Mouse genetic models of cleft lip with or without cleft palate
Abstract
Nonsyndromic cleft lip and palate (CLP) is among the most common human birth defects. Transmission patterns suggest that the causes are “multifactorial” combinations of genetic and nongenetic factors, mostly distinct from those causing cleft secondary palate (CP). The major etiological factors are largely unknown, and the embryological mechanisms are not well understood. In contrast to CP or neural tube defects (NTD), CLP is uncommon in mouse mutants. Fourteen known mutants or strains express CLP, often as part of a severe syndrome, whereas nonsyndromic CLP is found in two conditional mutants and in two multifactorial models based on a hypomorphic variant with an epigenetic factor. This pattern suggests that human nonsyndromic CLP is likely caused by regulatory and hypomorphic gene variants, and may also involve epigenetics. The developmental pathogenic mechanism varies among mutants and includes deficiencies of growth of the medial, lateral or maxillary facial prominences, defects in the fusion process itself, and shifted midline position of the medial prominences. Several CLP mutants also have NTD, suggesting potential genetic overlap of the traits in humans. The mutants may reflect two interacting sets of genetic signaling pathways: Bmp4, Bmpr1a, Sp8, and Wnt9b may be in one set, and Tcfap2a and Sox11 may be in another. Combining the results of chromosomal linkage studies of unidentified human CLP genes with insights from the mouse models, the following previously unexamined genes are identified as strong candidate genes for causative roles in human nonsyndromic CLP: BMP4, BMPR1B, TFAP2A, SOX4, WNT9B, WNT3, and SP8. Birth Defects Research (Part A), 2008. © 2008 Wiley-Liss, Inc.
INTRODUCTION
Nonsyndromic cleft lip with or without cleft palate, CLP, is among the most common serious birth defects in all human populations (Fig. 1). The causes appear to be complex, mostly genetic, and to involve interaction of genotype with environmental factors. The genes and environmental factors are largely unknown. In contrast to isolated cleft secondary palate, for which many mutations are known, only a handful of mouse mutations are known to cause CLP and the CLP is nearly always part of a severe multiorgan syndrome. There are two mouse models with nonsyndromic CLP that resemble human CLP in genetic complexity and environmental interaction. Together the CLP-causing mutations in mice point directly to candidate genes for human CLP and indirectly to several related candidate genes, and they illuminate developmental mechanisms that can lead to CLP. Most of these candidate genes have not been examined in human cases.
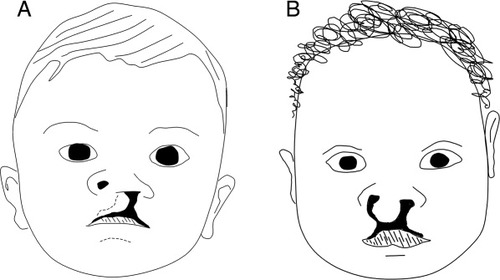
Drawings of unilateral cleft lip on patient's left side (A) and bilateral cleft lip (B). The cleft extends through the upper lip into the nostril and internally through the upper jaw. Often patients with cleft lip also have a cleft secondary palate (not shown).
HUMAN CLP GENETICS
Typical occurrence rates of CLP are 1–2 per thousand births (Fraser, 1970). Although several rare Mendelian syndromes include CLP among the combinations of defects (reviewed in OMIM; www.ncbi.nlm.nih.gov/omim/), most CLP cases are nonsyndromic and non-Mendelian (Calzolari et al., 2007; Fraser, 1970). Elevated recurrence risks in relatives of probands demonstrate that there is a strong hereditary basis to risk of CLP (e.g., sib risk is 3–5%; Fraser, 1970; Mitchell, 1997). The pattern of elevated risk levels across relatives is consistent with polygenic inheritance, where the level of risk of CLP in an individual is due to the combined effects of multiple genes, plus the effects of environmental variables (Carter, 1969; Falconer, 1965; Fraser, 1970; Mitchell, 1997). This mode of heredity also has been indicated for other common birth defects, such as the neural tube defects (NTD), anencephaly and spina bifida.
Population-based studies of recurrence of clefting in families indicate that the elevated recurrence risks are specific to type of cleft: CLP versus isolated cleft palate (CP). Rates of occurrence of CP usually are not elevated in relatives of nonsyndromic CLP probands, and rates of occurrence of CLP usually are not elevated in relatives of nonsyndromic CP probands (Fraser, 1970; Woolf et al., 1963). The simplest explanation for this pattern is that the gene variants causing risk of nonsyndromic CLP are mostly different than those in nonsyndromic CP. This contrasts with the genes that cause a few rare Mendelian syndromes that can include either CLP or CP. Van der Woude's syndrome is caused by IRF6 mutations (Kondo et al., 2002); variants at IRF6 also contribute to a small proportion of cases of nonsyndromic CLP and CP (Chakravarti, 2004; Zucchero et al., 2004). MSX1 mutations also cause either CLP or CP as part of a syndrome (van den Boogaard et al., 2000) and variants of MSX1 may contribute to a small proportion of cases of nonsyndromic CLP and CP (Jezewski et al., 2003; Modesto et al., 2006). PVRL1, a gene that causes a Mendelian ectodermal dysplasia syndrome with CLP, also contributes to a small proportion of clefts in pooled CLP and CP cases (Avila et al., 2006). P63 gene mutations cause another ectodermal dysplasia syndrome with either CLP or CP (Celli et al., 1999) and P63 variants might have a small role in nonsyndromic CLP in some populations (Leoyklang et al., 2006). Variants of genes in the FGF signaling pathway (FGF3, FGF7, FGF10, FGF18, or FGFR1) are also associated with nonsyndromic CLP or CP in small proportions of human clefting cases (Riley et al., 2007). Lastly, PTCH mutations cause a complex syndrome that includes a low rate of CLP or CP (Cohen, 1999; Gorlin, 1995) and uncommon variants may contribute to a few cases of nonsyndromic CLP (Mansilla et al., 2006). In contrast to the human mutants, none of these genes seems to cause CLP in mouse mutants. The Irf6 (Ingraham et al., 2006; Richardson et al., 2006) and Msx1 (Satokata and Maas, 1994) mouse mutants have only CP, not CLP; no clefts have been reported for p63 mutants (Mills et al., 1999; Yang et al., 1999) or Pvrl1 (nectin-1) mutants (Inagaki et al., 2005). Mouse mutants for Fgf10, Fgf18, or Fgfr1 have CP but not CLP (cited in Riley et al., 2007). The mouse mutant for Ptch (Ptch1) has a homozygous syndrome lethal before lip formation (Goodrich et al., 1997).
Of course, focusing on human syndromes with both CLP and CP as a source of candidate genes for nonsyndromic CLP probably creates an ascertainment bias in the types of genes studied. The statistical independence of CLP from CP in population-based studies of recurrence of nonsyndromic clefts predicts that in the majority of cases, the as-yet-unknown genes contributing to risk of nonsyndromic CLP are mostly different than those contributing to nonsyndromic CP.
Despite many genetic analyses, linkage and gene association studies, and sequencing of candidate genes in probands, most of the genetic etiology of common human nonsyndromic CLP remains unknown.
NON-MENDELIAN AND ENVIRONMENTAL PHENOMENA IN HUMAN CLP
Maternal race might influence risk of CLP according to a study of interracial crosses (Khoury et al., 1983). Maternal MTHFR genotype might affect risk of CLP, but studies are inconsistent (Chevrier et al., 2007; Pezzetti et al., 2004; Prescott et al., 2002). Periconceptional maternal folic acid supplementation appears to reduce risk of CLP (Badovinac et al., 2007; Wilcox et al., 2007). Maternal cigarette smoking increases risk of CLP, particularly if the embryo has a GSTT1 deficiency genotype (Shi et al., 2007). Agents that are known teratogens, such as maternal alcohol or anticonvulsant consumption, also increase risk of CLP (Carinci et al., 2003). Risk of CLP is higher for males than for females (Hay, 1971; Mitchell and Christensen, 1996). Season of conception may affect risk (Fraser and Gwyn, 1998). The frequency of non-right-handedness appears to be elevated in CLP probands and their relatives (Wentzlaff et al., 1997). There may be increased developmental instability in CLP probands and their relatives as assessed by degree of asymmetry of dermatoglyphics (Neiswanger et al., 2005; Woolf and Gianas, 1977). These phenomena may be clues to the nature of major etiological factors in CLP and may point to involvement of some nontraditional or epigenetic factors.
ETIOLOGICAL OVERLAP OF CLP AND NTD IN HUMAN POPULATIONS
Population frequencies, patterns of recurrence risks, and apparent multifactorial inheritance are similar for CLP and neural tube closure defects (NTD) (reviewed in Mitchell, 1997 and Harris and Juriloff, 2007). There are two types of intriguing evidence that CLP may have some etiological overlap with NTD. First, presence of one of these two traits in a family seems to increase risk of occurrence of the other trait in other individuals. Fraser et al. (1982) found that prevalence of NTDs was consistently and significantly elevated (over twofold) in sibs and parents of CLP probands in each of four geographically widespread medical centers. A review of the reciprocal relationship in the literature by Toriello and Higgins (1983) showed that the prevalence of CLP was significantly elevated (about twofold) in sibs of probands with NTD. Second, risk of both NTD and CLP can be reduced by maternal supplementation with folic acid; the data for NTD are well established (Mitchell, 2005); the consistent data for CLP are more recent (Badovinac et al., 2007; Wilcox et al., 2007).
DEVELOPMENT OF THE UPPER LIP
The development of the embryonic face is similar in mice and humans (Diewert and Wang, 1992). The development of the upper lip has been well-reviewed recently (Jiang et al., 2006), and a general outline is presented here, for use in interpretation of the CLP mutants.
Part of the development of the upper lip and nose begins at the induction of a pair of nasal (olfactory) placodes by the bilaterally divergent frontolateral aspects of the prosencephalon (Sulik and Johnston, 1982) at the end of the 4th week of human gestation or early GD10 in mouse (Sadler, 2000). The medial and lateral aspects of each of the nasal placodes, areas whose mesenchyme is largely derived from forebrain and midbrain neural crest cells (Osumi-Yamashita et al., 1994), grow to become prominences (processes) projecting frontally, surrounding a pair of pits (“nasal grooves”) that will become the nostrils (Fig. 2). This takes place at GD10–11 in mouse embryos and week 5 in human (Jiang et al., 2006; Trasler, 1968). At the same time, the dorsal cranial region of the first branchial arch (see Fig. 1g in Sulik and Johnston, 1982) expands frontally below the developing eye to form the maxillary prominence (process) (Fig. 2) while the ventral and more caudal region of the first arch becomes the lower jaw. The mesenchyme of the maxillary prominence is partly derived from neural crest cells originating in the midbrain and anterior rhombencephalon, a source tending to be more caudal than those going to the medial and lateral nasal prominences (Osumi-Yamashita et al., 1994). The neural crest cells migrate to the face from the regions of the cranial neural folds that are typically involved in cranial NTD, during cranial neural tube closure.
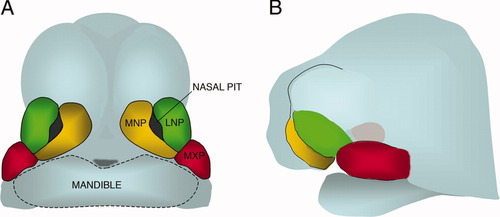
Diagram representing a frontal view (A) and lateral view (B) of the embryonic mouse face and head at the time of formation of the upper lip and pair of nostrils. A Medial Nasal Prominence (MNP) fuses with a Lateral Nasal Prominence (LNP) and a Maxillary Prominence (MXP) around each nasal pit.
The medial nasal prominences (MNP), lateral nasal prominences (LNP), and maxillary prominences (MXP) undergo directional growth to converge and fuse in a three-way junction (Fig. 2). At the external surface in mouse, the MNP and LNP seem to meet first, followed by meeting of the MNP and MXP (Trasler, 1968), and the contact in the internal area of the nasal groove is between the MNP and LNP. At the external surface in human, the MNP and MXP seem to meet first, at the frontal portion of the nasal grooves, followed by meeting of the MNP and LNP in the internal area of the nasal groove (Diewert and Wang, 1992; Jiang et al., 2006).
The fusion process involves death of periderm cells of the apposed prominences, bridging of the gap by epithelial filopodia, formation of the “nasal fin” or seam comprised of the apposed epithelia, regression of the nasal fin by an uncertain mechanism (apoptosis, migration to adjacent epithelia, or transformation to mesenchyme) and consolidation of the union by a mesenchymal bridge (Diewert and Wang, 1992; Jiang et al., 2006).
Cleft lip (CL) arises in failure of fusion of the MNP with the MXP and the LNP, resulting in a gap, lateral to the midline, extending through the upper lip and jaw into the nostril, as shown in Figure 1 (Diewert and Shiota, 1990; Jiang et al., 2006; Trasler, 1968; Wang et al., 1995b). The cleft can be unilateral or bilateral.
Various mechanisms might be predicted to cause cleft lip (CL): an abnormally small prosencephalon that induces more centrally placed nasal placodes (Sulik and Johnson, 1982) and more medially placed MNP, enlarging their distance from the MXP and delaying contact; delayed or deficient growth of the MNP so that they do not diverge towards the LNP and MXP sufficiently for contact; delayed or deficient growth of the LNP, so that the part of the MNP that would initiate fusion does not contact its posterior edge; delayed or deficient growth of the MXP, so that it fails to make adequate contact with the MNP and LNP; an impaired fusion process (cell–cell recognition at contact, breakdown of apposed epithelia, and infiltration by mesenchyme). The multifactorial nature of CLP could reflect combinations of these factors in individuals. The mouse models seem to demonstrate some of these mechanisms.
At the time of fusion of facial prominences, the outgrowth of the pair of MNP has created a V-shaped gap in the midline of the embryonic face. Subsequent growth of the midline and MNP tissue fills out the gap “from behind,” along with a relative narrowing of the midline by the movements of the surrounding tissue (Diewert and Shiota, 1990; Jiang et al., 2006). Failure of this midline merging leads to a midline cleft that is developmentally distinct from CLP. Sometimes a midline cleft reflects an underlying defect in closure of the most rostral aspect of the neural tube (Harris and Juriloff, 1999).
The secondary palate develops later (GD12-15 in mouse; weeks 6–7 in human) from outgrowths of the medial aspect of the MXP in the oral cavity (Ferguson, 1988; Sadler, 2000). The palatal shelves develop vertically, elevate to a horizontal position, make contact and fuse. Factors that reduce shelf size, block or delay elevation, or cause lack of the fusion process itself, cause CP (Ferguson, 1988; Juriloff, 2002). The CP in CLP may be due to mechanical interference due to the CL or to a defect intrinsic to the palate.
OVERVIEW OF THE MOUSE MODELS FOR CLP
In mice, for CLP, there are 10 spontaneous or induced Mendelian mutants, 2 conditional (tissue specific) knockout mutants, 1 multifactorial genetic model represented by several related strains, and 1 new multifactorial model based on a compound mutant (Table 1). The mouse CP mutants have been reviewed recently (Gritli-Linde, 2007). This review will discuss only the mouse mutants and strains that cause CLP, based on the stance that the mouse CLP mutants and human population data indicate that many of the genes and signaling pathways that cause CLP are different from those that cause CP. These CLP models (Table 1) offer insights into the etiology of human CLP; most involve different genes than those chosen as candidate genes for sequencing in human CLP cases (e.g., Vieira et al., 2005). These mouse CLP mutants, with the support of positive human linkage studies, point to several excellent human candidate genes that have not been studied in CLP cases.
| Gene or strain | Type of mutation | Syndrome? | Gene function | Cleft frequency† | Mouse/human gene location | Human linkage? |
|---|---|---|---|---|---|---|
| Bmp4 | Conditional KO: complete in MXP, partial in MNP and LNP* | No other defects | Signaling | 20% | Chr 14/14q22–q23 | Yes |
| Bmpr1a | Conditional KO: complete in MXP, partial in MNP, LNP* | No other defects | Signaling | 100% | Chr 14/10q22 | No |
| Bn | Bent tail spontaneous multi-gene deletion; includes Zic3 | Bent short tail, EX, omphalocele, situs defects | UN | 5% | Chr X/Xq26 | No |
| Folr1 | Null KO: partial rescue by folic acid | Polydactyly, EX, agnathia, anophthalmia, body wall defects | Folate transport | Some | Chr 7/11q13 | No |
| XtBph | Brachyphalangy, Bph, radiation-induced mutation; includes Gli3 | Polydactyly, EX | Transcription | 20% | Chr 13/7p14 | Yes |
| Sox11 | Null KO | Heart defects (VSD), lung hypoplasia, asplenia, open eyelids, omphalocele | Transcription | 70% | Chr 12/2p25 | No |
| Sp8lgl | Legless, lgl, spontaneous multi-gene deletion | Limb defects, midface deficiency, forebrain protrusion, situs defects | Transcription | 50% | Chr 12/7p15 | Yes |
| Tbx10 | Dancer, Dc, spontaneous insertion, ectopic expression in facial region | Inner ear defect | Transcription | 100% | Chr 19/11q13 | Yes (broad region) |
| Tcfap2a | Null/normal chimera | Polydactyly, EX, body wall and eye defects | Transcription | 40% | Chr 13/6p24 | Yes |
| Tw | Twirler spontaneous mutation | Inner ear defect | UN | Most | Chr 18/10p12 | No |
| Recessive in “Line 2” | ENU-induced, point mutation? | EX, body wall defects,severe craniofacial defects | UN | Not stated | Chr 15/5p or 8q | Yes (8q) |
| Wnt9b | Null KO | Vestigial kidneys; no reproductive ducts | Signaling | 50% | Chr 11/17q21 | Yes |
| A/− strain (digenic) clf1, clf2 | clf1, spontaneous hypomorph of Wnt9b; clf2, unknown | No other defects | clf1, signaling clf2, UN | 5–30% | Chr 11/17q21; Chr 13/5p or 9q | Yes Yes (9q) |
| Wnt9b/clf1, clf2 | Compound mutant at Wnt9b, modified by clf2 | No other defects | Wnt signaling clf2 UN | 10–90% | Chr 11/17q21; Chr 13/5p or 9q | Yes Yes (9q) |
- MNP, medial nasal prominences; LNP, lateral nasal prominences; MXP, maxillary nasal prominences; KO, gene knockout; EX, exencephaly; SBA, spina bifida aperta; VSD, ventricular septal defects; UN, unknown.
- * Conditional mutation expressed only in cells of facial prominences; nonconditional mutation causes early embryonic lethality.
- † Cleft frequency in homozygotes.
The models suggest that the variants contributing to human nonsyndromic CLP are often regulatory variants with reduced activity (hypomorphs). Many of the mouse CLP models are recessive “null” mutants (complete loss of function of the mutant gene) that cause syndromes of multiple defects with high penetrance. Two are facial-prominence-specific conditional mutations that produce nonsyndromic CL or CLP. For one of the genes, Wnt9b, two types of mutant allele have been studied: a null allele, and an allele with some residual gene activity. The null allele causes a severe syndrome including CLP. The hypomorph causes nonsyndromic CLP with lower penetrance and subject to a “modifier” at another gene locus, and is the model that most closely resembles human CLP in other aspects of etiology.
The models demonstrate a variety of genetic and developmental mechanisms underlying CLP, and this heterogeneity may have clinical significance if present in human CLP. They also demonstrate genetic etiological overlap with NTD. One multifactorial model introduces possible prevention by maternal folate supplementation and epigenetics (methylation of DNA) into the etiology of CLP.
By standard convention, mouse genes are written with an upper case first letter and lower case subsequent letters, all italicized (e.g., Wnt9b), whereas human genes are written entirely in uppercase letters, italicized (e.g., WNT9B). This serves to distinguish genes (italicized) from proteins and human species (upper case) from mouse when all are under discussion.
MODELS WITH MENDELIAN INHERITANCE
Bmp4 Conditional Mutant
The Bmp4 gene codes for an extracellular growth factor, required for mesoderm formation. The null mutant is an embryonic lethal, dying by GD 9.5 (Winnier et al., 1995). Bmp4 also has roles in cardiac morphogenesis (cited in Liu et al., 2005) and limb development (Bandyopadhyay et al., 2006).
In the embryonic face, Bmp4 is expressed in the MNP, LNP (Gong and Guo, 2003), and MXP (Fig. 6A in Liu et al., 2005) before contact and fusion, in the areas that will fuse these three prominences together, strongest in the ectoderm of the posterior half of the nasal pit. During fusion, Bmp4 is expressed in the ectoderm at the site of contact and ventral to the site of fusion, and within the disintegrating epithelial seam. After fusion of the facial prominences, Bmp4 expression diminishes and appears to be localized to the rim of the nasal pit and has shifted to the mesenchyme.
A conditional mutation was created in which the Bmp4 gene is probably completely inactivated in the epithelium and mesenchyme of the MXP by GD10.5, and partially inactivated in the epithelium of the LNP and MNP (Liu et al., 2005). The inactivation is irreversible and therefore descendants of these cells, such as those from the MXP that will contribute to the palate shelves, also lack Bmp4. The Bmp4 conditional mutant demonstrated bilateral delay of fusion of the MNP and MXP, and later about 20% (2/9) had isolated unilateral CL. The secondary palate was closed. The incomplete penetrance of CL could be due to incomplete inactivation of Bmp4 in the MNP and LNP epithelium or to redundant pathways that also facilitate fusion of the facial prominences, or both.
RNAi knockdown of Bmp4 caused delay in outgrowth of the MNP and LNP, but embryos subsequently recovered and fusion was accomplished, again perhaps pointing to redundancy of mechanisms involved in this process (Shuman and Gong, 2007).
Interestingly, another gene from the same gene superfamily, Tgfb2, is expressed in a similar, but more restricted pattern than Bmp4, in the ectoderm of the leading edges of the MNP, LNP, and MXP, at the prospective site of fusion and during fusion, and disappearing with the disintegration of the epithelial seam (Behnan et al., 2005). Later it is expressed in the mesenchyme of the developing secondary palate shelves, and the null mutant for Tgfb2 has low penetrance CP, but not CLP (Sanford et al., 1997). One could speculate that there is redundancy between Bmp4 and Tgfb2 function during the fusion step of the developing upper lip. TGFB2 itself (1q41) does not seem to be implicated in human CLP by linkage or association studies (Lidral et al., 1997; Marazita et al., 2004). However, other related genes, TGFB1 and TGFB3, are implicated. Both seem to be expressed abnormally in CLP patients (Bodo et al., 1999). The genomic regions have shown linkage (TGFB3; 14q24) or suggestion of linkage (TGFB1; 19q13) to CLP (Marazita et al., 2004), but gene sequence changes in CLP have not been found (Vieira et al., 2005).
BMP4 maps to human chromosome 14q22–q23 (MGD, 2007) a region that is implicated in risk of nonsyndromic CLP by human linkage studies (Marazita et al., 2004). BMP4 hypomorphs, in combination with other factors, would be excellent candidates for a role in CLP etiology. Surprisingly, BMP4 does not appear to have been examined for variants in CLP cases (e.g., Vieira et al., 2005).
Bmpr1a Mutants
Bmpr1a (Alk3), codes for a receptor for Bmp4 and Bmp2 and is required for formation of mesoderm. It is widely expressed in early embryonic and adult tissues. The null mutant is an early embryonic lethal that dies around the time of gastrulation (Mishina et al., 1995).
A conditional mutation was studied in which the Bmpr1a gene is partially and irreversibly inactivated in the epithelium of the LNP and MNP and more fully inactivated in epithelium and mesenchyme of the MXP (Liu et al., 2005), and later in the epithelium and mesenchyme of the secondary palate shelves. All of the conditional mutant embryos/fetuses developed bilateral CLP. Upregulation of apoptosis was detected in the ectoderm and mesenchyme of the MNP in the fusion zone, and expression of other genes in the ectodermal fusion zone of the MNP and LNP was altered. For example, Fgf8, known to interact with Bmp signaling and to promote cell survival in other tissues, did not upregulate normally in the fusion zone of the mutant, nor did its possible target Pitx1, nor did P63. This pattern suggests that Bmpr1a is required for survival of the edge epithelium and mesenchyme of the MNP and that the cleft lip is caused by this premature apoptosis in edge epithelium or by reduced mesenchyme. The cause of the cleft secondary palate was identified as a deficiency of palatal shelf growth after elevation, and traced back to reduced cell proliferation rates in the MXP mesenchyme on GD10.5 and 11.5. The palatal shelves were shown to retain the capacity to fuse if placed in contact with each other in vitro.
BMPR1A maps to human chromosome 10q22, a region not implicated in CLP linkage studies (Marazita et al., 2004) and therefore BMPR1A is probably not itself a good candidate gene. A related gene, Bmpr1b, is expressed in overlapping domains with Bmpr1a. The null mouse mutant has appendicular skeletal defects but not CLP (Yi et al., 2000). However, a species difference in which BMPR1B is the member of the gene pair that retained an essential developmental craniofacial role would not be surprising. Human BMPR1B (4q31) is implicated in human CLP by a linkage study (Schultz et al., 2004) and a deletion study (Brewer et al., 1998), but a search for mutations in BMPR1B in CLP cases does not seem to have been done.
The Bmpr1a mouse model, like the Bmp4 model, strongly points to the BMP signaling pathway in etiology of human CLP.
Tcfap2a Mutants
The Tcfap2a gene codes for various isoforms of the AP2-alpha protein, a DNA-binding transcription factor (Donner and Williams, 2006; Meier et al., 1995). Tcfap2a is expressed in cranial neural crest cells and their derivatives including the mesenchyme of the MNP, LNP, and MXP. Other embryonic expression domains include sensory ganglia, limb bud mesenchyme, the meso-metanephric region and epidermis (Mitchell et al., 1991).
Tcfap2a null mutants (Schorle et al., 1996; Zhang et al., 1996) develop a severe lethal syndrome including midline cleft face and lower jaw, or apparent lack of mouth and snout, exencephaly, deficient ear and eye structures, lack of ventral body wall, and forelimb deficiencies. However, chimeric embryos that are a mixture of Tcfap2a null cells and normal cells have various isolated defects such as CLP (Nottoli et al., 1998). These point to a potential role in human CLP of TFAP2A (“TCFAP2A”) hypomorphs or regulatory mutants. The clefts in the chimeras (see Figs. 3A, 3B, 4A in Nottoli et al., 1998) seem to have deficiencies in midface and MNP outgrowth or deficient LNP tissue.
The expression of Tcfap2a in its various developmental domains is controlled by a variety of regulatory elements in the DNA sequence (enhancers) that direct expression in specific tissues. A highly conserved enhancer located in intron 5 directs expression to MNP and LNP and limb bud mesenchyme on GD10.5, and sub-regions of the enhancer responsible for the facial prominences are partially distinct from those for limb (Donner and Williams, 2006). In the facial sub-region of this enhancer, there are binding sites for Sox and Stat transcription factors, both of which contribute to driving the expression of Tcfap2a in the MNP and LNP (Donner and Williams, 2006). Mutations in the binding sites for Sox or Stat would be expected to reduce or eliminate the level of expression level of Tcfap2a, specifically in the MNP and LNP, and to cause their reduced growth, and thereby, risk of CLP. The regulation of Tcfap2a by Sox and Stat in the facial prominences also points to a role of the Sox and Stat signaling pathways in CLP.
This model provides a clear example of a noncoding DNA region (enhancer) in the TFAP2A gene that should be screened for mutations in CLP patients. TFAP2A maps to human chromosome 6p24, a region repeatedly implicated in linkage studies of human CLP (Moreno et al., 2004; Prescott et al., 2000; Scapoli et al., 1997; Schultz et al., 2004). TFAP2A seems an excellent candidate gene for human CLP. There do not seem to have been studies of TFAP2A sequence in CLP patients.
Sox11 Mutant
Sox11 is one of about 20 Sox genes that code for transcription factors. Sox11, Sox4, and Sox22 (“Sox12”) comprise the “C” subfamily based on sequence similarity. Sox proteins are involved in numerous developmental processes including establishment and maintenance of lineages of the neural crest and epithelial-mesenchymal inductions (Hargrave et al., 1997; Hong and Saint-Jeannet, 2005). Sox genes may regulate each other. Sox11 in mouse embryos has widespread expression during gastrulation and early postgastrulation; it is expressed in the neural crest and its derivatives (Sock et al., 2004) and in various nonneural crest sites. On E9.5–10.5, its expression domains include the embryonic face, limb buds, and CNS (Hargrave et al., 1997; Sock et al., 2004).
Sox11 null mutants die at birth, with a syndrome that often (70%) includes CLP (Sock et al., 2004). The mutant pups also usually have heart defects, lung hypoplasia, asplenia, open eyelids, sometimes omphalocele, and other defects.
Based on photographs in Sock et al. (2004), the CLP of the Sox11 mutant appears to be morphologically similar to the CLP of the Wnt9b null mutant and the A/WySn strain (see below). No study of the embryonic face during lip and palate formation has been reported for the Sox11 mutant. It is intriguing that Sox genes may regulate Tcfap2a expression in the facial prominences (see the Tcfap2a mutant, above).
SOX11 maps to human 2p25 and linkage studies do not appear to have detected association of CLP to this location, although linkage has been reported for 2p13–14 (Pezzetti et al., 1998). Other C subfamily Sox genes share similar expression domains with Sox11. SOX22 maps to 20p13, a region not implicated in CLP by linkage studies. No mouse mutant for Sox22 (Sox12) seems to have been reported.
The other C family member is SOX4. SOX4 maps to human chromosome 6p23, a region implicated by several CLP linkage studies (Marazita et al., 2004; Moreno et al., 2004; Prescott et al., 2000; Scapoli et al., 1997). The Sox4 mouse null mutant has heart septation defects; no CLP was reported (Schilham et al., 1996). However, the Sox11 mutant points to a potential role of Sox genes in human CLP, as does the Sox-specific enhancer in Tcfap2a. Therefore, SOX4 should be considered a good candidate gene in the etiology of human nonsyndromic CLP, and studies of its coding and regulatory sequence do not seem to have been reported.
Wnt9b Mutants
Wnt9b is one of 19 mammalian Wnt genes that code for extracellular signaling molecules important in embryonic development. Wnt signaling uses various signal transduction pathways, of which the “canonical Wnt pathway” is best known, and involves activation of Tcf/Lef transcription factors in the nucleus (Lan et al., 2006; Logan and Nusse, 2004).
Wnt9b is expressed in embryonic mouse craniofacial development (Lan et al., 2006). In GD 9.5 embryos (after neural tube closure and before formation of the facial prominences), Wnt9b is expressed broadly in the head ectoderm. At GD 10.5, a stage shortly before fusion of the facial prominences, Wnt9b is expressed strongly in the distal epithelium of the MNP, LNP, and MXP at the region that will subsequently fuse, and in the MXP mesenchyme. At GD 11.5, Wnt9b is strongly expressed in the ectoderm of the facial prominences and in the epithelial seam between the fusing facial prominences.
The signaling by Wnt9b during development of the upper lip likely involves the canonical Wnt signaling pathway. Using the TOPGAL reporter transgene, Lan et al. (2006) demonstrated that the canonical Wnt signaling pathway is activated in various specific craniofacial cell types during GD 9.5 to GD11.0. These include the migrating neural crest cells, craniofacial ganglia, and all of the regions of Wnt9b expression described above. The canonical Wnt signaling pathway has been shown to activate Bmp4 expression (Lan et al., 2006), suggesting that Wnt9b may act through Bmp4 signaling to regulate lip fusion (see Bmp4 mutant, above).
Homozygotes for a targeted mutation inactivating Wnt9b have a lethal syndrome of fully penetrant vestigial kidneys and lack of reproductive ducts; some also have CLP with “incomplete penetrance” (Carroll et al., 2005). Embryological studies of the developing lip in this mutant have not been published. The expression pattern of Wnt9b suggests a role in CLP via the fusion step, but also the potential of additional earlier roles in CLP risk via neural crest cell migration and cell proliferation in the facial prominences.
A spontaneous recessive hypomorphic mutation of Wnt9b, called clf1, is present in all individuals of the A/WySn mouse strain and other closely related strains (Juriloff et al., 2005, 2006). These strains have 5–30% nonsyndromic CLP at birth. The genetics of CLP in these strains is multifactorial (Juriloff et al., 2001, 2004). They have been studied extensively and will be discussed separately below.
The compound mutant, Wnt9b−/clf1 (one null allele, one hypomorphic allele), has a CLP phenotype indistinguishable from the CLP of the A/WySn strain and, unlike the null mutant, has apparently normal kidneys and reproductive organs. Penetrance of CLP ranges between 10% and 90% depending on genotype of a modifier gene, clf2 (Juriloff et al., 2006). This model will also be discussed separately, below.
Another Wnt gene, Wnt3, located adjacent to Wnt9b on mouse and human chromosomes, is also expressed in the normal embryonic mouse face during development of the upper lip (Lan et al., 2006). The Wnt3 expression domains include the distal MNP and MXP, therefore partly overlapping with Wnt9b expression domains, raising the interesting possibility of some redundant involvement in growth of the facial prominences. In contrast to Wnt9b, however, Wnt3 is not expressed in the epithelial contact sites between the MNP and LNP, and therefore seems unlikely to be involved in the fusion process. The mouse null mutant for Wnt3 is lethal before the face forms (Liu et al., 1999). A WNT3 nonsense mutation has been reported in a lethal syndrome that includes absence of the limbs, CLP, and numerous other defects (Niemann et al., 2004).
The human WNT9B and WNT3 genes are located at 17q21, a region that was one of the first to be implicated by human linkage studies of nonsyndromic CLP (Chenevix-Trench et al., 1992) and that has been independently detected in several other linkage and association studies of CLP (listed in Juriloff et al., 2006) and by meta-analysis (Marazita et al., 2004). Screens for mutations in coding or regulatory sequence of WNT9B and WNT3 in CLP cases do not seem to have been reported.
Sp8/legless Mutant
The recessive legless (lgl) mutation causes a lethal syndrome of absent distal limb structures, transposition of thoracic and abdominal organs, and craniofacial defects (McNeish et al., 1990). The mild defects include mid-facial clefts with cleft secondary palate and shallow lateral clefts or grooves in the upper lip, whereas the severe spectrum includes wide midline cleft face with frontonasal encephalocele, cleft secondary palate, and overt lateral clefts of the upper lip.
The legless mutation affects three contiguous genes: Sp4 and lrd are deleted, and Sp8 function is impaired (Bell et al., 2003). The limb and face defects are probably due to deficiency of Sp8 function, as demonstrated by homozygotes for a targeted Sp8 knockout. The Sp8 null mutant has, in addition, exencephaly and spina bifida.
Sp8 codes for a transcription factor with specific expression domains during embryogenesis, including the developing neural tube, the precursor cells of the AER in the limbs, and the LNP and MNP (Bell et al., 2003). In the limb, Sp8 is an ectodermal target of Fgf10 signaling from mesenchyme, is positively regulated by Wnt3/beta-catenin signaling, is downstream of Bmp signaling cascades, and acts via Fgf8 (Bell et al., 2003; Kawakami et al., 2004). It seems reasonable to hypothesize a similar regulatory pathway for Sp8 in the embryonic facial prominences.
The severe craniofacial defects in the lgl/Sp8− mutants appear to be mostly midline defects: lack of closure of the most rostral region of the neural tube (Closure 3; Juriloff and Harris, 2000) leading to a frontonasal encephalocele and split face, and lack of growth of the MNP and the merging area between them. In addition, the LNP appear to fuse incompletely with the MXP, leaving obvious grooves or clefts.
The severe facial defects due to the Sp8 mouse mutations do not directly model common nonsyndromic CLP, but it seems possible that hypomorphic variants of Sp8 could contribute to nonsyndromic CLP. It is interesting that components upstream of Sp8 in the signaling pathway (Wnt, Bmp) are involved in CLP in mice (see above).
Meta-analysis of linkage studies from several populations did not implicate the chromosomal region containing human SP8 (7p15) in the etiology of CLP (Marazita et al., 2004), but one of the populations, West Bengal India, did suggest CLP linkage to this site (Field et al., 2004). SP8 therefore seems to be a candidate gene that should be examined further in CLP cases from some populations.
Folr1 Mutants
The Folr1 (Folbp1) gene codes for a membrane-bound protein that mediates transport of folic acid into epithelial cells. It is expressed in embryonic neural folds and neuroepithelium (Saitsu et al., 2003); a description of later craniofacial expression does not seem to have been published. Mouse embryos homozygous for a null mutation of Folr1 die around GD 10, with numerous defects including failed cranial neural tube closure (NTD) and heart defects (Piedrahita et al., 1999; Tang et al., 2004). Maternal supplementation with folate reduces the severity of defects. Some have exencephaly, failure of closure of the abdomen, and facial defects including medial clefts that appear to be due to a deficiency of tissue, unilateral or bilateral CLP, and absent eyes (Spiegelstein et al., 2004; Tang and Finnell, 2003). Some appear to have a combination of a medial cleft and lack of fusion of the MXP with the MNP, producing a multilobed complex of clefts.
This model clearly demonstrates an essential role of the folate pathway in facial development. However, FOLR1 maps to human 11q13, a region not implicated by linkage studies as having a role in risk of CLP (Marazita et al., 2004; Scapoli et al., 2005).
Tbx10 Ectopic Expression and the Dancer Mutation
The Tbx10 gene codes for a putative transcription factor that is not normally expressed in craniofacial development (Bush et al., 2003).
Dancer (Dc), a semidominant spontaneous mutation of Tbx10, causes abnormalities of the inner ear, some CLP in heterozygotes (Trasler and Leong, 1982), and increased susceptibility to teratogen-induced CLP (Trasler et al., 1984). Heterozygotes have deficient growth of the MNP and LNP (Jacobson and Trasler, 1992; Trasler and Leong, 1982). Homozygotes nearly always have CLP and inner ear defects (Deol and Lane, 1966).
The Dancer mutation is a spontaneous insertion of part of another gene, p23, into the Tbx10 gene and leads to a chimeric protein (Bush et al., 2003, 2004). The ectopic expression of the chimeric transcript in the developing facial prominences causes the CLP phenotype (Bush et al., 2004).
The human TBX10 gene maps to 11q13, a region that has not been specifically associated with CLP (Marazita et al., 2004), but is included in a larger region (11p12–q13) that has been associated with CLP in two studies (Moreno et al., 2004; Prescott et al., 2000). The type of mutation that leads to ectopic expression of a particular Tbx10 chimeric protein in the facial prominences seems likely to be a unique event, and the same mutation at TBX10 in humans seems unlikely. Therefore, TBX10 does not seem a strong candidate for the CLP risk gene on human chromosome 11 and the variants found in a few CLP patients and controls may be polymorphisms unrelated to CLP (Vieira et al., 2005). The particular value of Dancer may be the demonstration that CLP can be caused by a gain-of-function mutation in a gene that is not normally expressed in the embryonic face.
Twirler Mutation
Twirler (Tw) is a semidominant spontaneous mutation on mouse Chr 18 which causes heterozygotes to have inner ear defects and adult obesity. Homozygotes die soon after birth and usually have CLP (Gong et al., 2000; Lyon, 1958) or CP (Lyon, 1958). The severity of clefts is variable (Gong et al., 2000; Lyon, 1958). Studies of the developmental cause of the cleft lip do not seem to have been published. Studies of the developing secondary palate indicate that the palate shelves from GD 11.5 onward are deficient, late elevating, and too small to meet in the midline to fuse (Gong and Eulenberg, 2001). Twirler maps to a small chromosomal region (Liu et al., 2006) whose human homologous genes seem to be mostly on human 10p11–12 (http://genome.ucsc.edu). The Mkx gene (formerly Irxl1; MGI, 2007) has been proposed as a candidate gene for the Twirler mutation (Liu et al., 2006).
Sloan Kettering ENU Mutagenesis “Line 2”
Various programs to create a large catalogue of new point mutations caused by ENU (N-ethyl-N-nitrosourea) at random gene loci in mice are in progress (Abbott, 2000). Examples are The European Mouse Mutant Archive (http://www.emmanet.org/), RIKEN (http://www.gsc.riken.go.jp/Mouse/), and the mutagenesis program at Sloan Kettering (http://mouse.ski.mskcc.org/). Although mutants with CP have been reported (e.g., Herron et al., 2002), CLP seems to be conspicuously absent in listings of phenotypes of new recessive mutations. However, Zohn et al. (2005) reported a collection of 32 new recessive mutations causing the NTD, exencephaly, often as part of a syndrome. One of these, “Line 2,” was reported to also have “craniofacial defects” (see Table 1 in Zohn et al., 2005). The supporting website (http://mouse.ski.mskcc.org/mutant/category/viewCategory.php) shows an embryo with apparent bilateral cleft lip, omphalocele, and exencephaly, and indicates that the gene has been mapped to a large region of mouse Chr 15. However, a very severe disruption of craniofacial development is more typical of the mutant [L. Niswander, personal communication]. More precise mapping is needed to enable identification of the human homologous chromosomal region.
Gli3 Gene, Bph Mutant
Gli3 is a zinc-finger transcription factor that functions in the Sonic hedgehog signaling pathway (Johnston et al., 2005). Among 12 independent mutations of the Gli3 gene in mice (MGI, 2007) one, the brachyphalangy (Bph) allele (now called Gli3Xt−3H), was reported to have CLP in homozygotes (Johnson, 1969). No further studies of this mutant seem to have been reported and it seems to be extinct. Most Gli3 mutant alleles cause polydactyly with a variety of other defects in homozygotes, including exencephaly. The Gli3Xt−3H mutation was caused by radiation and, like many radiation-induced mutations, could be a multigene deletion, raising the possibility that a deficiency of a gene near Gli3, not Gli3 itself, causes CLP.
Mutations of GLI3 on Chr 7p14 in humans are known to cause Greig cephalopolysyndactyly syndrome and Pallister Hall syndrome, both of which are dominantly inherited (Johnston et al., 2005). CLP does not seem to be a typical feature, although it has been noted “occasionally” (Jones, 1997).
The 7p12 region, near 7p14, has been linked to risk of nonsyndromic CLP (Marazita et al., 2004). Given that numerous genes near Gli3 have homologs that map to human 7p14 and the possibility that Gli3Xt−3H was a large deletion, the Bph mutant may have pointed, not to GLI3, but to an unidentified gene whose homolog maps to 7p14.
Bn Mutation
Bent tail, Bn, is a multigene deletion on the X chromosome (Klootwijk et al., 2004) which causes tail deformities, NTD, omphalocele, and situs abnormalities (Klootwijk et al., 2000), as well as a low frequency of “unilateral cleft lip.” The published illustration (Fig. 2E in Klootwijk et al., 2000) is suggestive of a median cleft due to deficiency of medial prominences, reminiscent of the median clefts in the Folr1 and Sp8 mutants, rather than a true unilateral cleft. One of the genes in the Bn deletion, Zic3, probably causes the NTD and situs abnormalities, but Zic3 mutants do not seem to have cleft lip or omphalocele, suggesting that other genes in the deletion may cause these defects (Purandare et al., 2002).
The Bn deletion may point to an X-linked gene that can contribute to risk of CLP through a reduction of size of the MNP. A common polymorphic variant at a human homologous gene in the Bn deletion region could be hypothesized to account for the higher risk of CLP in males than females.
MULTIFACTORIAL MODELS: (1) THE “A” STRAINS AND THEIR RELATIVES
The “A” strains and a few derivative strains are the genetic model that most closely resembles human nonsyndromic CLP in genetic etiology and environmental sensitivity. The CLP is nonsyndromic. The genetic cause is complex. Two gene loci, clf1 and clf2, acting jointly in embryos create the risk of CLP. clf1 is a mutation of the Wnt9b gene, a very good candidate gene for human CLP (see above). Genetic and nongenetic maternal factors influence penetrance, including possibly folic acid nutrition. The genetics and embryology of CLP in some members of this group of strains have been studied extensively over the past 70 years, and the cumulative results are the experimental foundation of most of the understanding of the developmental etiology of CLP. The most important recent finding in this multifactorial model may be the indication that deficient methylation of an element in the DNA at Wnt9b is the basis of penetrance of the CLP (Juriloff et al., 2007), and introduces the issue of epigenetics into the causation of CLP.
The following strains all produce nonsyndromic CLP in their embryos and fetuses at various strain-specific frequencies and are all genetically related to each other: A/WySn, A/HeJ, A/J, CL/Fr, the “L Line,” AXB-6/Pgn, BXA-8/Pgn, and the A.SW/Sn and A.BY/Sn congenic strains.
Relationships among the “A” Strains and their Derivative Strains
Strain “A,” created by Leonell Strong in the 1920s was probably the first inbred strain of mice ever created by geneticists (Strong, 1978). The A/J, A/WySnJ, A/HeJ and other “A/−” substrains are all descendants of the original “A” strain mice distributed to other researchers in the early generations shortly after the A strain was inbred. Although nearly all polymorphic marker loci and genes are the same among the A/− substrains, there are a few known differences (see MGI, 2007). All A/− substrains examined produce some CLP in their progeny but the frequencies of CLP differ (Kalter, 1979) and currently range between about 2–4% and 25%. The CLP frequency differences seem to be largely due to different maternal effects, originally thought to be genetic (Juriloff, 1982), but current hypotheses should include heritable epigenetic (methylation) differences among the substrains.
The A/− strains share common ancestors, and segments of chromosomes (“haplotypes”) with the BALB/c, DBA, CBA, and C3H strains (Strong, 1978). None of these non-A strains have CLP in their offspring, but some received the ancestral haplotypes that now contain the clf1 mutation and clf2 polymorphism. This situation enables a strategy for identifying the CLP-causing mutations/polymorphisms in the A/− strains (Juriloff et al., 2005).
The creation of the “A” strain is a colorful story (Strong, 1978). Early generations were housed in the Strong household; later they were in a former chicken coop where toxic gases from a malfunctioning coal-burning stove caused a near-extinction at F7 (1924), the survivor being one pregnant mouse, from which all A/− strain mice are descended. This history may have imposed inadvertent genetic selection for various metabolic and behavioral traits that now characterize the A/− strains.
Genetic and developmental studies of the CLP of the “A” strain probably began in the late 1920s. Beginning in about 1925, Strong's “A strain” mice were housed by W. E. Castle at the Bussey Institute (Strong, 1978). Mice were obtained from the Bussey Institute by Reed and Snell soon thereafter, probably a sample of the partially inbred “A strain,” and were used in genetic and embryological studies of CLP (Reed, 1933, 1936a, b; Reed and Snell, 1931). In 1933, Steiniger obtained mice related to Reed's mice from C. C. Little (Steiniger, 1939) for several studies of CLP, including embryological studies (Steiniger, 1942). The basic developmental findings of Reed, Snell, and Steiniger were essentially the same as those of later studies of the A/− strains and related strains. The mode of inheritance clearly involved more than one gene (Reed, 1936a, b).
The CL/Fr strain was created by F. C. Fraser in the 1960s. A heterogeneous stock was crossed with A/J and the subsequent generations were selected for production of CLP while being inbred (Bornstein et al., 1970). CL/Fr typically produces 20–25% CLP (Juriloff and Fraser, 1980). DNA analysis has shown that CL/Fr has the clf1 mutation from the A/− strains (Juriloff et al., 2005) and its haplotype indicates that it also has the second causal gene, clf2, from A/J (Juriloff et al., 2004). The higher frequency of CLP in CL/Fr compared to A/J is due to a maternal effect (Juriloff and Fraser, 1980).
The “L line” created by D. G. Trasler in the early 1970s from a four-way cross among CL/Fr, DBA/1J, C57BL/6Fr, and NS/Fr, was selected in early generations for occurrence of CLP after maternal treatment with 6-aminonicotinamide (Trasler et al., 1978). By F15, 9% CLP was observed to occur in the absence of treatment. The L Line is now extinct. The haplotype of polymorphic markers in archived DNA indicates that the clf1 mutation and clf2 polymorphism of A/− strain origin had been homozygous in the L Line [Juriloff, unpublished].
AXB-6/Pgn and BXA-8/Pgn are Recombinant Inbred (RI) strains created from a cross between A/J and C57BL/6J; each have both the clf1 and clf2 homozygous mutations from A/J. AXB-6/Pgn produces about 8% CLP and BXA-8/Pgn produces about 4% CLP; the lower frequency than A/WySn is a maternal effect (Juriloff et al., 2001).
A.SW/Sn and A.BY/Sn are congenic strains that are essentially A/WySn mice into which the major histocompatibility locus (H-2) from other strains has been substituted. These congenic strains produce CLP at about the same rate as A/WySn, demonstrating that the H-2 gene complex does not influence the CLP trait (Juriloff, 1982).
Multifactorial Genetic and Epigenetic Cause of CLP in the A/− Strains and Related Strains
The genetic cause of CLP in the A/− strains and related strains is the combined effects of two gene loci (“digenic”): the recessive clf1 mutation on Chr 11 and the semi-dominant clf2 polymorphic variant on Chr 13 (Juriloff et al., 2001, 2004, 2005, 2006). Neither homozygous gene alone can cause CLP. Superimposed on the effects of clf1 and clf2 in embryos is an apparently genetic maternal effect that strongly alters penetrance of CLP (Davidson et al., 1969; Juriloff, 1982; Juriloff and Fraser, 1980; Juriloff et al., 2001).
The clf1 mutation is a defect of the Wnt9b gene (Juriloff et al., 2006), caused by the insertion of a transposable element of the “IAP” class, outside the gene's coding sequence (Juriloff et al., 2005). In similar mutations at other genes, such as Avy or AxinFu, individuals in which the IAP is well-methylated have normal transcription of the adjacent gene, whereas individuals in which the IAP is under-methylated have poor function of the gene due to interference by the IAP (Morgan et al., 1999; Waterland et al., 2006). In short, these mutations exhibit large differences between genetically identical individuals in transcription levels from the mutant gene. Recent evidence indicates that clf1 is a mutation of this type (Juriloff et al., 2007). The chromosomal location of the second locus, clf2, encompasses several genes (Juriloff et al., 2004), and the specific gene variant is not yet known.
Dichotomous Gene Expression within the Inbred A/− Strains and Relatives
Given that individuals within a highly inbred strain are virtually identical twins, the level of expression of any given gene is expected to be similar. But the clf1 mutation appears to be a member of a class of mutations that produce widely variable expression levels from the affected gene, ranging from almost normal to very deficient. This predicts a wide range of transcription of Wnt9b and of any genes downstream of Wnt9b in the regulatory pathway, and also predicts that embryos with CLP would be more deficient in Wnt9b and downstream gene products than would phenotypically normal genetically identical littermates. Studies of expression of Wnt9b in A/− strain mice have not yet been reported. However, the expression patterns for a variety of genes expressed in the embryonic face follow these predictions.
Msx1, a gene normally expressed in the mesenchyme of the MLP, LNP and MXP, showed an interesting dichotomy in A/WySn embryos: immediately postfusion stage, Msx1 expression declined in the normal embryos but persisted at high levels in their prospective CLP littermates (Gong, 2001). Tgfb3 was expressed normally in the epithelial seam and mesenchyme of the facial prominences of normal embryos, but was not detected in incipient cleft lip littermates in CL/Fr (Muraoka et al., 2005). The ras protein was detected in mesenchyme and ectoderm of normal control strain and A/WySn strain facial prominences before the fusion stage, was deficient in the A/WySn strain after the fusion stage, and was most deficient in an A/WySn incipient CL embryo (Wang et al., 1995a). Only one study did not report a dichotomy of gene expression: the Erbb4 protein was reported to be deficient in A/WySn embryos after the fusion stage (Wang et al., 1998).
One would expect gene expression differences to lead to cellular differences. At the cellular level, epithelial changes in the nasal pit that are associated with the fusion of the MNP and LNP were lacking in many, but not all, CL/Fr embryos, at a frequency similar to the CLP rate (Millicovsky et al., 1982). Thyroxin administered to the mother induces differential mortality of CLP embryos in mid-gestation, as observed in CL/Fr, A/HeJ, and A/WySn (Juriloff, 1981; Juriloff and Harris, 1985). The development of the atrial septum appears to be retarded in CLP embryos relative to normal littermates from GD 11.5 onward (Fraser and Rosen, 1975).
Developmental Studies
Reed (1933) and Steiniger (1942) observed that cleft lip arose in a failure of fusion between the MNP and LNP and between the MNP and MXP, both attributed to a retarded growth of the MXP. Trasler (1968) reported that A/J embryos differ in face shape from embryos from the normal C57BL/6J strain, as follows: “…just before and when the adjacent epithelia of medial nasal and lateral nasal processes meet and fuse at the posterior end of the nasal pit, the medial nasal and lateral nasal processes of A/J embryos were more prominent, more medially placed, and diverged less than those of C57BL/6J.” This finding was supported by studies of the L line (Juriloff and Trasler, 1976) and of CL/Fr (Millicovsky et al., 1982). In addition, the LNP of CL/Fr appeared to be hypoplastic and the consolidation of the MXP with the LNP was delayed (Millicovsky et al., 1982). A morphometric analysis comparing A/WySn with C57BL/6J (Wang and Diewert, 1992) confirmed that the relation of width between the nasal pits to head growth was smaller in A/WySn during early stages and that the MXP had deficient forward growth (Wang and Diewert, 1992). Comparisons between CL/Fr and C57BL/6J for formation of the mesenchymal intrusion through the apposed epithelia, which fuses the MXP with LNP and MNP, found delayed and small or deficient formation of the mesenchymal bridge in CL/Fr (Diewert and Wang, 1992). A recent application of more advanced morphometric analyses to archived embryos (Young et al., 2007) produced complex findings that were basically in accord with previous studies and indicated that during formation of the upper lip, A/WySn, A/J, and CL/Fr all differ from three normal strains (C57BL/6J, BALB/c, CD1) in having “more medially located nasal pores and very long and narrow maxillaries (i.e., underdeveloped), especially at the medial and superior junction with the nasals.” The embryonic differences in face shape may lead to detectable postnatal differences in face shape (Trasler and Machado, 1979), supporting the observation that normal relatives of human CLP probands have detectable differences from average face dimensions (Chatzistavrou et al., 2004; Fraser and Pashayan, 1970).
Currently it is not known which of the morphological differences can be attributed to Wnt9b deficiency, and which to clf2. Based on its expression domains, it is possible that Wnt9b influences both facial prominence growth and the fusion step.
In the A/− strains and related strains, the cleft lip is usually accompanied by cleft secondary palate, but not always. The secondary palate shelves, outgrowths of an internal area of the MXP, form the secondary palate by growth and rotation during GD 12–15. Cleft upper lip (CL) is established by the end of GD 11, and therefore the CP develops in the context of abnormal facial morphology. Studies have demonstrated that the palate shelves in CL embryos are capable of fusing if they are brought into contact with each other (Pourtois, 1967; Sasaki et al., 2004). The reasons for the lack of contact of palate shelves potentially include smaller shelves in CL embryos than their normal littermates, delayed elevation, and mechanical interference with elevation by the tongue due to the abnormal position of the premaxilla in CL (Trasler and Fraser, 1963). Notably, neither Wnt9b nor the activation of the canonical Wnt signaling pathway were detected in the developing secondary palate shelves (Lan et al., 2006), suggesting that CP is probably indirectly caused by the presence of CL (Trasler and Fraser, 1963). There is, however, some evidence that the palate shelves may be abnormal; the proliferative rate of mesenchyme cells in the pre-closure secondary palate shelves may be reduced in BCL embryos compared with normal littermates in the CL/Fr strain (Sasaki et al., 2004), and it should be borne in mind that the role of clf2 is unknown.
Liability of the A/− Strains to Teratogen-Induced CP
The A/− strains are more susceptible than many other strains to teratogen-induced CP and, because of the extensive past research on this phenomenon, they became the canonical model of genetically determined liability to a teratogen-induced birth defect (reviewed in Juriloff, 1980). The elevation of palate shelves in non-CL A/J embryos is delayed relative to other strains (Walker and Fraser, 1956), and the delay was thought to contribute to the liability to teratogen-induced CP (Vekemans and Fraser, 1979). Whether the susceptibility to teratogen-induced CP in the A/− strains is biologically related to their genetic liability to CLP is not known. The one genetic study that tested the relationship indicated that the genetic basis of liability to teratogen-induced CP is due to different genes than is the genetic liability to CLP in the A/J strain (Biddle and Fraser, 1986).
Non-Mendelian and Environmental Phenomena in A/− Strain CLP
In contrast to the consistently higher risk of males for human CLP, a consistent sex effect on CLP risk in the A/− strains and crosses has not been detected. Maternal strain influences the penetrance of CLP. This has been demonstrated in contrasts of A/J or A/WySn or CL/Fr versus C57BL/6 (Bornstein et al., 1970; Davidson et al., 1969; Juriloff et al., 2001), A/J versus CL/Fr (Juriloff and Fraser, 1980), and A/WySn versus A/J (Juriloff, 1982). The role of an IAP-type transposable element in the clf1 mutation raises the possibility that the maternal effects on penetrance of CLP may be due to epigenetic methylation of the IAP at Wnt9b as discussed above. Dietary factors can affect methylation of the IAP in other mutants (Morgan et al., 1999; Waterland et al., 2006). Maternal folinic acid supplementation may reduce risk of CLP in A/WySn mice (Paros and Beck, 1999), but the study needs to be confirmed in mothers of matched ages, because maternal age affects risk of CLP (Kalter, 1978; Loevy, 1969).
MULTIFACTORIAL MODELS: (2) THE COMPOUND Wnt9b−/clf1 MUTANT
The compound mutant, Wnt9b−/clf1, has one null allele and one hypomorphic allele at Wnt9b, and is expected to produce a lower level of Wnt9b than most A/− strains. The frequency of CLP in this compound mutant is influenced by the A/− strain maternal effect [Juriloff, unpublished data] and by clf2 genotype (Juriloff et al., 2006). The frequency of CLP in the compound mutant is about 90% if clf2 is in the A/− strain state and the embryo is in an A/WySn mother, but is only about 10% if clf2 is heterozygous for the C57BL allele, and is lower if the mother is not an A/− strain. The CLP appears to be nonsyndromic. As this compound mutant has one clf1 allele, the CLP rate would be expected to be susceptible to factors that alter levels of IAP methylation (see above).
In this model, the genotypes with high frequency of CLP have mostly bilateral clefts, whereas the genotypes with lower CLP frequency have more unilateral clefts (Juriloff et al., 2006), supporting the long-standing hypothesis for human CLP that bilateral clefts reflect higher genetic risk of CLP.
A large proportion of human CLP cases would be expected to be compound mutants for the genes causing their cleft, because the parents are mostly not related and a variety of mutations at any given gene are usually present in a population. This mouse compound mutant with one null, syndrome-causing, allele and one hypomorphic allele susceptible to modification by environmental factors and other genes, is likely a very good model of some of the genetic complexities of human CLP cases.
HUMAN CANDIDATE GENES BASED ON THE MOUSE MODELS
Table 2 lists the best candidate genes for roles in human nonsyndromic CLP based on the combination of mouse models and cumulative human CLP linkage studies. The genes are: BMP4, BMPR1B, SOX4, SP8, TFAP2A, WNT9B, and WNT3. Most of these genes have not been examined in human nonsyndromic CLP cases.
| Gene | Human location* | Function/pathway | Human linkage | Strength of candidacy |
|---|---|---|---|---|
| BMP4 | 14q22–q23 | Signaling | Yes | ++++ |
| BMPR1B | 4q31 | Signaling | Yes | +++ |
| SOX4 | 6p23 | Transcription | Yes | +++ |
| SP8 | 7p15 | Transcription | Yes | ++ |
| TFAP2A | 6p24 | Transcription | Yes | ++++ |
| WNT3 | 17q21 | Signaling | Yes | +++ |
| WNT9B | 17q21 | Signaling | Yes | ++++ |
- * Gene locations can be found at http://genome.ucsc.edu/
DISCUSSION
One of the striking patterns in the mouse CLP mutants as a group is that a large proportion also produce NTD in the same or different individuals. Almost half (6/14) of the mutants (Tcfap2a, Folr1, Bph, Line 2, Sp8, and Bn) produce cranial NTD as well as CLP. The mutants therefore support the evidence that there may be genetic etiological overlap between human NTD and CLP. Folic acid seems to prevent CLP in at least one model, as well as in some NTD models, paralleling the human data for folic acid response and suggesting an environmental overlap between the defects. The developmental origin of NTD is earlier than that of CLP, but a subpopulation of cranial neural crest cells is in common between both tissues. The relatively small number of mutants with CLP contrasts sharply with the large numbers that cause NTD (Harris and Juriloff, 2007) or CP (Gritli-Linde, 2007). The reasons for this difference are unclear.
All of the Mendelian mutants cause severe syndromes as well as CLP in homozygotes. It would be interesting to know whether there is any frequency of nonsyndromic CLP in heterozygotes, modeling human haploinsufficiency. In contrast to the Mendelian mutants, nonsyndromic CLP occurs in conditional face-specific mutants that model tissue-specific regulatory mutations, in chimeras with normal cells (modeling hypomorphs), and in multifactorial models with some normal gene product from the mutant locus (the A/− strains and the Wnt9b−/clf1 compound mutant). This pattern suggests that the mutations involved in human nonsyndromic CLP may be more likely to be in the regulatory regions of genes than in the coding sequence. The complex inheritance pattern of common human nonsyndromic CLP is consistent with regulatory or hypomorphic mutations. It is also possible that a large proportion of nonsyndromic CLP cases are compound mutants of a null allele and a hypomorph, like the Wnt9b−/clf1 model, as has been discussed elsewhere (Juriloff et al., 2006).
The mouse multifactorial CLP models underline the possibility that the genetic complexity of nonsyndromic CLP in individual humans might be as simple as combinations of mutations at two gene loci from the same or interconnected signaling pathway(s), acting together, as has been found for cases of another multifactorial trait, holoprosencephaly (Ming and Muenke, 2002).
The mouse models as a group demonstrate the variety of causative genes and mutations that can lead to CLP, and indicate that human CLP is likely genetically and developmentally heterogeneous. The mutants offer some insights into the heterogeneity of developmental mechanisms that can cause CLP independently or together in various combinations. Based on the mutants, the MNP misplaced towards the midline, or lacking in growth, small LNP, small MXP, and a defective ability to fuse all seem likely to contribute to risk of CLP.
It is noteworthy that the heterogeneity of causative genes might narrow down to relatively few gene regulatory pathways. Several of the CLP mutants seem to reflect interconnected gene regulatory pathways: Bmp4 may be regulated by canonical Wnt signaling (Wnt9b), Bmpr1 is the receptor for Bmp4, and Sp8 is downstream of Bmp signaling; Tcfap2a expression is regulated in the embryonic face by Sox genes (Sox11).
The involvement of epigenetics in the mouse multifactorial CLP models demonstrates the potential role of epigenetics in human nonsyndromic CLP. Although the specific IAP element is not present in human DNA, an element with similar potential, L1, is present (discussed in Juriloff et al., 2005). Epigenetics, particularly DNA methylation, is an attractive mechanism to explain a preventative response to folic acid and other phenomena such as season-of-conception effects.
Most of the best candidate genes for human CLP have not been examined in human nonsyndromic CLP cases. The genes are: BMP4, BMPR1B, SOX4, SP8, TFAP2A, WNT9B, and WNT3. Regulatory mutations and hypomorphs are predicted.
Acknowledgements
Research funding for many years to D. M. J. and M. J. H. from the Medical Research Council of Canada and from the Canadian Institutes of Health Research for studies of the genetics and development of CLP in the A/− strains is gratefully acknowledged.




