Compound craniosynostosis, intellectual disability, and Noonan-like facial dysmorphism associated with 7q32.3-q35 deletion
Funding information: Polish National Science Centre, Poland, Grant/Award Number: UMO-2016/23/N/NZ5/02577
Abstract
Objective
Craniosynostosis (CS) is the premature fusion of the cranial sutures, occurring either in isolated or syndromic form. Syndromic CS, which was described in over 180 genetic syndromes, accounts for 15–30% of all CS cases and usually originates from mutations within the FGFR1, FGFR2, FGFR3, and TWIST1 genes. However, causative alterations in other genes, or rarely copy number variations (CNVs) were also reported. In this article, we describe a patient with Noonan-like facial dysmorphism accompanied by intellectual disability and compound CS, involving coronal, sagittal, and squamous sutures.
Methods
We applied karyotyping, copy number variations analysis using array comparative genomic hybridization, and microarray-based genes expresion analysis.
Results
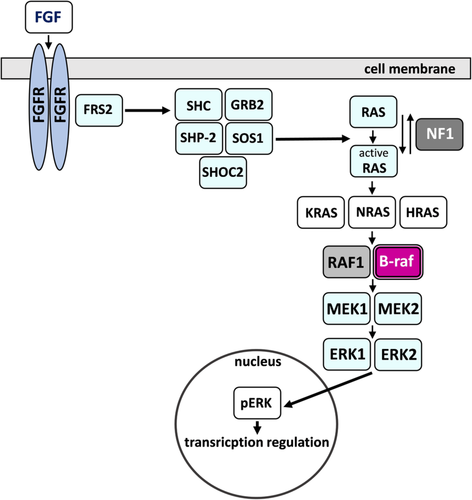
We have shown that the index carried a large and rare heterozygous deletion, which encompassed 12.782 Mb and mapped to a chromosomal region of 7q32.3-q35 (HG38 – chr7:131837067-144607071). The aberration comprised 109 protein-coding genes, including BRAF, that encodes serine/threonine-protein kinase B-Raf, being a part of the RAS/MAPK signaling pathway.
Discussion
The RAS/MAPK pathway plays an essential role in human development; hence, its dysregulation not surprisingly results in severe congenital anomalies, such as phenotypically overlapping syndromes termed RASopathies. To our best knowledge, we report here the first CNV causing haploinsufficiency of BRAF, resulting in dysregulation of the RAS/MAPK cascade, and consequently, in the phenotype observed in our patient. To conclude, with this report, we have pointed to the involvement of the RAS/MAPK signaling pathway in CS development. Moreover, we have shown that the molecular analysis based on both DNA and RNA profiling, undoubtedly constitutes a comprehensive diagnostic and research strategy for elucidating a cause of genetic diseases.
1 INTRODUCTION
Adjacent cranial bones articulate with one another through soft fibrous junctions called sutures that, in a state of their patency, allow the neonatal skull for the passage through the birth canal and for synchronized growth of the neurocranium during development (Baer, 1954; Opperman, 2000). Both ossification of sutures and a state of suture patency are tightly regulated by numerous genetic factors, belonging either to specific signaling pathways or acting independently during morphogenesis (Opperman, 2000).
The impaired function of sutures, defined as their single or compound premature closure, results in cranial defects referred to as craniosynostosis (CS) (Morriss-Kay & Wilkie, 2005). CS occurs with the incidence of 1 per 2,100–2,500 live births, which makes it one of the most common craniofacial defects worldwide (Cohen, 1979; French, Jackson, & Melton, 1990). A considerable number of genes that are affected in patients presenting with CS belong to FGF, WNT, BMP, MAPK, STAT, EPH/EPHRIN, or hedgehog signaling pathways (Katsianou, Adamopoulos, Vastardis, & Basdra, 2016; Poot, 2019; Timberlake et al., 2017; Timberlake & Persing, 2018). Currently, only 21–62% of all affected individuals obtain a reliable genetic diagnosis, depending on the size of the study, ethnicity, and range of the performed genetic testing (Paumard-Hernández et al., 2015; Wilkie et al., 2010). In one-third of the patients, pathogenic variants occur within the genes that encode fibroblast growth factor receptors (FGFRs), which act upstream of MAPK, also known as RAS–RAF–MEK–ERK, signaling cascade (Goos & Mathijssen, 2019; Twigg & Wilkie, 2015). The CS condition may also be associated with pathogenic variants in other genes or rarely with copy number variations (CNVs) (Lattanzi, Barba, Di Pietro, & Boyadjiev, 2017; Massalska et al., 2014; Poot, 2019; Sowińska-Seidler, Olech, Larysz, & Jamsheer, 2018; Wilkie et al., 2010).
The classification of CS is usually based on three criteria. First, depending on the number of involved sutures, we distinguish single or compound CS. Second, based on the etiology, CS is classified either as a primary defect, resulting from abnormalities directly affecting formation or maintenance of the suture(s), or secondary, which arises as a consequence of other disorders, such as central nervous system, metabolic, endocrine, or hematological conditions (Khanna, Thapa, Iyer, & Prasad, 2011; Lattanzi et al., 2017; Wilkie et al., 2010; Wilkie, Bochukova, Hansen, Taylor, et al., 2007). Finally, the absence of additional features allows classifying CS as an isolated, non-syndromic defect. Such type of CS is more common and occurs in about 70–85% of cases, whereas its less common syndromic form accompanies additional congenital abnormalities (Greenwood, Flodman, Osann, Boyadjiev, & Kimonis, 2014; Heuzé, Holmes, Peter, Richtsmeier, & Jabs, 2014; Wilkie et al., 2010).
2 MATERIALS AND METHODS
All procedures were performed under the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. We granted ethics approval from the Institutional Review Board of Poznan University of Medical Sciences (no. 742/17 obtained on June 22, 2017). This research involved a young female patient under the age of 18 years. Hence, we obtained the parental informed consent for the patient's participation in this study and involvement in the analysis and data report.
2.1 Karyotyping
We performed karyotyping on peripheral blood lymphocytes of the female 10.5 years old patient and her healthy parents. We used the Giemsa-banding technique at 550 band resolution per haploid genome.
2.2 Copy number variation analysis using array comparative genomic hybridization
Array comparative genomic hybridization (aCGH) was performed on the DNA of the index. We extracted genomic DNA from the peripheral blood lymphocytes using the MagCore® HF16 Automated Nucleic Acid Extractor and Genomic DNA Large Volume Whole Blood Kit (RBC Bioscience Corp.). gDNA was quantified with both Agilent 2200 TapeStation system (Agilent Technologies) and NanoDrop 2000 Spectrophotometer (ThermoFisher Scientific). We performed aCGH with the use of SurePrint G3 Human CGH Microarray 4 × 180 (Agilent Technologies) with 25.3 kb overall median probe spacing according to the standard protocol provided by the manufacturer. The hybridization signals were detected with SureScan Dx Microarray Scanner (Agilent Technologies) and visualized with the use of Agilent CytoGenomics software (Agilent Technologies).
2.3 Microarray-based gene expression analysis
To analyze the expression profile of the deleted genes, we extracted total RNA from whole blood of the index patient and one gender- and age-matched standard control, following the RNA and miRNA isolation protocol with the use of TRIreagent and chloroform. RNA concentration and RNA integrity number (RIN) was assessed using NanoDrop 2000 Spectrophotometer (ThermoFisher Scientific) and Agilent 2200 TapeStation system (Agilent Technologies), respectively. We used one-color SurePrint G3 Human Gene Expression v3 Microarray (Agilent Technologies) to analyze gene expression profile following the standard protocol provided by the manufacturer. The hybridization signals were detected with SureScan Dx Microarray Scanner (Agilent Technologies).
2.4 Bioinformatic analysis
We visualized and analyzed the aberrant genomic locus with the use of Cytoscape v3.3.0 software and ClinTAD online available tool (https://www.clintad.com/) using the following HPO terms as an input: HP:0001363—craniosynostosis, HP:0001249—intellectual disability, HP:0000316—hypertelorism, HP:0000384—preauricular skin tag, HP:0001999—abnormal facial shape, HP:0004484—craniofacial asymmetry, HP:0001263—global developmental delay (Shannon et al., 2003; Spector&Wiita, 2019). The gene expression profile was analyzed with the use of both GeneSpring 14.9 software (Agilent Technologies) and Database for Annotation, Visualization and Integrated Discovery (DAVID; https://david.ncifcrf.gov/home.jsp) online available tool.
3 RESULTS
3.1 Clinical assessment
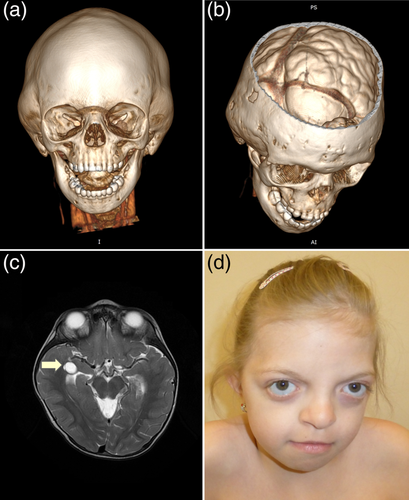
The index was a 10.5 years old sporadic female patient referred to our clinic due to genetically undiagnosed compound CS, intellectual disability, and facial dysmorphism (Figure 1). Both parents were healthy, non-consanguineous, and the three-generation family pedigree did not show any overt genetic diseases, congenital malformations, intellectual disability, or reproductive failures. The patient was born from a second pregnancy complicated with cervical insufficiency treated with pessary at 33 weeks of gestation. The proband was spontaneously delivered at 38 weeks of pregnancy with body mass 2,650 g (25th percentile), birth length 48 cm (50th percentile), and head circumference (HC) of 32 cm (10th percentile). After birth, echocardiography and abdominal ultrasound revealed no abnormalities. Her psychomotor development was delayed with independent sitting and walking achieved at 10 and 22 months, respectively. Speech development was also delayed with the first single words spoken at 2 years of age. The patient was sent for the first genetic evaluation in a different clinic because of facial dysmorphism, preauricular skin tag, and psychomotor retardation at the age of 2.5 years and received no diagnosis at that time. At the age of 4 years, the index had a computer tomography (CT) scan of the head, which revealed deformation of the calvarial bones resulting from the premature fusion of the sagittal, coronal, and squamous sutures (Figure 1a). In addition, the beginning of increased intracranial pressure with indentations in the skull was noted (Figure 1b). Therefore, the patient underwent neurosurgical treatment involving incision of the prematurely fused sutures, followed by remodeling of the skull to its normal anatomical shape. Furthermore, a CT scan showed a lack of the fusion of the posterior arch of the first cervical vertebra, whereas brain magnetic resonance imaging (MRI) detected a small left-sided choroid fissure cyst (Figure 1c). At the age of 10.5 years, the patient presented with moderate intellectual disability and facial dysmorphism, strikingly resembling features of Noonan syndrome (NS), including proptosis, hypertelorism, down-slanted palpebral fissures, broad nasal bridge, and bulbous nasal tip (Figure 1d; for clinical symptoms and dysmorphic characteristics, see Table 1). The body measurements of 10.5 years of age were as follows: height 138 cm (between 25th and 50th percentile), body mass 33 kg (between 25th and 50th percentile), and HC 50 cm (below third percentile).
| Index case | |
|---|---|
| Deletion region | 7q32.3-q35 |
| Sex | F |
| Short stature | − |
| Neonatal growth failure | + (IUGF) |
| Dolichocephaly | − |
| Compound craniosynostosis | + |
| Bitemporal narrowing | + |
| Prominent forehead | + |
| Low set ears | − |
| Preauricular ear tags | + |
| Thick helix | − |
| Hypertelorism | + |
| Epicanthal folds | + |
| Flat nasal bridge | + |
| Bulbous nose | + |
| Short neck | + |
| Skin/ectodermal abnormalities | − |
| Broad chest | − |
| Pectus carinatum | − |
| Congenital heart defects | − |
| Psychological delay | + |
| Development delay | + |
| Speech delay/disorder | + |
| Abnormal brain MRI | + |
| Seizures/epilepsy | − |
3.2 Genetic analyses
3.2.1 Karyotyping and CNV analysis using aCGH
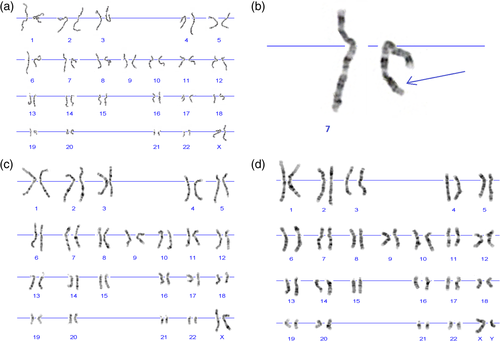
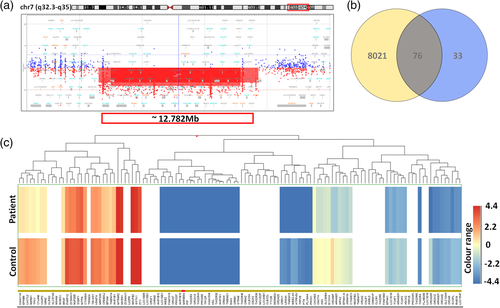
Karyotyping revealed an interstitial deletion on chromosome 7 in the patient (Figure 2a and b) and excluded this chromosomal aberration in both healthy parents (Figure 2c and d). aCGH allowed us to refine the size and the exact location of the deletion, which encompassed 12.782 Mb and mapped to a chromosomal region of 7q32.3-q35 (HG38 – chr7:131837067-144607071) (Figure 3a). We presented the detailed list of all deleted genes in Supplementary File 1. Cytoscape and ClinTAD have indicated that BRAF, PTK1, CALD1, and NUP2005 genes are responsible for the phenotype observed in the index case.
3.2.2 Microarray-based gene expression analysis
Using GeneSpring 14.9 software, we have shown that the expression profile varied between our patient and a standard RNA sample. Because only one unique patient sample and one control sample was available, it was not possible to apply statistical methods for differential gene expression analysis. Instead, we compared normalized signal intensities for probes in the patient sample with normalized signal intensities for probes in the control sample and designated genes with normalized signal intensity fold-change higher than or equal to 1.2 as differentially expressed. The fold-change analysis performed on normalized, log-transformed signal intensities revealed lower signal for 8097 annotated protein-coding genes in the patient versus control sample (Supplementary File 2). Vienn diagram demonstrated a total number of detected, annotated putatively downregulated protein-coding genes—8097 (yellow and gray), the number of annotated protein-coding genes located within the deleted region according to literature—107 (gray and blue), and a number of annotated putatively downregulated protein-coding genes located within the deletion—76 (gray) (Supplementary File 3) (Figure 3b). The hierarchical combined tree (non-averaged) of downregulated genes located within the deletion was presented in Figure 3c. Moreover, both DAVID and GeneSpring 14.9 tools pointed to the dysregulation of the RAS/MAPK signaling pathway (Figure 4).
4 DISCUSSION
In this article, we have described a female patient presenting with compound CS that involves coronal, sagittal, and squamous sutures accompanied by facial dysmorphic features reminiscent of NS. The index harbored a heterozygous deletion, which encompassed 12.782 Mb and mapped to a chromosomal region of 7q32.3-q35 (HG38 – chr7:131837067-144607071). The aberration comprised 109 protein-coding genes (Supplementary File 1), including BRAF that encodes serine/threonine-protein kinase B-Raf, being a part of the RAS/MAPK signaling pathway (Figure 4). Germline mutations within BRAF link to phenotypically overlapping syndromes that belong to RASopathies such as cardio-facio-cutaneous (CFC) syndrome (MIM 115150), LEOPARD syndrome 3 (MIM 613707), and Noonan syndrome 7 (MIM 613706), known as RASopathies.
RASopathies result from germline mutations dysregulating RAS/MAPK pathway, which plays an essential role in human development. Furthermore, somatic usually activating mutations are found in cancer diseases. The phenotype of each RASopathy is unique; however, commonly recognized clinical features include craniofacial dysmorphism, neurocognitive impairment, cardiac and musculoskeletal defects, and hypotonia (Rauen, 2013; Tidyman & Rauen, 2016). Recently, a few researchers have described individuals with RASopathy and CS as an additional phenotype (Davis et al., 2019; Kratz et al., 2009; Takenouchi et al., 2014; Ueda, Yaoita, Niihori, Aoki, & Okamoto, 2017). For example, CS has been reported in patients presenting with NS as a result of germline alterations within KRAS, SHOCK2, and PTPN11 (Kratz et al., 2009; Takenouchi et al., 2014; Ueda et al., 2017). Moreover, pathogenic variants in BRAF and KRAS were also noted among six individuals with CFC and various forms of CS (Ueda et al., 2017).
There are some possible mechanisms of interaction between altered FGFR signaling, that is associated with CS, and RAS/MAPK pathway; but, no comprehensive explanation accounting for the co-occurrence of both CS and RASopathy was proposed thus far. Noteworthy, a reduced dosage of both ERF1 and ERF2 that downregulates RAS/MAPK signaling was found to cause the complex CS in humans as well as in a mouse model (Kim et al., 2003; Twigg et al., 2013). Growing evidence of the occurrence of CS in patient with RASopathies, however, inclines further investigation of possible interaction between RAS/MAPK and FGFR pathway.
Our index carried a large CNV encompassing 109 deleted protein-coding genes, which we regard causative for the patient's phenotype. The pathomechanism of CNVs can be explained by gene dosage effect, giving rise to either overexpression or haploinsufficiency of genes, and/or by cis-regulatory effect altering the local regulatory landscape (Klopocki & Mundlos, 2011). Here, we propose a possible link between haploinsufficiency of BRAF, dysregulation of the RAS/MAPK signaling pathway, and Noonan-like facial dysmorphism with CS. Importantly, in a contiguous gene deletion syndrome, when numerous genes are affected, it is often difficult to find a precise link between patient's phenotype and a single gene causative for most or all of the symptoms (Dilzell, Darcy, Sum, & Wallerstein, 2015; Malmgren, Malm, Sahlén, Karlsson, & Blennow, 2005; Petrin et al., 2010; Sowińska-Seidler et al., 2018). In addition to BRAF haploinsufficiency, the deletion detected by us contained genes involved in nervous system development or neural cell functioning—AGK, AKR1B1, CHCHD3, CASP2, DGKI, EPHA1, LUC7L2, and TPK1. Therefore, some of the additional anomalies, especially psychomotor retardation and moderate intellectual disability, could arise from haploinsufficiency of one or more of the listed genes. Another limitation of our study is an inability to exclude the involvement of other genes with currently unknown function or a change of a regulatory landscape within the deleted locus.
In conclusion, with this report as well as based on the relevant literature review, we have pointed to the possible involvement of the RAS/MAPK signaling pathway in CS development and its co-occurrence with RASopathies. Moreover, we have shown that the molecular analysis based on both DNA and RNA profiling, undoubtedly constitutes a comprehensive diagnostic and research strategy for elucidating a cause of genetic diseases. With such an integrated approach, we will be able to broaden our understanding of molecular and biological processes underlying structural abnormalities and impaired function of the cranial sutures.
ACKNOWLEDGMENTS
We are grateful to all participants of this study. We also thank Aleksandra Szczepankiewicz from the Department of Genetics in Psychiatry, Poznan University of Medical Sciences, for her support in gene expression profile analysis.
AUTHORS' CONTRIBUTIONS
AJ and EB-O designed the study; EB-O performed the aCGH and genes expression profiling analysis, wrote the manuscript, and prepared the figures; MDW performed genes expression profiling analysis; DL recruited the patient and wrote the part of the clinical description; BW performed the part of gene expression analysis; JWS prepared the part of the figures; DS performed karyotyping analysis; AJ diagnosed the patient clinically and critically revised and corrected the manuscript. All authors have read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All procedures performed in this study were under the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethics approval was granted by the Institutional Review Board of Poznan University of Medical Sciences. This research involved a young female patient under the age of 18 years. Hence, we obtained the parental informed consent for the patient's participation in this study and involvement in the analysis and data report.
CONSENT FOR PUBLICATION
The additional consent for publication of identifying information/images in an online open-access publication was obtained.
CONFLICT OF INTEREST
Bartosz Wojciechowicz is a full-time employee of Perlan sp. z o.o., and Perlan Technologies is an authorized distributor of Agilent Technologies in Poland.
Open Research
DATA AVAILABILITY STATEMENT
Expression analysis' data were submitted on Gene Expression Omnibus (GEO) NCBI https://www.ncbi.nlm.nih.gov/geo/ (GEO BioProject: PRJNA633506); samples no. GSM4558105 and GSM4558106. Release date: June 1, 2020.




