Selected gene polymorphisms and their interaction with maternal smoking, as risk factors for gastroschisis†‡
Presented in part at the 45th Annual Meeting of the Teratology Society, June 25–30, 2005, St. Pete Beach, Florida.
Although this work was partially supported by the U.S. Environmental Protection Agency (EPA), it has not been subjected to any EPA review and therefore does not necessarily reflect the views of the EPA, and no official endorsement should be inferred.
Abstract
BACKGROUND: Gastroschisis is a severe birth defect in which the infant is born with a portion of the intestines extruding through a small tear in the abdominal wall, usually to the right of the umbilical cord. Its etiology is unknown, but the prevailing hypothesis is that it results from a vascular accident at the time of involution of the right umbilical vein or of the development of the superior mesenteric artery. METHODS: In a case-control study of 57 cases of gastroschisis and 506 controls, we tested DNA for polymorphisms of 32 genes representing enzymes involved in angiogenesis, blood vessel integrity, inflammation, wound repair, and dermal or epidermal strength. RESULTS: In logistic regression, controlling for maternal ethnicity, and using the homozygote wild-type as referent, the following gene polymorphisms were associated with an increased risk for a gastroschisis for heterozygotes: ICAM1 gly241arg (odds ratio [OR], 1.9; 95% confidence interval [CI], 1.1 –3.4); NOS3 glu298asp (OR, 1.9; 95% CI, 1.1–3.4); NPPA 2238T > C (OR, 1.9; 95% CI, 1.0–3.4); and ADD1 gly460trp (OR, 1.5; 95% CI, 0.8–2.8). Additionally, for the NPPA and ADD1 single-nucleotide polymorphisms (SNPs), the homozygote variants had a significantly higher risk than the heterozygotes (OR, 7.5; 95% CI, 1.7–33.5 and OR, 4.9; 95% CI, 1.9–12.9, respectively). Three SNPs showed a strong interaction with maternal smoking. The risk for smokers with 1 or 2 variant alleles compared to nonsmokers with the wild-type allele were: NOS3 (OR, 5.2; 95% CI, 2.4–11.4); ICAM1 (OR, 5.2; 95% CI, 2.1–12.7); and NPPA (OR, 6.4; 95% CI, 2.8–14.6). CONCLUSIONS: These results support the hypothesis of a vascular compromise as part of a multifactorial etiology of gastroschisis involving both genes and environmental factors. Birth Defects Research (Part A) 76:723–730, 2006. © 2006 Wiley-Liss, Inc.
INTRODUCTION
Gastroschisis is a severe birth defect in which the infant is born with a portion of its intestines protruding outside the body through a small opening in the abdominal wall. The prevalence is approximately 3 per 10,000 live births and is highest among young mothers (Bugge and Hauge, 1983). The prevailing hypothesis is that the defect results from a vascular compromise. The location of the abdominal tear, usually to the right of the umbilicus, is an area where there are important vascular changes during early embryogenesis. In humans, between the fifth and eighth weeks of gestation, the right umbilical vein involutes and its nutritive function is then replaced by that of the omphalomesenteric artery (deVries, 1980). Hoyme et al. (1981) have postulated an early gestational interruption of the omphalomesenteric artery as the underlying pathogenesis of gastroschisis. Either of these pathogenic mechanisms could involve both apoptosis and angiogenesis, placing the overlying dermis and epidermis at risk for a tear in the skin, followed by inflammation, poor wound repair, and ultimately necrosis. Several environmental and maternal factors have supported the hypothesis of a vascular compromise in the etiology of gastroschisis, among them maternal smoking of cigarettes (Goldbaum et al., 1990; Werler et al., 1992; Torfs et al., 1994), the use of aspirin or ibuprofen (Torfs et al., 1996), and the lack of specific nutrients (Torfs et al., 1998). Furthermore, associated defects, such as amyoplasia, Möbius syndrome, intestinal atresia (Robertson et al., 1992), porencephaly (Hoyme et al., 1983), and Poland Sequence (Der Kaloustian et al., 1991) are also thought to have a vascular etiology, although the intestinal atresia may sometimes be a sequence of the gastroschisis. Although familial cases have been reported (Torfs and Curry, 1993; Nelson and Toyama, 1995; Reece et al., 1997), there are no gene studies that we know of for cases of gastroschisis. In the present study, we tested DNA from neonatal blood spots, employing a panel of 32 gene polymorphisms affecting the activity of enzymes involved in blood pressure regulation, cell-cell interaction, coagulation, and homocysteine metabolism. Several of these genes' enzymatic pathways are involved in angiogenesis, blood vessel integrity, wound repair, and dermal or epidermal strength. A gene involved in homocysteine metabolism, methylenetetrahydrofolate reductase (MTHFR), was of interest as a negative control because its variant is associated with neural tube defects (NTDs) and orofacial clefts, defects that are not associated with gastroschisis. Thus we predicted there should be no association with this gene. Because the enzymatic activity level of several candidate genes may be modified by smoking (Ponthieux et al., 2003; Brown et al., 2004), we evaluated the interaction between the gene polymorphisms and maternal smoking during the trimesters around conception. For the analyses, we used data from 2 population-based case-control studies conducted in California between 1987 and 1990.
MATERIALS AND METHODS
Interviews and genotyping of the blood specimens for this study met with the approval of the State of California Health and Welfare Agency Committee for the Protection of Human Subjects and of the Internal Review Board of the Children's Hospital in Oakland.
Cases were infants with gastroschisis who were ascertained for a study that was conducted in California during the years 1988–1990 (Torfs et al., 1994). Cases included all white infants, either Hispanic or non-Hispanic, born in the area of surveillance of the California Birth Defects Monitoring Program (CBDMP). Gastroschisis was confirmed by the surgical description of the defect and reviewed by a geneticist and a dysmorphologist. Within 3–6 months after the infant's birth, a case mother was given an in-home interview that covered the mother's pregnancy, medical and occupational history, and the parents' lifestyle. Of the 140 cases that met the ethnicity criteria, 110 (79%) were interviewed.
Cigarette smoking was reported for the 3-month period immediately preceding the date of conception and for each of the 3 trimesters of pregnancy. For this study, mothers had to have smoked during either the trimester before or the trimester after the time of conception to be considered as smokers, because gastroschisis is thought to occur between the fifth and eighth week of gestation, before the time when the pregnancy is confirmed and the mother is encouraged to stop smoking. Originally, the amount of cigarettes smoked was divided into 2 categories: >1 cigarette and up to 1 pack per day, and more than 1 pack per day. However, previous analysis showed no difference in risk for these 2 categories, which were then grouped for further analysis (Torfs et al., 1994).
As controls, 582 infants were randomly selected from all infants born alive in a similar geographic area and time period (1987–1989) for a parallel large study of several different structural birth defects (Shaw et al., 1996). Control infants had no major structural congenital anomalies identified before their first birthday. Phone interviews of control mothers were given within 4 years of delivery and mothers were queried about medical history and lifestyle, including cigarette smoking. Mothers were considered smokers in the current analyses if they reported smoking in the period from 1 month before conception to 2 months after conception. Interviews were completed for 75% of control mothers.
Of the 582 control infants ascertained who had an available newborn screening blood sample from their Guthrie spot, we included in the present analysis only those controls who met the same ethnic criteria as the cases. This selection resulted in 506 controls, all of whom were genotyped. Of the 110 gastroschisis cases in the original study we genotyped the first 57 cases whose Guthrie spots were pulled by the Genetic Disease Branch. Because this was a pilot study, we limited our genotyping to those cases. There were no selection criteria, and we verified that these cases had the same demographic and maternal characteristics as the remaining 53 cases.
Genetic Analysis
Recently, a multilocus allele-specific hybridization assay including 32 gene polymorphisms developed by Roche Molecular Systems (Alameda, CA) (Cheng et al., 1999) to evaluate enzymes and factors that are related to blood pressure regulation, cell-cell adhesion, coagulation, inflammatory response, and homocysteine metabolism was made available to the study. The panel included: MTHFR, factor II or prothrombin (F2), factor V (F5), factor VII (F7), plasminogen activator inhibitor 1 (SERPINE1), fibrinogen beta (FGβ), glycoprotein 1a (ITGA2), glycoprotein IIIa (ITGB3), E-selectin or endothelial leukocyte adhesion molecule (SELE), intracellular adhesion molecule 1 (ICAM1), matrix metalloproteinase 3 (MMP3), tumor necrosis factor alpha or TNF-α (TNF), tumor necrosis factor beta or lymphotoxin alpha (LTα); endothelial nitric oxide synthase (NOS3, also known as eNOS), angiotensin II receptor type 1 (AGTR1), angiotensinogen (AGT), atrial natriuretic peptide (NPPA), alpha adducin (ADD1), epithelial sodium channel alpha subunit (SCNN1A), G nucleotide binding protein beta 3 subunit (GNβ3), and beta 2 adrenergic receptor (ADRβ2). Several genes were represented by more than 1 single-nucleotide polymorphism (SNP), for example NOS3 (3 SNPs) and TNF (4 SNPs).
DNA was extracted from newborn blood specimens by an adaptation of the salting out methodology (Iovannisci, 2000). Each DNA sample was tested using the above multilocus allele-specific hybridization assay. All genotyping was performed blinded to subjects' case or control status.
Statistical Analysis
Risk for gastroschisis associated with each gene SNP was calculated for both the homozygotes and the heterozygotes, with the wild-types as the referent. For several analyses, the homozygote and the heterozygote variants were grouped. Risks were estimated as odds ratios (ORs) with 95% confidence intervals (CIs) by logistic regression using SAS software (SAS Institute, Cary, NC).
RESULTS
Of the 57 case mothers with an infant born with gastroschisis, 47.4% were Hispanic and 52.6% were non-Hispanic. Of the 506 controls, 32.2% were Hispanic and 67.8% were non-Hispanic. The proportion of case mothers and control mothers who smoked was 42.1% and 24.2%, respectively.
The distributions of gene polymorphisms among controls were evaluated for consistency with expectations under Hardy-Weinberg equilibrium. Only ADRβ2 gln27glu did not conform to Hardy-Weinberg equilibrium.
Several polymorphisms on the panel had too few cases with the variant allele to analyze, or had distributions that were similar in cases and controls, with associations with a P value higher than .15. Their genotype distributions are listed at the end of the text, in the Appendix. However, we retained the MTHFR gene results in Table 1 for reasons given in the introduction and in the discussion.
| Gene polymorphism (common name/SNP ID/reference sequence and nt alteration) | Genotype | Cases (n) | Controls (n) | OR | 95% CI |
|---|---|---|---|---|---|
| MTHFR 677C>T/rs1801133/NM_005957.3:c.849C>T | Heterozygote | 28 | 249 | 0.9 | 0.5–1.5 |
| Homozygote | 4 | 65 | 0.5 | 0.2–1.4 | |
| Wild-type | 25 | 192 | Ref | ||
| NOS3 glu298asp/rs1799983/D26607:g.G7002G>T | Heterozygote | 30 | 192 | 1.8 | 1.0–3.3 |
| Homozygote | 4 | 44 | 1.1 | 0.4–3.2 | |
| Wild-type | 23 | 270 | Ref | ||
| AGT met235thr/rs699/NM_000029.2:c.916T>C | Heterozygote | 28 | 251 | 1.4 | 0.7–3.0 |
| Homozygote | 19 | 128 | 1.9 | 0.8–4.2 | |
| Wild-type | 10 | 127 | Ref | ||
| NPPA 2238T>C/rs5065/NM_006172.1:c.553T>C | Heterozygote | 20 | 129 | 1.7 | 0.9–3.1 |
| Homozygote | 3 | 5 | 6.6 | 1.5–28.7 | |
| Wild-type | 34 | 372 | Ref | ||
| ADD1 gly460trp/rs4961/AH003627.1:c.1378G>T | Heterozygote | 21 | 156 | 1.5 | 0.9–2.8 |
| Homozygote | 7 | 18 | 4.5 | 1.7–11.5 | |
| Wild-type | 29 | 332 | Ref | ||
| SCNN1A trp493arg/rs5742912/NM_001038.4:c.1576T>C | Heterozygote | 2 | 25 | 0.7 | 0.2–3.2 |
| Homozygote | 3 | 0 | — | ||
| Wild-type | 52 | 481 | Ref. | ||
| SERPINE1 (–675)G5/G4/rs1799768/J03764.1:g.2491dupG | Heterozygote | 21 | 244 | 0.5 | 0.3–1.0 |
| Homozygote | 13 | 121 | 0.6 | 0.3–1.3 | |
| Wild-type | 23 | 136 | Ref | ||
| SERPINE1 11053G>T/rs7242/J03764.1:g.14216G>T | Heterozygote | 23 | 238 | 1.3 | 0.5–3.3 |
| Homozygote | 28 | 183 | 2 | 0.8–5.1 | |
| Wild-type | 6 | 80 | Ref | ||
| SELE leu554phe/rs5355/NM_000450.1:c.1839C>T | Heterozygote | 8 | 35 | 2.2 | 1.0–5.0 |
| Homozygote | 0 | 1 | — | — | |
| Wild-type | 49 | 470 | Ref | ||
| ICAM1 gly241arg/rs1799969/NM_000201.1:c.778G>A | Heterozygote | 19 | 111 | 1.8 | 1.0–3.3 |
| Homozygote | 2 | 8 | 2.7 | 0.6–13.1 | |
| Wild-type | 36 | 387 | Ref | ||
| SELE ser128arg/rs5361/NM_000450.1:c.561A>C | Heterozygote | 3 | 85 | 0.3 | 0.1–0.9 |
| Homozygote | 0 | 9 | |||
| Wild-type | 54 | 412 | Ref. |
Table 1 gives the unadjusted results of the SNP analyses for the remaining genes. Of note, gastroschisis was not associated with the MTHFR 677C > T variant, which is associated with NTDs and orofacial clefts.
The SCNNIA trp439arg SNP showed an unusual distribution: 3 cases and no controls were homozygous for the variant allele while 5 (9%) cases and 25 (5%) controls had at least 1 variant allele. Although there was a strong association between gastroschisis and the homozygotes for the variant, no further analysis was done because of the small number of cases with the variant SNP.
Several gene polymorphisms showed a significant or borderline significant (P < .15) association with gastroschisis, and those SNPs were used in further analyses when the number of cases permitted their analysis.
Because the distributions of several of the variants in controls differed between the 2 ethnic groups, we adjusted for ethnicity in all further analyses. A separate analysis of the genes of interest with a difference in ethnic distribution shows that the direction of the association is the same in both ethnic groups (Table 2). Because the number of cases was small, and because the heterozygote and homozygote variants were combined, the associations are not as precise.
| Gene | Non-Hispanic Whites | Hispanic Whites | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| NOS3 glu298asp | 2.0 | 0.9–4.5 | 1.6 | 0.7–3.6 |
| NPPA 2238T > C | 2.4 | 1.1–5.0 | 1.7 | 0.7–4.3 |
| AGT met235thr | 1.2 | 0.5–2.9 | 1.8 | 0.4–8.4 |
| SERPINE1 (–675)G5/G4 | 0.5 | 0.2–1.2 | 0.7 | 0.3–1.6 |
| ICAM1 gly241arg | 1.4 | 0.6–3.3 | 2.2 | 1.0–5.1 |
- Heterozygote and homozygote variants were combined for the analyses.
Table 3 shows the results of the analyses adjusted for ethnicity. Six SNPs showed significant or borderline associations for either the heterozygote variant or the homozygote variant. They are: 1) NOS3 gly298asp, a gene variant that produces a reduced level of NO; 2) NPPA 2238T > C, a gene variant that has previously been associated with cardiac hypertrophy; 3) ADD1 gly460trp, a variant of the gene essential for epidermal differentiation and cell proliferation, and for wound repair; 4) SERPINE1 (–675)G5/G4, a variant associated with secondary ischemia and possibly defects in cell adhesion and cell migration; 5) SELE ser128arg, a variant associated with early atherosclerosis and inflammation; and 6) ICAM1 gly214arg, a variant associated with peripheral arterial occlusion (PAOD) and possibly defects in intercellular adhesion and cell migration.
| Gene | Genotype | OR | 95% CI |
|---|---|---|---|
| NOS3 glu298asp | Heterozygote | 1.9 | 1.1–3.4 |
| Homozygote | 1.2 | 0.4–3.7 | |
| NPPA 2238T>C | Heterozygote | 1.9 | 1.0–3.4 |
| Homozygote | 7.5 | 1.7–33.5 | |
| ADD1 gly460trp | Heterozygote | 1.5 | 0.8–2.8 |
| Homozygote | 4.9 | 1.9–12.9 | |
| SERPINE1 (-675)G5/G4 | Heterozygote | 0.6 | 0.3–1.1 |
| Homozygote | 0.7 | 0.3–1.5 | |
| SELE ser128arg | Heterozygote | 0.3 | 0.1– 0.9 |
| Homozygote | — | — | |
| ICAM1 gly241arg | Heterozygote | 1.7 | 1.0–3.2 |
| Homozygote | 2.1 | 0.4–10.3 |
- Referents have the wild-type genotype.
Three SNPs had a significant positive association of the heterozygote variant with gastroschisis: ICAM1 gly241arg (OR, 1.7; 95% CI, 1.0–3.2); NOS3 glu298asp (OR, 1.9; 95% CI, 1.1–3.4); and NPPA 2238T > C (OR, 1.9; 95% CI, 1.2–3.7). ADD1 gly460trp had a nonsignificant association (OR, 1.5; 95% CI, 0.8–2.0). Additionally, for the NPPA and ADD1 SNPs, the homozygote variants had significantly higher risks than the heterozygotes: for NPPA the OR increased from 1.9 to 7.5 (95% CI, 1.7–33.5) and for ADD1 the OR increased from 1.5 to 4.9 (95% CI, 1.9–12.9).
Table 4 gives the results of multivariate analyses, adjusted for ethnicity, that evaluate a possible interaction of maternal smoking with several of the gene variants. It also shows the association of gastroschisis with the SNPs among nonsmokers. There were enough cases for the analysis of an interaction for only 4 of the SNPs after the heterozygote and homozygote variants were combined. For smokers without a variant allele, smoking had an independent effect on the risk for a gastroschisis. In the case of ADD1, the risk was higher for smokers without the variant than for nonsmokers with the variant; and smokers with the variant had the same risk as smokers without the variant. However, smoking increased the risk substantially for carriers of the variant alleles of NOS3, NPPA, and ICAM1, although the risk estimates were imprecise.
| Gene variant | 1or 2 variant alleles | No variant allele | ||||
|---|---|---|---|---|---|---|
| Nonsmoker | Smoker | Smoker | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| NOS3 glu298asp | 1.3 | 0.6–2.7 | 5.2 | 2.4–11.4 | 1.6 | 0.6–4.2 |
| NPPA 2238 T > C | 1.6 | 0.7–3.4 | 6.4 | 2.8–14.6 | 2.1 | 1.0–4.6 |
| ADD1 gly460trp | 2.7 | 1.3–5.6 | 4.3 | 1.7–10.8 | 4.3 | 2.0–10.0 |
| ICAM1 gly241arg | 1.6 | 0.8–3.4 | 5.2 | 2.1–12.7 | 2.5 | 1.2–5.2 |
- Referents are nonsmokers without a variant allele.
DISCUSSION
To our knowledge this is the first report of a role for gene polymorphisms in the etiology of gastroschisis. Because of the exploratory design of this study, the results should be considered preliminary and the analyses should be replicated.
The polymorphisms tested in this study were originally assembled for evaluation of cardiovascular disease risk. Therefore, some of the genes (e.g., those involved in blood pressure regulation) were not expected to be of interest to our hypothesis. MTHFR 677C > T was considered as a negative control because it is associated with NTDs and orofacial clefts. However, no epidemiological study of gastroschisis that we have knowledge of has reported either NTDs or orofacial clefts as associated defects. Thus one would not have expected this SNP to be associated with gastroschisis, which our data seem to confirm.
A limitation of the study was the relatively small number of cases, which required that heterozygote and homozygote variants be combined in some of the analyses.
We based our evaluation on the vascular hypothesis, which suggests that the defect occurs early in development, at the time when, in the area of the defect, there is a shift in vascularization (deVries, 1980), or that there may be an interruption of the blood flow in the vascular plexus that will form the right vitelline arteries (Hoyme et al., 1981), We hypothesized that either process might involve apoptosis, angiogenesis, cell migration, cell adhesion, and possibly inflammation. Furthermore, we hypothesized that several environmental factors could adversely influence the process, among them anoxia, thrombosis, inflammation, and wound formation with defective wound repair. Several of the polymorphisms associated with gastroschisis in our study belong to genes that intervene in some of those hypothesized pathogenetic mechanisms. 1
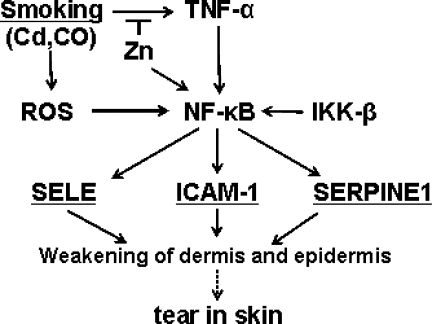
Diagram of one of the hypothetical pathogenetic pathways in the development of gastroschisis that would involve several of the studied polymorphisms.
SCNN1A's function in development can be suggested by studies of knockout mice for the SCNN1A gene who demonstrate abnormal epidermal differentiation (Hummler and Beermann, 2000). The fact that there were no homozygote variants for this gene among the 506 controls and that there were 3 homozygote variants among the 57 cases is of interest and needs replication in another study. However, the small number of cases and controls with this SNP in our study precluded further analyses.
ADD1 is important in epidermal differentiation, cell proliferation and wound repair (Guo et al., 2005). Several authors have reported seeing cases with gastroschisis on ultrasound in utero, but no abdominal wall defect at birth; most such cases were diagnosed at birth with jejunal atresia or short bowel syndrome (Celayir et al., 1999; Kimble et al., 1999; Barsoom et al., 2000; Tawil et al., 2001). In most cases of “vanishing gastroschisis,” the wound healing of the abdominal wall was complete. The importance of wound repair is also shown by a study of the knock-out mouse for the ACLP gene: these mice cannot repair an abdominal wound artificially inflicted during embryogenesis, and the majority of mice are born with extruded intestines (Layne et al., 2001). Furthermore, mice with the wild-type variant repair the inflicted wound and are normal at birth. Although not a perfect model of gastroschisis, the study emphasizes the importance of genes that are active in wound repair. The ACLP gene was not included on the candidate gene panel and is cited here only to emphasize the possible role of wound repair in the etiology of gastroschisis. The model fits with the idea that, in some cases, gastroschisis may occur and be repaired before birth, and that some of the intestinal atresias could be the result of self-repaired gastroschisis. Both these defects share vascular risk factors (Werler et al. 2003), which strengthens the argument.
Endothelial cell activation is induced or controlled by several of the genes whose polymorphisms we examined, specifically ICAM-1, SELE, and SERPINE1; all have roles in the integrity of the dermis. SERPINE1 plays a crucial role in cell migration, cell adhesion, and angiogenesis (Chorostowska-Wynimko et al., 2004). The insertion/deletion polymorphism of its promoter region is associated with increased SERPINE1 activity (Nauck et al., 1999), resulting in impaired fibrinolytic activity and risk of microthrombi and secondary ischemia (Vergouwen et al., 2004). As observed in our study, differences in allele frequencies between Hispanics and non-Hispanic whites have been reported by others (Festa et al., 2003). The SELE SNP is associated with inflammation and early atherosclerosis (Wenzel et al., 1996). The SELE ser128arg polymorphism was inversely associated with the risk for a gastroschisis. The ICAM-1 SNP is associated with PAOD (Gaetani et al., 2002).
NOS3 is essential for neovascularization (Aicher et al., 2003) through production of nitric oxide (NO), a strong vasodilator, and the NOS3 glu298asp variant is a risk factor for carotid atherosclerosis (Lembo et al., 2001). Smoking reduces NOS3 activity. Mice deficient in NOS3 show reduced levels of mobilization of endothelial progenitor cells. NO also activates cyclooxygenase-2 (COX-2), and NOS inhibitors prevent the formation of prostaglandins (Kim et al., 2005). Both COX-1 and COX-2 have been shown to be essential for wound healing of skin in rats (Futagami et al., 2002) and of dermis in mice (Laulederkind et al., 2002). Cyclooxygenase inhibition also leads to the formation of leukotrienes, which affects the microvasculature, in particular, by contracting the mesenteric vasculature (Campbell, 1993), thus affecting the vasculature that supports the abdominal wall. Interestingly, a previous epidemiological study showed a strong association of gastroschisis with COX inhibitors, such as aspirin and ibuprofen (Torfs et al., 1996).
We attempted to integrate these findings with results from previous environmental studies to understand the interrelation between several of these risk factors. Whether the pathogenesis of gastroschisis follows these paths is not known, but they are of interest for further interpretation. The expression of three of the genes associated with gastroschisis, ICAM-1, SELE, and NOS3, is induced by NF-kappa B, a key gene in inflammation. NF-kappa B itself is induced by TNF, an important inflammatory cytokine that also has a role in apoptosis. Although there was no significant association of gastroschisis with the TNF variants we studied, the important point is that smoking induces the expression of TNF, which in turn results in the expression of ICAM-1, SELE, and NOS3. The induction of TNF may be caused by the cadmium (Cd) contained in cigarette smoke: Cd has been shown to stimulate the expression of ICAM-1 in brain endothelial cells via NF-kappa B (Jeong, 2004). Thompson (Thompson and Bannigan, 2001) reported the effect of Cd on ventral body wall formation in FFF mice, and showed that Cd induced cell death in the mesoderm by disrupting peridermal cell adhesion. One cigarette contains 1–2 μg of Cd and at least 5–10% is assimilated into the blood stream during smoking (Lewis et al., 1972). Hovland et al. (1999) report gastroschisis in 6% of C57BL/6N mice exposed to Cd on day 8 of gestation (GD 8). Those results differed according to the strain of mice, emphasizing the genetic susceptibility to environmental factors. Thus, the effect of smoking on the risk associated with ICAM-1, SELE, and NOS3 might be mediated by the effect of Cd on the expression of these genes. Zinc (Zn) antagonizes the effect of Cd by preventing Cd's excess production of reactive oxidative species (ROS). We have previously reported that the combination of poor maternal nutrition and low Zn significantly increased the risk for having offspring with gastroschisis (Torfs et al., 1998). Additionally, Cd accumulates in the body, leading to depletion of glutathione (McMurray and Tainer, 2003). In a previous work, low maternal intake of glutathione was associated with the risk for a gastroschisis in offspring (Torfs et al., 1998).
Cigarette smoke also carries carbon monoxide (CO) to the fetal circulation. An animal model has shown the significant interaction of CO and protein-Zn deficiency on the risk for gastroschisis (Singh, 2003). A subsequent analysis of the gastroschisis data confirmed this interaction in humans (Lam and Torfs, 2006).
These observations suggest that cigarette smoke may act through different pathways, either through Cd or CO, to cause a gastroschisis. The relatively strong interaction of maternal smoking with 4 of the studied polymorphisms, with about a doubling to tripling of the risk, underlines the hypothesis that many, if not most, human diseases result from gene-environment interactions (Botto and Khoury, 2001). The challenge is to find among the many known genes and environmental factors, those that interact in the pathogenesis of the defect. Appendix 1 provides a list of genetic variants that did not meet the criteria for further analysis. This study and most previous studies suggest that there may be many factors that predispose a mother to have an infant with gastroschisis. As is the case for the majority of birth defects (Emanuel and Sever, 1973), gastroschisis probably has a multifactorial etiology.
Our results show an association of gastroschisis with several gene variants and a significant interaction of an environmental factor, smoking, with several of the studied SNPs. It also suggests possible pathogenetic pathways and examines the possible effect of several other reported environmental risk factors for gastroschisis, such as COX-inhibitors or a low protein, glutathione or Zn diet, on the suggested pathways.
The significant interaction between maternal smoking and SNPs in the infant reported here supports previous results that showed an interaction between maternal smoking and another environmental factor, maternal malnutrition, as a risk for gastroschisis (Lam and Torfs, 2006), but the present study adds a genetic component to the analysis.
Acknowledgements
We are grateful to Drs. Cunnigham and Lorey for making newborn specimens available for genotyping experiments, and to Dr. Suzanne Cheng for providing the Roche panels of gene polymorphisms and for thoughtful review of the manuscript. We acknowledge Brett Cohen and Christa Haun for their technical assistance in genotyping the study samples. We also thank the families who participated in the study.
Appendix
| Gene polymorphism (common name/SNP ID/reference sequence and nt alteration) | Genotype | Cases (n) | Controls (n) | OR | 95% CI |
|---|---|---|---|---|---|
| NOS3 (–922)A>G/rs1800779/D26607:g.498A>G | Heterozygote | 27 | 225 | 1.0 | 0.6–1.8 |
| Homozygote | 3 | 55 | 0.5 | 0.1–1.6 | |
| Wild-type | 27 | 226 | Ref | ||
| NOS3 (–690)C>T/rs3918226/D26607:g.730C>T | Heterozygote | 9 | 51 | 1.7 | 0.8–3.6 |
| Homozygote | 0 | 3 | — | ||
| Wild-type | 48 | 452 | Ref | ||
| AGTR1 1166A>C/rs5186/Z11162.1:c.1629A>C | Heterozygote | 23 | 223 | 0.9 | 0.5–1.6 |
| Homozygote | 5 | 36 | 1.2 | 0.4–3.3 | |
| Wild-type | 29 | 247 | Ref | ||
| NPPA 664G>C/rs5063/NM_006172.1:c.193G>A | Heterozygote | 8 | 56 | 1.3 | 0.6–2.9 |
| Homozygote | 0 | 1 | — | ||
| Wild-type | 49 | 449 | Ref | ||
| SCNN1A Ala663Thr/rs2228576/NM_001038.4:c.2096G>A | Heterozygote | 23 | 219 | 0.9 | 0.5–1.5 |
| Homozygote | 6 | 58 | 0.9 | 0.3–2.1 | |
| Wild-type | 28 | 229 | Ref | ||
| GNB3 825C>T/rs5443/U47924.1:g.57721C>T | Heterozygote | 25 | 219 | 1.0 | 0.6–1.8 |
| Homozygote | 6 | 63 | 0.8 | 0.3–2.1 | |
| Wild-type | 26 | 224 | Ref | ||
| ADRB2 Arg16Gly/rs1042713/M15169.1:c.1633A>G | Heterozygote | 23 | 240 | 0.7 | 0.3–1.6 |
| Homozygote | 24 | 188 | 1.0 | 0.4–2.1 | |
| Wild-type | 10 | 75 | Ref | ||
| ADRB2 Gln27Glu/rs1042714/M15169.1:c.1666C>G | Heterozygote | 25 | 215 | 1.0 | 0.6–1.8 |
| Homozygote | 8 | 78 | 0.9 | 0.4–2.1 | |
| Wild-type | 24 | 210 | Ref | ||
| MMP3 (–1171)A5>A6/rs3025058/J04732.1:g.138insdelA | Heterozygote | 26 | 258 | 1.0 | 0.5–2.2 |
| Homozygote | 22 | 153 | 1.4 | 0.6–3.3 | |
| Wild-type | 9 | 90 | Ref | ||
| F2 20210G>A/rs1799963/M17262:g.26784G>A | Heterozygote | 0 | 10 | — | — |
| Homozygote | 0 | 0 | — | — | |
| Wild-type | 57 | 479 | |||
| F5 Arg506Gln/rs6025/NM_000130.3:c.1746G>A | Heterozygote | 4 | 23 | 1.6 | 0.5–4.7 |
| Homozygote | 0 | 1 | — | — | |
| Wild-type | 53 | 480 | Ref | ||
| F7 (–323)10-bp del-ins/rs5742910/J02933:g.198_199delinsCCTATATCCT | Heterozygote | 12 | 119 | 0.9 | 0.5–1.7 |
| Homozygote | 2 | 8 | 2.2 | 0.5–10.7 | |
| Wild-type | 43 | 379 | Ref | ||
| F7 Arg353Glu/rs6046/NM_000131.2:c.1289G>A | Heterozygote | 12 | 98 | 1.1 | 0.6–2.2 |
| Homozygote | 2 | 9 | 2.1 | 0.4–9.8 | |
| Wild-type | 43 | 398 | Ref | ||
| FGB (–455)G>A/rs1800790/X05018.1:g.1045G>A | Heterozygote | 20 | 150 | 1.3 | 0.7–2.4 |
| Homozygote | 4 | 24 | 1.7 | 0.6–5.1 | |
| Wild-type | 33 | 330 | Ref | ||
| ITGA2 873G>A/rs1062535/NM_002203.2:c.867G>A | Heterozygote | 24 | 227 | 0.9 | 0.5–1.6 |
| Homozygote | 9 | 82 | 1.0 | 0.4–2.0 | |
| Wild-type | 24 | 197 | Ref | ||
| ITGB3 Leu33Pro/rs5918/NM_000212.2:c.196T>C | Heterozygote | 11 | 109 | 0.9 | 0.4–1.7 |
| Homozygote | 0 | 10 | — | — | |
| Wild-type | 46 | 387 | Ref | ||
| TNF (–376)A>G/rs1800750/X02910.1:g.240G>A | Heterozygote | 2 | 15 | 1.2 | 0.3–5.3 |
| Homozygote | 0 | 0 | — | — | |
| Wild-type | 55 | 491 | Ref | ||
| TNF (–308)A>G/rs1800629/X02910.1:g.308G>A | Heterozygote | 10 | 126 | 0.6 | 0.3–1.3 |
| Homozygote | 1 | 8 | 1.0 | 0.1–8.3 | |
| Wild-type | 46 | 372 | Ref | ||
| TNF (–244)G>A/rs673/M16441:c.3851G>A | Heterozygote | 0 | 4 | — | — |
| Homozygote | 0 | 0 | — | — | |
| Wild-type | 57 | 502 | |||
| TNF (–238)>A/r361525/X02910.1:g.378G>A | Heterozygote | 3 | 47 | 0.5 | 0.2–1.8 |
| Homozygote | 0 | 0 | — | — | |
| Wild-type | 54 | 459 | |||
| LTA Thr26Asn/rs1041981/X01393.1:c.258C>A | Heterozygote | 29 | 238 | 1.2 | 0.7–2.2 |
| Homozygote | 6 | 52 | 1.1 | 0.4–2.9 | |
| Wild-type | 22 | 216 | Ref |




