Sensory experiences questionnaire unravels differences in sensory profiles between MECP2-related disorders
Bernhard Suter and Davut Pehlivan contributed equally to this study.
Abstract
The methyl CpG-binding protein-2 (MECP2) gene is located on the Xq28 region. Loss of function mutations or increased copies of MECP2 result in Rett syndrome (RTT) and MECP2 duplication syndrome (MDS), respectively. Individuals with both disorders exhibit overlapping autism symptoms, yet few studies have dissected the differences between these gene dosage sensitive disorders. Further, research examining sensory processing patterns in persons with RTT and MDS is largely absent. Thus, the goal of this study was to analyze and compare sensory processing patterns in persons with RTT and MDS. Towards this goal, caregivers of 50 female individuals with RTT and 122 male individuals with MDS, between 1 and 46 years of age, completed a standardized measure of sensory processing, the Sensory Experiences Questionnaire. Patterns detected in both disorders were compared against each other and against normative values. We found sensory processing abnormalities for both hyper- and hypo-sensitivity in both groups. Interestingly, abnormalities in MDS were more pronounced compared with in RTT, particularly with items concerning hypersensitivity and sensory seeking, but not hyposensitivity. Individuals with MDS also exhibited greater sensory symptoms compared with RTT in the areas of tactile and vestibular sensory processing and for both social and nonsocial stimuli. This study provides a first description of sensory symptoms in individuals with RTT and individuals with MDS. Similar to other neurodevelopmental disorders, a variety of sensory processing abnormalities was found. These findings reveal a first insight into sensory processing abnormalities caused by a dosage sensitive gene and may ultimately help guide therapeutic approaches for these disorders.
Key points
What is known
- Individuals with both MDS and RTT exhibit overlapping autism symptoms, few studies have dissected the differences between these opposite gene dosage disorders. Sensory processing patterns in individuals with RTT and MDS have not been well-studied.
What is new
- Clear sensory processing abnormalities were observed in both RTT and MDS, including atypical hyposensitivity and sensory seeking behaviors. Notably, MDS individuals showed more pronounced abnormalities, particularly in hypersensitivity.
What is relevant
- This study sheds light on sensory symptoms in RTT and MDS, contributing valuable insights. This research helps advance our understanding of these rare genetic disorders and may enlighten potential therapeutic interventions.
INTRODUCTION
Gene dosage disorders, in which either too much or too little of a gene cause disease, are strongly linked to psychiatric, cognitive, and behavioral impairments (Genovese & Butler, 2023; Levitis et al., 2024; Morris, 2023; Vicari, 2006). One well-studied example of gene dosage disorders are diseases caused by the X-linked MECP2 (methyl CpG-binding protein-2) gene, a transcriptional regulator of thousands of genes (Collins & Neul, 2022). MECP2 is associated with two major neurodevelopmental disorders: Rett syndrome (RTT) and MECP2 duplication syndrome (MDS) (Sandweiss et al., 2020).
RTT (MIM# 312750), caused by loss of function mutations in MECP2, is characterized by seemingly normal postnatal development in the first 6–18 months, followed by regression in communication and fine motor function and the emergence of characteristic midline hand stereotypies along with an abnormal or absent gait. The disorder leads to severe to profound intellectual disability, global developmental delay, autistic behavior, sleep disturbances, hypotonia progressing to spasticity/contractures, growth failure, gastrointestinal problems, epilepsy, autonomic system dysfunction, and insomnia (Sandweiss et al., 2020). It is predominantly seen in females with an incidence of 1 in 10,000–15,000 live born female individuals (Hagberg, 1985). Historically, RTT was initially classified as an autism spectrum disorder (Hagberg et al., 1983), as these individuals often exhibit signs of autism including poor sociability, lack of communication, absent/poor speech, sensory symptoms, and stereotypies. However, as knowledge on the phenotype of RTT has increased, it has been recognized that autistic features may be transient or variably present in individuals with RTT, leading to its removal as an autism spectrum disorder in the DSM-5 (Oberman & Kaufmann, 2020). Nevertheless, given the overlapping features, autism may be the initial label or descriptive diagnosis that is given until further etiologic evaluation via genetic testing reveals a diagnosis of RTT.
MDS (MIM# 300260) is caused by genomic duplications or triplications of the Xq28 region containing MECP2 (Meins et al., 2005; Van Esch et al., 2005). The most common clinical features include infantile hypotonia leading to severe developmental delay, progressive spasticity, absent to little speech, recurrent respiratory infections, refractory epilepsy, autistic features, behavioral problems such as anxiety and compulsion, and stereotypic hand movements (Ta et al., 2022). The frequency of MDS is not comprehensively studied; however, a recent study reported the birth prevalence of MDS as 0.65/100,000 live births in Australia (Giudice-Nairn et al., 2019). A study utilizing gold-standard autism diagnostic measures (Autism Diagnostic Observation Schedule, ADOS; Autism Diagnostic Interview-Revised, ADI-R) found autism to be universally present in MDS individuals, characterized by deficits in maintaining eye gaze, limited initiation of social interactions, absent or repetitive language, and the presence of motor stereotypies, repetitive behaviors, and sensory interests or aversions (Ramocki et al., 2009). Larger cohort studies, while still showing a preponderance of the autistic features described, do not find autism universally present in MDS (Miguet et al., 2018). Peters et al. (2013) compared core symptoms of autism in individuals with MDS to those of individuals with idiopathic autism in a small but thorough cohort study (N = 10 in each group) and showed that hyposensitivity to pain and temperature is a distinct feature of MDS, but other core features of autism (social affect, repetitive behavior) are similar in MDS and idiopathic autism.
Thus, patients with RTT and MDS have many overlapping features including components of autism such as sensory behaviors. However, the specific patterns of sensory changes in each disorder are not yet well characterized. Although sensory behaviors have not been characterized in clinical patients, work in animal models has demonstrated a role for MECP2 in sensory processing across multiple sensory modalities. For example, auditory pathways in a Mecp2 duplication mouse model had differences in cortical activity patterns compared with wild type mice, but not differences in brainstem responses (Zhou et al., 2019). Similarly, Rett mice models exhibit significant latency differences in auditory pathways (Liao et al., 2012). Mouse models of Rett also exhibit deficits in tactile sensory perception as measured in the primary somatosensory cortex (Mykins et al., 2023), postnatal refinement of olfactory circuits (Degano et al., 2014), and discriminating stimulus patterns (LeBlanc et al., 2015). All these studies provide evidence that sensory pathways are globally affected in MECP2-related disorders, although direct comparison of MECP2 duplication to loss of MECP2 has not been reported.
Thus, we sought to investigate if differences in sensory features could distinguish two gene dosage disorders. Towards this purpose, we aimed to explore sensory behavior differences in MDS and RTT by applying the widely used Sensory Experiences Questionnaire (SEQ) to measure patterns of sensory behaviors in children with MDS and RTT. We additionally compared sensory features with idiopathic autism given the overlap of features.
METHODS
Study design and participants
This survey was conducted as a cross-sectional study. The survey was administered in three different ways: in-person after clinical appointments for both RTT and MDS, in-person during RTT family camp for RTT individuals, and through an online secure portal for MDS individuals. Pilot survey data, including signed consent by caregivers, were collected under Baylor College of Medicine (BCM) Institutional Review Board (IRB) protocol H-35943. For subsequent in-person data, IRB waived the requirement for signed consent to participate in the study (protocol number: H-51705). In-person surveys were completed between December 2021 and April 2023.
The online Health Insurance Portability and Accountability Act (HIPAA)-compliant registry portal was developed for MDS individuals to coordinate and implement studies. The BCM IRB approved to create and maintain this registry, and to conduct survey studies (protocol number: H-46176). Participants provided a written consent form for the portal registration, participation in survey studies, and publication of the results. All the collected data were stored in password-protected BCM and Texas Children's Hospital-secured computers. This survey was hosted at https://mds.nrihub.org and was available to caregivers between August 16 and September 6, 2022.
Measurement tools
We used the SEQ (Lee et al., 2022) to investigate sensory behaviors in MDS and RTT. This 37-question parent survey was chosen based on validation for individuals in the developmental age range of 2–12 years, which matched our patient population, ease of administration, and ability to query hyper- and hypo-sensitivity separately. Given the developmental level of participants, and as is consistent with research within other similar developmental disorders, the SEQ was utilized outside of the normative age range. The SEQ has been used to measure sensory symptoms in a wide range of populations including autism (Baranek et al., 2006) as well as Angelman, Cornelia de Lange and Fragile X syndromes (Heald et al., 2020). The SEQ was scored per the instruction manual provided by Dr. Baranek, the creator of the instrument, without any adjustments or corrections. The approximate duration to complete the survey was 8–10 min.
Statistical approach
Descriptive statistics using mean +/− standard error are reported. Student's t-test was used to compare means between MDS and RTT. Chi-squared test was used to compare nominal data. To measure variability, we calculated population variance. p-value <0.05 was considered to be statistically significant. Statistical calculations were performed in Prism 10.
RESULTS
Participants
We collected sensory data from 50 patients with RTT and 122 patients with MDS using a combination of in-person and electronic surveys (Table 1). Patients with RTT were slightly older (mean RTT = 13.62 +/− 8.22; mean MDS = 9.84+/− 7.87, t-test p = 0.0039). There was no difference in percent of surveys completed by mother (RTT = 33 [66%], MDS = 88 [72%], chi-squared = 0.6391, p = 0.424). As expected based on their diagnosis, all participants in the RTT group were female while almost all participants in the MDS group were male.
| Rett syndrome | MECP2 duplication syndrome | |||
|---|---|---|---|---|
| Total (N) | 50 | 122 | ||
| Number | (%) | Number | (%) | |
|---|---|---|---|---|
| Survey completed by | ||||
| Mother | 33 | 66% | 88 | 72% |
| Father | 9 | 18% | 18 | 15% |
| Both Father and Mother | 6 | 12% | 13 | 11% |
| Other | 2 | 4% | 3 | 2% |
| Survey completed in | ||||
| Clinic | 41 | 82% | 15 | 12% |
| Rett Camp | 9 | 18% | ||
| Online | 107 | 88% | ||
| Gender of the child | ||||
| Male | 0 | 119 | 98% | |
| Female | 50 | 100% | 3 | 2% |
| Age | ||||
| Mean (years) | 13.62 | 9.84 | ||
| Median (IQR) | 12.25 (9.58) | 8 (8.71) |
- Abbreviation: IQR, interquartile range.
Both patients with Rett and MECP2 duplication exhibit atypical sensory symptoms
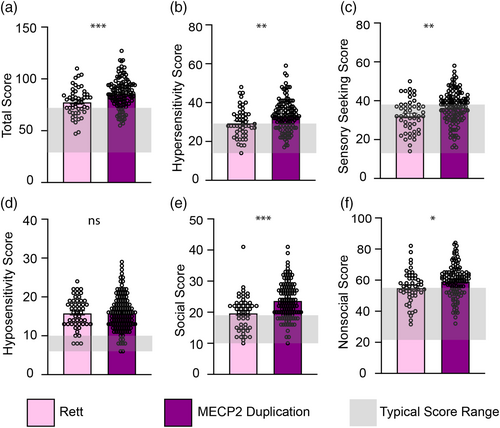
Given the frequent comorbid diagnosis of autism in both these populations, we hypothesized that both would exhibit atypical sensory symptoms. Indeed, total sensory score was in the atypical range for both RTT (mean +/− SEM = 77.89 +/− 1.907) and MDS (mean +/− SEM = 86 +/− 1.27) compared with the cut off for a typical score which is 74 (62.49+/−11.50). Interestingly, individuals with MDS were significantly more impacted (unpaired t-test, p = 0.0006) compared with individuals with RTT (Figure 1a). This difference in sensory symptoms was driven by selective worsening in hypersensitivity and sensory seeking, but not hyposensitivity (Figure 1b–d). Notably, these sensory differences were noted for both social (mean +/− SEM RTT = 19.80 +/− 0.798, MDS = 23.80 +/− 0.5211, p < 0.0001, Figure 1e) and nonsocial (mean +/− SEM RTT = 55.23 +/− 1.485, MDS = 59.58 +/− 0.9309, p = 0.0133, Figure 1f) stimuli.
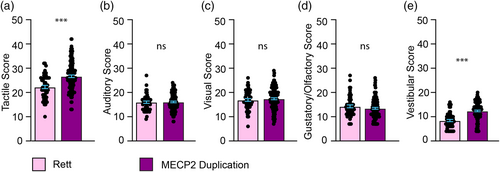
We next asked if there was a difference in affected sensory modalities between individuals with RTT versus MDS. Individuals with MDS exhibited greater sensory symptoms compared with RTT in the areas of tactile and vestibular sensory processing, but not auditory, visual or gustatory/olfactory (Figure 2).
Distribution of sensory scores in RTT is more homogeneous
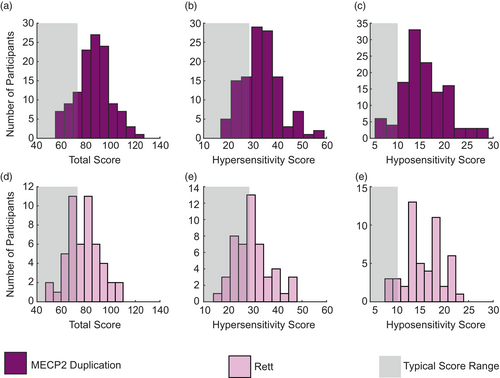
Given that we identified significant differences in sensory phenotypes between individuals with RTT and those with MDS, we next evaluated the heterogeneity of these phenotypes. Patients with MDS exhibited a similar mean score to previously published mean scores in autism (Baranek et al., 2006) for total sensory score (Figure 3a), hypersensitivity (Figure 3b), and hyposensitivity (Figure 3c) with a broad distribution (variance (𝜎2) total = 196, hypersensitivity = 65, hyposensitivity = 20). In contrast, patient scores from individuals with RTT for total sensory (Figure 3d), hypersensitivity (Figure 3e), and hyposensitivity (Figure 3e) were skewed towards the typical range (gray boxes) with a much narrower distribution (variance total = 178, hypersensitivity = 56, hyposensitivity = 15). Thus, we conclude that sensory symptoms are more homogeneous in RTT compared with MDS, and that sensory symptom scores in MDS are nearer to those seen in idiopathic autism.
Sensory scores do not change with age in RTT or MDS
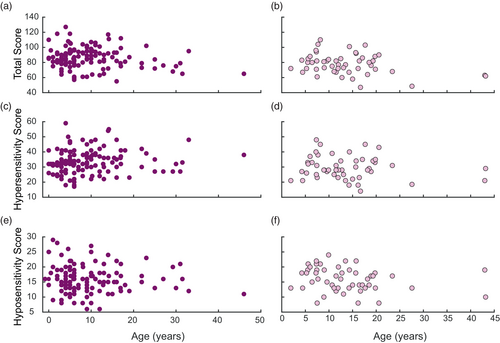
Prompted by recent work suggesting sensory differences can change with age (Lyons-Warren et al., 2024, Cakar et al., 2023), we next evaluated how sensory scores in RTT and MDS change with age. Interestingly, we saw no age dependent changes for either population for total sensory score (Figure 4a,b), hypersensitivity score (Figure 4c,d), or hyposensitivity score (Figure 4e,f).
DISCUSSION
We conducted one of the first studies comparing sensory symptoms in MECP2-related disorders to identify sensory behaviors that are different between RTT and MDS. Both diagnoses exhibited atypical sensory symptoms, but interestingly, individuals with MDS were more impacted, driven largely by more hypersensitivity and sensory seeking behavior. When looking across sensory modalities, individuals with MDS exhibited greater sensory symptoms in the tactile and vestibular domains. There was not a significant difference in hyposensitivity between RTT and MDS, nor were there significant differences in the auditory, visual, or olfactory domains. Sensory symptom scores for MDS individuals were closer to that of idiopathic autism, while RTT scores were nearer to the typical range.
Interestingly, high pain tolerance, which may be related to hyposensitivity, is one of the minor diagnostic criteria for RTT (Neul et al., 2010) and reported to be common in large MDS cohort studies (Lim et al., 2017; Miguet et al., 2018; Peters et al., 2019). Both groups exhibited atypical hyposensitivity scores, consistent with these clinical findings. Recent work assessing tactile response to a range of stimuli (light touch, temperature, pressure and pinprick) suggests that heart rate variability can be used as a marker of tactile detection in individuals with RTT (Merbler et al., 2020) suggesting more sensitive measures of tactile hyposensitivity could be elucidated. Future work in animal models quantitatively evaluating somatosensory sensitivity across light touch, temperature, and pain can help to discriminate the relationship between hyposensitivity and pain tolerance in MECP2-related disorders. Importantly, prior studies evaluating sensory circuits in animal models of MECP2-related disorders have looked only at loss or gain of function. This work suggests that it will be important for future studies to directly compare the two conditions.
We also observed that individuals with MDS had higher scores in the tactile and vestibular sensory domains as compared with patients with RTT. Though a diminished response to pain is one of the criteria for a diagnosis of (atypical) RTT, it is important to note that pain receptors evidently differ from those by which light touch is perceived. It thus remains even more intriguing that tactile domains appear more severely affected in MDS. Understanding what these differences mean clinically will require further work evaluating subdomains of touch sensitivity such as stereognosis, roughness, hardness, and heaviness (Liang et al., 2023). Interestingly, hyposensitivity in general appears related to pain perception with hyposensitivity corresponding with a known age dependent change in pain tolerance in, for example, cerebral palsy (Lyons-Warren et al., 2024). Further, stereotypies are associated with both syndromes, although the semiology differs between the two. Specifically, in our experience, patients with MDS appear to have more autistic-like side-flapping and body rocking, whereas patients with RTT exhibit more midline hand wringing stereotypies. Very little has been reported in the literature regarding the mechanisms behind midline hand ringing seen in RTT versus the sides-of-the-body stereotypies seen in MDS, but vestibular sensory differences might contribute as vestibular stimulation impacts body rocking (Bonadonna, 1981; Dave, 1992; MacLean Jr. & Baumeister, 1982). However, tactile and proprioceptive changes could also contribute. This will be an important area for further exploration in patients and animal models.
Integrating insights about sensory abnormalities found in these disorders into clinical care may also benefit outcomes. For instance, providing a controlled multisensory (Snoezelen) environment has been suggested to improve adaptive and functional behavior in Rett individuals (Lotan, 2006), and Ayres sensory integration therapy may help with the development of skills such as grasping (Drobnyk et al., 2019).
Our findings support the use of sensory behaviors as a distinguishing clinical phenotype for MECP2-related disorders. While there are no approved genetic-based treatments for MECP2-related disorders yet, preclinical studies using antisense oligonucleotide (Sztainberg et al., 2015) and gene therapy (Garg et al., 2013) demonstrate robust phenotype recovery in MDS and RTT mouse models, respectively. Genetic-based treatments hold great promise for ameliorating human genetic diseases, but in gene dosage sensitive disorders there is a potential risk of turning a disorder with too little functional gene (e.g., RTT) into one with too much (such as MDS), or vice versa. Thus, titration of dosage remains of utmost importance. We propose that differences in severity and presentation of distinct clinical features such as sensory behaviors may be helpful to guide treatment dosage. Importantly, circuit level changes are more likely to respond faster to an intervention compared with observed developmental steps. However, changes in sensory behaviors can be easily tracked over time (Baranek et al., 2019; McCormick et al., 2016; Peters et al., 2012), further suggesting that sensory behaviors may be a useful clinical biomarker. Thus, unraveling circuit level differences in visual, auditory, and somatosensory pathways between MDS, RTT, and neurotypicals are highly important.
There are several limitations to our study. First, we relied on the caregivers' report for the sensory symptoms due to cognitive and/or communication delays in our patient population. While this is a common technique (Jorquera-Cabrera et al., 2017), use of caregiver report is still less sensitive than quantitative measurements of sensory ability. This is particularly relevant in our population as this questionnaire was administered on its own without additional clinical exam to determine overall functional level of participants. It will be important to place findings within the context of a broader clinical evaluation in future studies. Differences in developmental age (our chronological age range was outside the stated validated age range of the tool), baseline fine, and gross motor skills as well as overall functioning could have impacted scores and could potentially account for the group differences between RTT and MDS. These factors will be important to examine in future studies. Second, the study was conducted as a cross-sectional study with a small difference in mean age between the groups. Symptoms may change over time, which might have influenced the differences we found. Importantly, regression status may also affect sensory differences. As a small number of RTT patients in our cohort were younger than 4 years of age at the time of assessment, they could potentially still have further regression in the future, leading us to underestimate their sensory scores. We note that these individuals had already been clinically diagnosed with RTT, and thus a significant part of their regression likely already had happened by the time of our assessments. The effect on sensory processing, as well as the effects of a regression period that accompanies refractory epilepsy in the MDS group, should be studied in the future. Third, by nature of their diagnosis, all participants in the RTT group were female and almost all participants in the MDS group were male. There is some evidence for sex-related differences in sensorimotor gating (Scaglioni-Solano & Aragón-Vargas, 2015), pain (Ravn et al., 2012), and tactile perception thresholds (Leong et al., 2010). However, a recent meta-analysis found no significant differences in sensory experiences between males and females with autism (Edwards et al., 2023). Finally, most of the participants in the survey were from North America and Europe, which may cause bias in the selection of their choices due to cultural and socioeconomic differences.
In summary, we conducted one of the first studies of sensory experiences in patients with MECP2-related disorders. Data from this study may help clinicians in managing individuals with MDS and RTT. Additionally, our study opens new areas of research into tactile and vestibular differences in individuals with MDS. Understanding sensory symptoms in neurodevelopmental disorders is also important for furthering scientific inquiry into biological mechanisms that may underlie these sensory behaviors. Finally, this study paves the path toward better understanding of sensory phenotypes in MDS versus RTT, adding to the detailed phenotypic characterization. This may be useful in future clinical trials as part of a combined clinical biomarker to prevent over or under treatment.
AUTHOR CONTRIBUTIONS
B.S. was responsible for study design; data acquisition and interpretation; drafting, reviewing, and approving the final version of the manuscript. D.P. was responsible for data acquisition and interpretation; drafting, reviewing, and approving the final version of the manuscript. M.A. was responsible for data acquisition, analysis, and interpretation; reviewing and approving the final version of the manuscript. H.K.H. was responsible for data analysis, and interpretation; reviewing and approving the final version of the manuscript. A.M.L-W. was responsible for study design; data acquisition, analysis, and interpretation; drafting, reviewing, and approving the final version of the manuscript; and agreeing to be accountable for all aspects of the study.
ACKNOWLEDGMENTS
We thank all families for their participation in this research. We thank Mohammed Sameeruddin and Shawntel David for their help with data gathering. We thank Grace Baranek for kindly sharing the Sensory Experience Questionnaire and providing valuable feedback on its use.
FUNDING INFORMATION
D.P. is supported by Rett Syndrome Research Trust (RSRT), International Rett Syndrome Foundation (IRSF grant #3701-1), Doris Duke Charitable Foundation with grant #2023-0235, and NINDS K23 NS125126-01A1. MA receives salary support from MECP2 Duplication Foundation. HKH is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number P50HD103555. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Blue Bird Circle supports research in Rett syndrome. The MECP2 Duplication Foundation funded the establishment and maintenance of the online MDS server.
CONFLICT OF INTEREST STATEMENT
Dr. B.S. received consulting fees for consultancy from Ionis Pharmaceuticals, Neurogene, Acadia, and Taysha; acted as investigator for clinical trials with Neurogene, Acadia, Marinus, and Newron. All remuneration has been paid to Baylor College of Medicine. He has funding from the NIH, IRSF, and IFCR. D.P. is a consultant for Ionis Pharmaceuticals and all remuneration has been paid to Baylor College of Medicine. Other authors declare no conflicts of interest related to this work.
ETHICS STATEMENT
Pilot survey data, including signed consent by caregivers, were collected under Baylor College of Medicine (BCM) Institutional Review Board (IRB) protocol H-35943. For subsequent in-person data, IRB waived the requirement for signed consent to participate in the study (protocol number: H-51705). In-person surveys were completed between December 2021 and April 2023. The online Health Insurance Portability and Accountability Act (HIPAA)-compliant registry portal was developed for MDS individuals to coordinate and implement studies. The BCM IRB approved to create and maintain this registry and to conduct survey studies (protocol number: H-46176).Participants provided a written consent form for the portal registration, participation in survey studies and publication of the results.
Open Research
DATA AVAILABILITY STATEMENT
This study collected survey responses. Individual data points are plotted on graphs. De-identified survey responses for each individual question are available from the authors upon request.




