyślony et al., 2004]. The experimental protocol of our current study was reviewed and approved by the 9th Local Ethics Committee of the Medical University, Lodz, Poland (17/LB514/2010).
Pooled lymphocytes were divided into six groups (in parentheses, the pooled number of samples): Group I—exposed to X-ray radiation and 0 mT SMF (6); Group II—exposed to X-ray radiation and 5 mT SMF (7); Group III—exposed only to X-ray radiation (12); Group IV—exposed only to 0 mT SMF (6); Group V—exposed only to 5 mT SMF (9); and Group VI—non-exposed (11). For each group, 3–8 samples of 0.4 ml lymphocyte suspensions (containing approximately 4 × 105 of lymphocytes) and 0.6 ml of RPMI 1640 medium without L-glutamine were added.
Groups I–III were exposed to X-ray radiation for 5 min, 20 s. At the same time, the remaining samples (Groups IV to VI) were incubated at approximately 22 °C. Next, SMF-exposed groups (I, II, IV and V) were exposed to the SMF, and the remaining groups (III and VI) were incubated as described below. Before exposure to the SMF, and after 15 min, 1 and 2 h of exposure to the SMF or a corresponding incubation time, the measurements of intracellular ROS were performed using a fluorescent probe. Fluorescence of the samples was determined in the same sequence for successive measurements. Each sample measurement time was recorded for further analysis. For the time of fluorescence measurement, exposure/incubation was interrupted. The experiment was repeated once and the resultant data were pooled for analysis. A schematic of the experiment is presented in Table 1.
| Group | Time (min)/Cumulative time of exposure to SMF or incubation (min) | |||||||
|---|---|---|---|---|---|---|---|---|
| 5.33/0 | 5/0 | 15/0 | 5/15 | 45/15 | 5/60 | 60/60 | 5/120 | |
| I | X | F | 0 | F | 0 | F | 0 | F |
| II | X | F | 5 | F | 5 | F | 5 | F |
| III | X | F | I | F | I | F | I | F |
| IV | I | F | 0 | F | 0 | F | 0 | F |
| V | I | F | 5 | F | 5 | F | 5 | F |
| VI | I | F | I | F | I | F | I | F |
| X | Exposure to X-rays |
| I | Incubation—geomagnetic field |
| 0 | Exposure to 0 mT SMF |
| 5 | Exposure to 5 mT SMF |
| F | Fluorescence measurements |
In our experiment, the lymphocytes were irradiated by X-ray radiation generated from a 300 kV Gulmay X-Ray Calibration System (Surrey, UK). The exposure data were as follows: 150 kV at 15 mA X-ray tube current, 4 mmAl total filtration, 56 cm focal distance, and 8 cm × 8 cm homogeneous irradiated fields over the samples. The main parameters of the X-ray spectra were also investigated. The effective energy of the applied radiation was 50 keV. The dose of 3 Gy was applied at a dose rate of 560 mGy/min.
For SMF exposure, 0 mT (provided by approximately 50 µT magnetic induction fields directed opposite to the geomagnetic field to effectively cancel the latter) and 5 mT magnetic field inductions were chosen. The 0 mT field was chosen because of the expected decrease in the ROS number (compared to geomagnetic exposure) resulting from a reduction in S–T (singlet–triplet) transitions by the 0 mT field––the basis for the low SMF influence on the radical pairs model [Timmel et al., 1998], while 5 mT was chosen because this exposure provided valuable data in our previous works. Exposure to the SMF was performed inside a pair of Helmholtz coils (35 cm in diameter), which provided a highly homogenous field (±5%). For measurements of flux density (magnitude and distribution), an F.W. Bell gaussmeter Model 9500 A, with an STF 99-0404 probe (Orlando, FL) was used; the range of measurements was 3 µT–30 T, with an accuracy of ±0.17%. Lymphocyte suspensions were exposed to the SMF in 1.5 ml light-proof Eppeddorf tubes in a tightly closed Plexiglas container (26 cm long, 17 cm diameter cylinder with no metal parts) placed inside the pair of Helmholtz coils. An identical container for non-exposed samples was placed outside the Helmholtz coils (in the natural geomagnetic field). Each container was coupled with a thermostat to form a closed system of heated (37 ± 1 °C) compressed air circulation and temperature control, which also prevented additional heating within the Helmholtz coils. In such an arrangement, the exposure system did not disturb the electromagnetic field uniformity inside the Helmholtz coils; the uniformity was confirmed by measurements.
A 2′,7′-dichlorofluorescin diacetate (H2DCFDA) fluorescent probe (Molecular Probes, Leiden, The Netherlands) was used to assess the level of ROS. H2DCF-DA is a non-polar compound that easily passes the cell membrane and is hydrolysed by intracellular esterases to the non-fluorescent polar derivative, DCFH. In the presence of ROS, DCFH is oxidised to fluorescent dichlorofluorescein (DCF). This method has been described in detail earlier [Zmyślony et al., 2004]. To minimise DCFH photooxidation, samples were kept in darkness throughout the experiment. The level of DCF fluorescence (proportional to the ROS level) was monitored using a spectrofluorimeter (LS-50B, Perkin-Elmer, Beaconsfield, UK) with a 488 nm excitation wavelength and 521 nm emission wavelength. The viability of the lymphocytes was monitored through the experiment and was not less than 95%. The fluorescence of the samples was determined just before (Fs) and immediately after (Ff) the exposure.
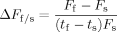 (1)
(1)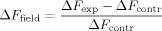 (2)
(2)The dimensionless data obtained this way were statistically analysed using two-way ANOVA, and significant differences between the groups were evaluated using Tukey's post hoc test; P < 0.05 was selected for statistical significance [Scheffe, 1959].
The exposure of lymphocytes to X-ray radiation (effective energy 50 keV, dose 3 Gy, dose rate 560 mGy/min) triggered an average 340% increase in fluorescence (533.1 ± 14.7 in groups exposed to X-rays compared to 121.2 ± 41.9 in groups non-exposed to X-rays). The increased ROS levels in lymphocytes exposed to X-ray radiation are expected as radiolysis is one of the ways of obtaining ROS (hydroxyl radical or hydrogen peroxide are common products of water irradiation); these results are consistent with results of other authors [Kalpana et al., 2010]. Figure 1 illustrates the effects of the applied SMF on ROS produced in lymphocytes (naturally and stimulated with X-ray radiation). For irradiated lymphocytes, we observed that the SMF effect in Group II was significantly higher than in Group III, both after 15 min and 2 h of exposure to the SMF (P = 0.032 and P < 0.001, respectively). Additionally, for 2 h of exposure, the SMF effect was lower in Group I than in Group III, with P = 0.005 (Fig. 1). In lymphocytes not exposed to X-ray radiation, for all times of exposure to SMF or corresponding incubation times, the SMF effect in Group V was higher than in Group VI, with P < 0.001 (Fig. 1).
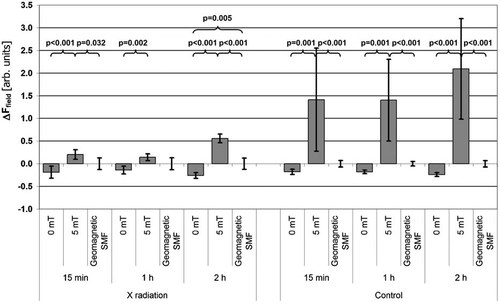
Effects of 0 and 5 mT SMF exposure on ROS level changes in rat lymphocytes for lymphocytes exposed to X-ray radiation and those non-exposed to X-rays (Control).
Additionally, for every time point, whether samples were exposed to X-ray radiation or not, the SMF effect for lymphocytes exposed to 5 mT (Groups II and V) was higher than for lymphocytes exposed to 0 mT (Groups I and IV), with significance levels not exceeding 0.002 (Fig. 1). Furthermore, the trend of SMF effects for 5 mT was always opposite to that for 0 mT. The first one increased the rate of fluorescence change, while the latter one decreased it.
There was some improvement in methodology in comparison to our earlier papers [Zmyślony et al., 2004]. Because oxidation naturally proceeds with time, even during the time of measurement, we decided to precisely record the time of successive fluorescence measurements. This improvement allowed us to eliminate the time factor as an interfering variable (see Eq. 1) and demonstrated the SMF influence on ROS levels in lymphocytes not stimulated to ROS overproduction by physical or chemical factors (e.g. UV radiation or the addition of Fe2+ ions to lymphocyte suspensions) [Zmyślony et al., 2004].
As expected, the exposure of lymphocytes to X-ray radiation caused an immediate increase in their ROS content. Our results also do not disqualify the hypothesis about SMF affecting ROS levels in rat lymphocytes in vitro by the radical pair mechanism. For the chosen inductions of SMF, 5 mT resulted in a stable increase in the rate of fluorescence change, while 0 mT resulted in a decrease in the same parameter compared to the geomagnetic field. It has been particularly interesting to note the effect of SMF in non-stimulated lymphocytes. Such an effect has been obvious in theory for some time, but this is the first time it has been confirmed in an experiment.
The conversion of DCFDA to a fluorescent product inside the cells is done via an intermediate radical, DCF [Eruslanov and Kusmartsev, 2010]. Therefore, it is plausible that the SMF may affect H2DCF-DA fluorescence measurements intracellularly, independent of ROS production. However, there are some other papers showing similar results without DCF use, for instance, measuring the influence of SMF on ROS levels with T-BARS [Zmyślony et al., 1998], which minimise the possibility of the SMF affecting only intermediate radical reactions.
The SMF values chosen for our experiment were very low. Values like 50 µT or even 5 mT are significantly lower than any worldwide general population exposure limits (e.g. the International Commission on Non-Ionizing Radiation Protection recommends 400 mT as the exposure limit for the general population [ICNIRP, 2009]). The potential implications of such a low SMF affecting ROS levels should not be underrated. Man-made SMF, usually much higher than the geomagnetic field, are produced wherever direct electric currents or permanent magnets are used. For example, the SMF may reach a few Tesla for magnetic resonance imaging devices, and 50 mT at the floor level of a magnetic levitation vehicle [Feychting et al., 2005]. Electrical equipment can contain loudspeakers with permanent magnets that produce SMF; the transmission and distribution of direct current electricity also produce SMF. Considering the outcomes of our experiment, the ubiquitous headphones and earphones, with permanent magnets located in close proximity to the brain and hearing organ and producing 3–5 mT SMF, should be regarded as very promising material for further investigations.




