Increased anticholinergic medication use in middle-aged and older autistic adults and its associations with self-reported memory difficulties and cognitive decline
Abstract
Many commonly used prescription and over-the-counter medicines have potent anticholinergic (AC) effects. Among older adults, AC medications are associated with cognitive impairment and risk for cognitive disorders, including Alzheimer's disease. Collectively, the impact of AC medications is known as anticholinergic cognitive burden (ACB). Because of the high rates of co-occurring medical and psychiatric conditions, autistic adults may have high AC exposure and, thus, may experience elevated ACB. However, no research has characterized AC exposure or examined its associations with cognitive outcomes in autistic adults. Autistic adults (40–83 years) recruited via Simons Powering Autism Research's (SPARK) Research Match service self-reported their medication use (N = 415) and memory complaints (N = 382) at Time (T)1. At T2, 2 years later, a subset of T1 participants (N = 197) self-reported on decline in cognition. Medications were coded using two scales of AC potency. A high proportion (48.2%–62.9%, depending upon the AC potency scale) of autistic adults reported taking at least one medication with AC effects, and 20.5% to 26.5% of autistic adults reported clinically-relevant levels of AC medication (potency ≥3). After controlling for birth-sex, and age, hierarchical linear regression models showed total ACB scores and AC potency values of ≥3 predicted greater memory complaints. Logistic regression models showed that AC medicines at T1 were associated with self-reported cognitive decline at follow-up 2 years later. Understanding AC medications—including potentially earlier AC polypharmacy—and their impacts on cognition (e.g., dementia risk) in autistic adults is warranted.
INTRODUCTION
Acetylcholine plays an important role in cognition, including learning and memory (Huang et al., 2022). Cholinergic neurons, which project widely to the cerebral cortex, arise in the basal forebrain (Mesulam, 2004). The hippocampus, a key medial temporal lobe structure implicated in memory (Dickerson & Eichenbaum, 2010), receives inputs from the basal forebrain cholinergic system (Mesulam, 2004). Cholinergic system dysfunction is implicated in aging-related cognitive decline and disorders like Alzheimer's disease (AD) (Bartus et al., 1982; Hampel et al., 2018). Among the evidence for cholinergic system involvement in AD is atrophy of basal forebrain cholinergic neurons in mild cognitive impairment and AD (Grothe et al., 2012; Teipel et al., 2011).
Based on the cholinergic hypothesis of AD, medications used in the symptomatic management of AD include cholinesterase inhibitors (e.g., donepezil, galantamine, and rivastigmine) (Marucci et al., 2021). These medications target the cholinergic system, preventing the enzymatic breakdown of acetylcholine (Lane et al., 2005). Unlike cholinesterase inhibitors, which allow for the increased availability of acetylcholine, anticholinergic (AC) medications block the action of the neurotransmitter at cholinergic receptors (i.e., muscarinic and nicotinic receptors) present in the central and peripheral nervous systems. AC medications are used to manage or treat an array of medical (e.g., allergies, asthma, epilepsy, and Parkinson's disease) and psychiatric (e.g., anxiety, bipolar disorder, depression, schizophrenia spectrum disorders) conditions. Many commonly used prescription (e.g., antidepressants) and over-the-counter (e.g., antihistamines) medicines have AC effects (Gerretsen & Pollock, 2011).
In aging, AC medication use is associated with decrements in cognitive functioning. For example, greater AC medication use has been associated with cognitive impairment as measured by the Mini Mental State Examination (Sargent et al., 2020) and poorer cognitive functioning as assessed via performance-based measures of memory and executive functioning (Risacher et al., 2016). AC medication use is associated with both acute and chronic cognitive impairment (Boustani et al., 2008; Fox et al., 2011), and greater AC exposure is associated with elevated risk for delirium (Egberts et al., 2021), dementia (Cai et al., 2013; Mur et al., 2022), mild cognitive impairment (Cai et al., 2013), and AD (Gray et al., 2015). Collectively, these associations of AC medications with cognitive problems are referred to as anticholinergic cognitive burden (ACB).
Because of high rates of co-occurring medical (e.g., seizure disorders, gastrointestinal) (Hand et al., 2020; Weir et al., 2021) and psychiatric (e.g., anxiety, depression) (Lai et al., 2019) conditions across the lifespan, autistic individuals are likely to have elevated AC exposure relative to non-autistic individuals, including exposure earlier in the lifespan. Autistic individuals are also likely to have increased AC exposure as they age. A study of Medicare enrollees aged 65 and older found that after adjusting for sex, age, ethno-racial identity, and rurality, relative to non-autistic adults, autistic adults showed significantly greater odds of having metabolic (e.g., diabetes and thyroid disorders), respiratory (including asthma), circulatory (including hypertension and heart disease), neurological (including epilepsy and Parkinson's disease), gastrointestinal (including upper and lower gastrointestinal disorders, gastroenteritis, and constipation), and psychiatric conditions (e.g., schizophrenia and psychotic disorders, mood disorders, and anxiety disorders) (Hand et al., 2020). Importantly, these older age autistic adults also showed a higher prevalence of cognitive disorders, including delirium and dementia, compared to non-autistic adults (Hand et al., 2020). Additionally, converging evidence suggests that middle-aged and older autistic adults are at increased risk of (a) having early onset AD, delirium, and other cognitive disorders (Vivanti et al., 2021) and (b) self-reported experiences of cognitive decline (Klein et al., 2023).
Cross-sectional work using neuropsychological testing to investigate aging-related cognitive trajectories in autistic and non-autistic adults has presented discrepant results. Some show patterns of parallel cognitive aging (Torenvliet et al., 2022) between autistic and non-autistic adults. Other studies have shown accelerated cognitive aging (Geurts & Vissers, 2012), suggesting vulnerabilities to aging-related cognitive decline. Yet others show preserved cognitive aging, suggesting possible resilience to aging impacts in autistic adults relative to non-autistic adults (Lever & Geurts, 2016).
An emerging body of longitudinal work investigating cognitive aging in autistic versus non-autistic adults has also presented differing patterns of results. Some longitudinal work provides evidence for parallel cognitive aging across a range of measures, including verbal and visual recall, working memory, and prospective memory, among autistic and non-autistic adults, suggesting that autistic adults neither show increased nor decreased risk for age-related cognitive decline relative to non-autistic adults (Torenvliet et al., 2023). Other longitudinal studies, however, suggest that a subset of middle-aged and older autistic adults evidence steeper age-related decline (relative to non-autistic adults) in long-term visual memory (Walsh et al., 2022) and short-term verbal memory (Pagni et al., 2022). These differences in findings may be related to differences in sample size or methods across the studies. These differences, however, also point to the possibility that other factors could distinguish a subset of autistic adults at increased risk of experiencing cognitive decline.
While most of the extant work on autistic adult samples has utilized performance-based measures of memory and cognition, another potentially effective approach is self-ratings of memory complaints and self-reported experiences of cognitive decline. In non-autistic samples of older adults, self-reported cognitive decline is correlated with performance-based assessments of cognitive decline (Passler et al., 2021) and is associated with MCI and dementia (Kasai et al., 2021). Furthermore, subjective memory complaints are associated with the development of MCI and dementia (Jessen et al., 2014; Mitchell et al., 2014). Though studies to date have included primarily non-autistic adults, this research suggests that self-report assessments of memory problems and of changes in cognition can be used to screen for and identify individuals who may be at risk for disorders of cognition such as MCI, dementia, and AD.
Taken together, while there is evidence for overall high rates of both general polypharmacy and psychotropic polypharmacy in autistic persons in childhood (Mandell et al., 2008; Ritter et al., 2021), adolescence (Esbensen et al., 2009; Frazier et al., 2011), and adulthood (Esbensen et al., 2009; Jobski et al., 2017; Lake et al., 2012; Vohra et al., 2016), to our knowledge, no research has characterized AC exposure in autistic individuals or examined associations between AC exposure and cognitive outcomes. Thus, the current study aimed to (1) characterize AC medication usage in a sample of middle-aged and older autistic adults (Study 1A); (2) examine associations between this AC medication use and concurrent self-reported memory problems (Study 1B); and (3) examine associations between AC medication use and self-reported cognitive decline at follow-up 2 years later (Study 2). We hypothesized that AC medication use would be: (1) common among autistic middle-aged and older adults (Study 1A); (2) positively correlated with self-reported memory problems (Study 1B); and (3) associated with self-reported cognitive decline at 2-year follow-up (Study 2).
METHODS
Participants
Autistic middle-aged and older adults aged 40+ years were recruited through Simons Powering Autism Research's (SPARK; The SPARK Consortium, 2018) Research Match service as part of a broader study of autistic adult outcomes (Project Number: RM0045Wallace4090).
Participants completed the initial survey battery in December 2019 and January 2020. A subset of these participants was invited to complete a follow-up survey battery 2 years later, in December 2021 and January 2022 (Project Number: RM0135Wallace_Adult). The Data S1 provide further details concerning the Study 1 and Study 2 samples (please see “Study 1 and Study 2: Details concerning participant recruitment and inclusionary/exclusionary study workflow,” Figure S1, Table S1).
Participants were designated by SPARK as “independent” adults, indicating that participants were able to consent for themselves and were thus unlikely to have a co-occurring intellectual disability. Further, as part of the detailed medical history collected in the present study, no participant reported an intellectual disability diagnosis. All participants self-disclosed a professional community-based autism diagnosis. SPARK partners with and recruits from expert autism clinical sites, in part, to increase the likelihood that participants will have a professional autism diagnosis (The SPARK Consortium, 2018). Using electronic medical records, a study of 254 SPARK participants independently confirmed an autism diagnosis in 98.8% of the sample. This study, which included “independent” adults like those in the sample here, confirmed “with high confidence” the validity of reported diagnoses in the SPARK registry (Fombonne et al., 2022). Additionally, for the purposes of sample characterization, autistic traits were queried using the 28-item Autism-Spectrum Quotient (AQ-28; Hoekstra et al., 2011). Consistent with the self-disclosed professional diagnoses reported by all participants in the current study, 97.7%–99.5% of participants who completed the AQ-28 scored at or above the cut-off of >65 (see Table 1).
| Study 1A | Study 1B | Study 2 | |
|---|---|---|---|
| N = 415 | N = 382 | N = 197 | |
| Age, years, T1 | |||
| Mean (SD) | 52.04 (9.1) | 51.9 (9.2) | 52.8 (9.3) |
| Median (range) | 50.08 (40.1–83.3) | 50 (40.1–83.3) | 50.8 (40.1–74.5) |
| Age, years, T2 | |||
| Mean (SD) | – | – | 54.8 (9.3) |
| Median (range) | 52.8 (42.1–76.5) | ||
| Sex assigned at birth, n (%) | |||
| Female | 243 (58.6%) | 222 (58.1%) | 115 (58.4%) |
| Male | 172 (41.4%) | 160 (41.9%) | 82 (41.6%) |
| Gender identity, n (%) | |||
| Gender diverse | 24 (5.8%) | 23 (6.0%) | 10 (5.1%) |
| Cisgender | 389 (93.7%) | 357 (93.5%) | 186 (94.4%) |
| Not reported | 2 (0.5%) | 2 (0.5%) | 1 (0.5%) |
| Age at autism diagnosis, years | |||
| Mean (SD) | 38.7 (15.8) | 38.5 (16.0) | 40.7 (14.7) |
| Median (range) | 41 (1.1–82.7) | 40.9 (1.1–82.7) | 42.8 (1.1–69.0) |
| Ethno-racial identity | |||
| Race | |||
| African American or Black | 11 (2.6%) | 11 (2.9%) | 3 (1.5%) |
| Asian | 5 (1.2%) | 5 (1.3%) | 5 (2.5%) |
| More than one race | 43 (10.4%) | 38 (9.9%) | 16 (8.1%) |
| Native American/Native Alaskan | 5 (1.2%) | 5 (1.3%) | 3 (1.5%) |
| Other | 9 (2.2%) | 9 (2.4%) | 4 (2.1%) |
| White | 340 (81.9%) | 312 (81.7%) | 165 (83.8%) |
| Not reported | 2 (0.5%) | 2 (0.5%) | 1 (0.5%) |
| Ethnicity | |||
| Latinx | 29 (7.0%) | 26 (6.8%) | 7 (3.6%) |
| Not Latinx | 379 (91.3%) | 350 (91.6%) | 188 (95.4%) |
| Unknown | 7 (1.7%) | 6 (1.6%) | 2 (1.0%) |
| Educational attainment, n (%) | |||
| Less than a bachelor's degree | 171 (41.2%) | 154 (40.3%) | 69 (35.0%) |
| Bachelor's degree or higher | 242 (58.3%) | 226 (59.2%) | 127 (64.5%) |
| Missing | 2 (0.5%) | 2 (0.5%) | 1 (0.5%) |
| AQ-28 cut-offa | |||
| Yes | 387 (93.2%) | 373 (97.6%) | 195 (99.0%) |
| No | 9 (2.2%) | 8 (2.1%) | 1 (0.5%) |
| Missing | 19 (4.6%) | 1 (0.3%) | 1 (0.5%) |
| CALS total score | |||
| Mean (SD) | 1.8 (2.2) | 1.8 (2.2) | 1.7 (2.0) |
| Median (range) | 1 (0–11) | 1 (0–11) | 1.0 (0–9) |
| CALS AC medication, dichotomous, n (%) | |||
| Yes | 261 (62.9%) | 246 (64.4%) | 124 (62.9%) |
| No | 154 (37.1%) | 136 (35.6%) | 73 (37.1%) |
| CALS AC ≥3, n (%) | |||
| Yes | 110 (26.5%) | 102 (26.7%) | 52 (26.4%) |
| No | 305 (73.5%) | 280 (73.3%) | 145 (73.6%) |
| ACB Scale total score | |||
| Mean (SD) | 1.2 (1.8) | 1.2 (1.8) | 1.1 (1.6) |
| Median (range) | 0 (0–10) | 0 (0–10) | 0 (0–7) |
| ACB Scale medication, dichotomous, n (%) | |||
| Yes | 200 (48.2%) | 189 (49.5%) | 94 (47.7%) |
| No | 215 (51.8%) | 193 (50.5%) | 103 (52.3%) |
| ACB Scale AC ≥3, n (%) | |||
| Yes | 85 (20.5%) | 80 (20.9%) | 39 (19.8%) |
| No | 330 (79.5%) | 302 (79.1%) | 158 (80.2%) |
| PRMQ total score | |||
| Mean (SD) | – | 45.4 (14.1) | – |
| Median (range) | 46 (16–80) | ||
| AD8 Cutoff (≥1), n (%) | |||
| Yes | – | – | 58 (29.4%) |
| No | 139 (70.6%) | ||
| AD8 Cutoff (≥2), n (%) | |||
| Yes | – | – | 46 (23.4%) |
| No | 151 (76.6%) | ||
- Abbreviations: AC, anticholinergic; ACB Scale, Anticholinergic Cognitive Burden Scale (Boustani et al., 2008; Campbell et al., 2013); AD8, Eight-item Interview to Differentiate Aging and Dementia (Galvin et al., 2005, 2007); CALS, CRIDECO Anticholinergic Load Scale (Ramos et al., 2022); PRMQ, Prospective and Retrospective Memory Questionnaire.
- a AQ-28 N = 396 for Study 1A.
For inclusion in the current study, participants must not only have a self-disclosed community-based professional autism spectrum diagnosis as described above but also have completed relevant measures. The final samples reported here included 415 autistic adults aged 40.1–83.3 years (Study 1A); 382 autistic adults aged 40.1–83.3 years (Study 1B); and 197 autistic adults aged 42.7–77.7 years (Study 2).
Because of high rates of (i) co-occurring medical and psychiatric conditions (Hand et al., 2020; Lai et al., 2019; Weir et al., 2021) and (ii) polypharmacy in autistic individuals (Esbensen et al., 2009; Frazier et al., 2011; Jobski et al., 2017; Lake et al., 2012; Mandell et al., 2008; Ritter et al., 2021; Vohra et al., 2016), we anticipated high rates of AC medication use among autistic adults and that AC medication use would be associated with self-reported cognitive challenges. Importantly, emerging evidence indicates that autistic adults both self-report more experiences of cognitive decline (Klein et al., 2023) and are at elevated risk for early-onset AD, delirium, and other cognitive disorders (Vivanti et al., 2021). Thus, given the hypotheses and their underlying rationale, self-reported neurological and/or neurodegenerative conditions were not exclusionary. However, in order to understand the potential impacts of excluding autistic adults with such conditions from the analyses reported here, the Data S1 present the results of the analyses for Study 1A, Study 1B, and Study 2 after having excluded these autistic adults from the sample.
The studies were approved by The George Washington University Institutional Review Board and followed procedures, including informed consent, in accordance with the Declaration of Helsinki.
MEASURES
Demographics
Socio-demographic information was collected, including age, gender identity, sex assigned at birth, ethno-racial identity, and educational level.
Medications
As part of a detailed health questionnaire collected at Time 1, participants reported on their current medication usage in a free-text field, including prescription (“List any prescription medications you are now taking”) and over-the-counter medications, as well as vitamins and/or supplements (“List any over-the-counter medications, dietary supplements, or vitamins you currently take regularly”).
Only participants with complete and unambiguous medication information available that allowed for coding were included in analyses reported on here (see Figure S1). Participants who indicated that they preferred not to answer or who indicated that they were taking medications but did not specify these medications by either the generic or brand name were not included in analyses. Similarly, participants who reported a type of medication use but did not specify the generic/brand name of the medication (e.g., if a participant reported “allergy medicine,” “antidepressant,” or “anxiety medicine” without further information) were not included in analyses.
Data were cleaned to eliminate duplicates (e.g., listing of both the generic and brand name of a medication; listing a medication more than once to indicate its dosage in the morning and evening; listing the same medication in both the field querying prescription medications and the field querying over-the-counter medications).
Coding of medications
After excluding participants with incomplete or ambiguous medication information (n = 24) and after removing duplicates for participants with complete and unambiguous medication information, medications were coded based on two scales of ACB: the CRIDECO Anticholinergic Load Scale (CALS; Ramos et al., 2022) and the Anticholinergic Cognitive Burden/ACB Scale (Boustani et al., 2008; Campbell et al., 2013). We used two scales to allow for a more rigorous and robust characterization of AC medications in the sample and to evaluate commonalities in findings across coding schemes. The ACB Scale has been widely used in studies of aging (see, inter alia, Fox et al., 2011; Pfistermeister et al., 2017; Ramos et al., 2022; Richardson et al., 2018), thereby providing for comparisons with characterizations of ACB in general aging samples, while the CALS offers more coverage of medications developed and in use since the publication of the ACB Scale in 2012.
The CALS characterizes AC potency on a scale from 1 to 3, where 1 corresponds to “low potency” and 3 to “high potency.” In developing the CALS, Ramos et al. (2022) sought to provide an up-to-date, comprehensive scale of ACB. Using a systematic literature review following the PRISMA guidelines, the authors identified seven AC scales used in Ecuador, Germany, Korea, New Zealand, and the United States of America. These seven scales were consulted in the creation of the CALS. In instances in which the seven scales agreed on the coding (e.g., 1, 2, 3) of a medication, the CALS adopted that coding. Where there were coding discrepancies between or among scales, the authors rounded up from the mean coding. Finally, a database of medications commercially available in Spain (the country in which the CALS was developed) was reviewed by a pharmacologist, a clinical pharmacist, and a community pharmacist to determine if any medications not included in the seven scales should be included in the CALS.
Among the seven scales consulted in the development of the CALS was the ACB Scale, formulated by Boustani et al. (2008) and updated in 2012 (Campbell et al., 2013). The ACB Scale ranks medications according to the nature of available evidence for their potential ACB, where higher scores are indicative of greater evidence for a medicine's ACB. Scores of 1 are assigned to medications for which there is in vitro evidence of cholinergic receptor activity in the peripheral nervous system (i.e., muscarinic receptors). Scores of 2 and 3 are assigned when there is evidence from (i) the literature, (ii) prescribers, or (iii) expert clinician determinations that the medication either demonstrates anticholinergic activity (Score of 2) or that the medication may result in delirium (Score of 3).
Based on the CALS and the ACB Scale, ACB was characterized in the current study's sample in three ways for each of the two scales: (i) dichotomous (yes/no) coding according to whether the participant reported any current AC medication; (ii) total ACB score for each participant (i.e., the sum of AC scores for all medications reported); and (iii) a dichotomous coding (yes/no) according to whether the participant's ACB score was ≥3, a cut-off identified as clinically important in the literature (Carnahan et al., 2006; Rudolph et al., 2008). Specifically, while AC medication exposure is linked to adverse effects, persons with ACB scores ≥3 appear to be at proportionately greater risk of experiencing these adverse AC effects, including cognitive impairments, relative to those with ACB <3 (Rudolph et al., 2008).
For participants in the current sample, each AC prescription and/or over-the-counter medication reported was coded according to the three-point scales of the CALS and ACB Scale, respectively, as follows: 1 = Low AC potency/ACB; 2 = Medium AC potency/ACB; 3 = High AC potency/ACB. Medications not classified as AC were coded as 0 = No AC potency/ACB; participants who reported that they were not taking any medication were also coded as 0 = No AC potency/ACB.
For Study 1A, all three characterizations (i.e., dichotomous coding for AC medication use; total ACB score; and dichotomous coding for clinically meaningful ACB score ≥3) were used to understand AC medication use and ACB in the sample. For Study 1B and Study 2, the total ACB score and the ACB cut-off score of ≥3 were independent variables of interest in the analyses.
In examining both a continuous total ACB and the dichotomous ACB ≥3, we follow existing work that quantifies AC medication use and characterizes its associations with cognitive impairments and aging-related disorders of cognition in terms of both ACB total score and ACB ≥3 (Egberts et al., 2021; Ramos et al., 2022; Zheng et al., 2021).
Self-reported memory complaints
At Time 1, participants completed the Prospective and Retrospective Memory Questionnaire (PRMQ; Smith et al., 2000). The PRMQ is a 16-item self-report measure of memory “failures.” Eight items probe retrospective memory failures, or memory failures related to previous events/occurrences (e.g., Do you forget something that you were told a few minutes before?; Do you repeat the same story to the same person on different occasions?” An additional eight items probe prospective memory failures, or failures in remembering to carry out a future action (e.g., Do you intend to take something with you, before leaving a room or going out, but minutes later leave it behind, even though it's there in front of you?; If you tried to contact a friend or relative who was out, would you forget to try again later?).
Due to a data collection oversight, in the current study, participants responded according to the following 6-point Likert scale: 1 = “Very Rarely,” 2 = “Rarely,” 3 = “Occasionally,” 4 = “Somewhat Often,” 5 = “Often,” and 6 = “Very Often.” The original PRMQ has a 5-point scale with slightly different anchors: 1 = “Never,” 2 = “Rarely,” 3 = “Sometimes,” 4 = “Quite Often,” and 5 = “Very Often”. Thus, participant responses in the current study were recoded to a 5-point Likert scale as follows: both 4 (“Somewhat Often”) and 5 (“Often”) were recoded as “4,” and 6 (“Very Often”) was recoded as “5”.
While, to our knowledge, the PRMQ has not been specifically validated for use with autistic adults, it has been used in previous studies with autistic adults (Cherkaoui & Gilbert, 2017; Landsiedel & Williams, 2020; Williams et al., 2014). In general population studies, the PRMQ total score has demonstrated acceptable internal consistency (Cronbach's α = 0.89) (Crawford et al., 2003) and acceptable construct validity (r = 0.75) (Rönnlund et al., 2008).
In the current study, of those participants with medication data (N = 415), 382 also had complete PRMQ data and were included in the analyses reported here. Internal consistency reliability for the PRMQ total score in this sample of middle-age and older autistic adults was good (Cronbach's α = 0.93). PRMQ mean item scores were used as the dependent variable in analyses for Study 1B.
Self-reported decline in cognitive abilities
At Time 2, a subset of the Time 1 sample completed the self-report version of the Eight-item Interview to Differentiate Aging and Dementia (AD8; Galvin et al., 2005, 2007). When administered as a self-report instrument, participants are asked to rate changes over the last several years in thinking and memory described in each of the items “without attributing causality” to the change. Participants respond by selecting one of the following three options: “YES, a change,” “NO, no change,” or “N/A, don't know.” A total score is generated by summing the number of items for which the participant responded, “YES, a change.”
The self-report AD8 has shown fair discriminant validity (area under the receiver operator curve = 0.78) (Galvin et al., 2007). In a national population-based study in the United States, the self-report AD8 demonstrated fair internal consistency reliability (Cronbach's α = 0.78), and both incident cognitive impairment and cognitive decline (changes over time in word list learning) were associated with a self-reported AD8 positive screen (Passler et al., 2021). Participants with complete medication information from Time 1 and complete AD8 data at Time 2 were included in the analyses reported here (N = 197). Internal consistency reliability for the AD8 total score in this sample of middleage and older autistic adults was good (Cronbach's α = 0.84).
From the total score, two dichotomous scores were generated and evaluated: a “traditional” cut-off score of 1, which is recommended for use of the AD8 as a self-report measure (Galvin et al., 2007), and a “conservative” cut-off score of 2, used in the informant report version of the AD8 (Galvin et al., 2005). Specifically, for self-report use of the AD8, Galvin et al. (2007) identified 1 (the “traditional” cut-off) as the cut-off with the best combination of sensitivity (80%) and specificity (59%). The use of 2 (the “conservative” cut-off) as a cut-off for the self-report AD8 was found to increase specificity (73%), while decreasing sensitivity (62%). The choice between 1 or 2 as a cut-point is then a question of psychometric prioritization, that is, whether specificity or sensitivity is prioritized. Previous studies using the self-report AD8, for example, have used the “conservative” cut-off of 2 in order to privilege specificity over sensitivity (Passler et al., 2021). Other studies using the self-report AD8, however, have included the “traditional” cut-point of 1 as well as the “conservative” cut-point of 2 (Chin et al., 2013).
To our knowledge, outside of our own prior study with a sample substantially overlapping with the sample reported here (Klein et al., 2023), the AD8 has not previously been used with autistic adult samples. Given this and given that there is no rationale in the current study for privileging sensitivity over specificity or vice versa, we elected to separately examine both cut-off scores as dependent variables in analyses. This analytic choice allows for a more comprehensive characterization of potential associations between self-reported cognitive decline and AC medication use.
Statistical analysis
Statistical analyses were conducted in R (v.4.2.0).
Study 1A: Characterization of AC medication use and ACB
Study 1A sought to characterize AC medication use and ACB in this sample of middle-aged and older autistic adults. This characterization took three forms: (i) percentage of participants reporting any AC medication usage (i.e., usage of at least one AC medication on the CALS or ACB scale); (ii) mean total ACB score (i.e., the sum of AC medications according to the CALS or ACB scale); and (iii) percentage of participants with total ACB score ≥3 on the CALS or ACB Scale.
Study 1B: Associations of ACB with self-reported memory “failures” at T1
Study 1B aimed to examine associations of ACB with self-reported memory “failures.” Hierarchical linear regression models examined associations of ACB with PRMQ total score, with sex assigned at birth and age at T1 entered in the first step and ACB total score or ACB ≥3 on the CALS or ACB Scale entered as the variable of primary interest in the second step.
Study 2: Associations of ACB at T1 with self-reported cognitive decline at T2
Study 2 aimed to examine associations between ACB at Time 1 and self-reported cognitive decline at follow-up 2 years later. Four logistic linear regression models for the CALS and ACB Scale separately examined associations of ACB with self-reported cognitive decline. For all models, age at T2 and sex assigned at birth were entered in the first step. Two logistic regression models were run using the AD8 cut-off of ≥1 and separately ≥2 as dependent variables, where participants who scored below the relevant cut-offs were coded as “0” and persons who were above were coded as “1.” In the second step, either the ACB total score or the ACB ≥3 score was entered as the primary variable of interest.
RESULTS
Study 1A results: Characterization of AC medication use and ACB
Table 1 presents the characteristics of Study 1A, Study 1B, and Study 2 participants. Study 1A revealed a high prevalence of AC medication use in autistic adults, with 62.9% reporting at least one AC medication according to the CALS and 48.2% according to the ACB Scale. The total range of ACB for participants was 0 to 11 with a mean of 1.8 (SD = 2.1) on the CALS and 0 to 10 with a mean of 1.2 (SD = 1.8) on the ACB Scale. The proportion of autistic adults reporting ACB ≥3 was 26.5% and 20.5% on the CALS and ACB Scale, respectively.
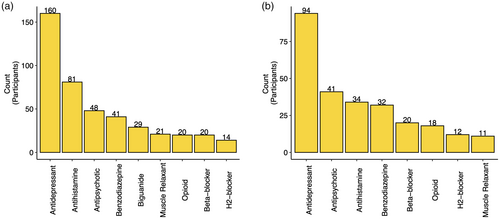
For the purposes of better understanding the types of AC medications reported by autistic adults in the Study 1A sample, Figure 1 presents the most common types of AC medications reported as indicated via the CALS (Figure 1a) and ACB Scale (Figure 1b). Psychotropic medications (particularly antidepressants, antipsychotics, and benzodiazepines) and antihistamines were the most commonly reported AC medication classes in the sample. The antidepressant medications reported included selective serotonin reuptake inhibitors (SSRIs; e.g., paroxetine), tricyclic antidepressants (TCAs; e.g., nortriptyline), and serotonin noradrenaline reuptake inhibitors (SNRIs; e.g., venlafaxine), as well as the serotonin modulator trazodone. Included among the antipsychotic medications reported were atypical (e.g., aripiprazole) as well as conventional (e.g., thorazine) antipsychotics. Reported antihistamines included medications such as diphenhydramine, hydroxyzine, and loratadine, and reported benzodiazepines included clonazepam, diazepam, and lorazepam. Also commonly reported were biguanides (e.g., metformin; note that biguanides are included only in the CALS and not the ACB Scale), beta-blockers (e.g., atenolol, metoprolol), opioids (e.g., morphine, oxycodone), H2-blockers (e.g., ranitidine, cimetidine), and skeletal muscle relaxants (e.g., baclofen, cyclobenzaprine). A list of AC medications reported in the sample can be found in the Data S1 (see Table S2).
Study 1B results: Associations of ACB with self-reported memory “failures” at T1
CALS results
Hierarchical regression modeling with ACB characterized using the CALS revealed that after accounting for the significant effects of age and sex assigned at birth, both total ACB (β = 0.12, t = 2.46, Δp = 0.01, ΔR2 = 0.02) and ACB ≥3 (β = 0.16, t = 3.17, Δp = 0.0016, ΔR2 = 0.02) predicted greater concurrent self-reported memory failures (Table 2).
| Study 1B | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PRMQ total score | PRMQ total score | ||||||||||
| B | SE | 95% CI | 𝛽 | t | B | SE | 95% CI | 𝛽 | t | ||
| Step 1: | R2 = 0.057, F(2,379) = 12.57, p = 0.000005 | Step 1: | R2 = 0.057, F(2,379) = 12.57, p = 0.000005 | ||||||||
| Sex at birth | 0.31 | 0.09 | [0.14, 0.49] | 0.36 | 3.51 | Sex at birth | 0.31 | 0.09 | [0.14, 0.49] | 0.36 | 3.51 |
| T1 age | −0.02 | 0.005 | [−0.03, −0.005] | −0.15 | −3.07 | T1 age | −0.02 | 0.005 | [−0.03, −0.005] | −0.15 | −3.07 |
| Step 2: | ΔR2 = 0.02, ΔF(3378) = 6.03, Δp = 0.01 | Step 2: | ΔR2 = 0.02, ΔF(3378) = 10.07, Δp = 0.0016 | ||||||||
| ACB total score | 0.05 | 0.02 | [0.010, 0.090] | 0.12 | 2.46 | ACB ≥3 | 0.31 | 0.099 | [0.119, 0.505] | 0.16 | 3.17 |
| Study 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AD8 traditional cut-off | AD8 traditional cut-off | ||||||||||
| B | SE | 95% CI for odds ratio | Odds ratio | z | B | SE | 95% CI for odds ratio | Odds ratio | z | ||
| Step 1: | Nagelkerke R2 = 0.02, AIC = 241.83 | Step 1: | Nagelkerke R2 = 0.02, AIC = 241.83 | ||||||||
| Sex at birth | 0.55 | 0.33 | [0.91, 3.34] | 1.73 | 1.66 | Sex at birth | 0.55 | 0.33 | [0.91, 3.34] | 1.73 | 1.66 |
| T2 age | 0.009 | 0.02 | [0.98, 1.04] | 1.01 | 0.51 | T2 age | 0.009 | 0.02 | [0.98, 1.04] | 1.01 | 0.51 |
| Step 2: | Nagelkerke R2 = 0.06, AIC = 238.89 Model comparison: χ2(1) = 4.94, p = 0.026 |
Step 2: | Nagelkerke R2 = 0.04, AIC = 241.54 Model comparison: χ2(1) = 2.29, p = 0.13 |
||||||||
| ACB total score | 0.17 | 0.08 | [1.02, 1.39] | 1.19 | 2.22 | ACB ≥3 | 0.53 | 0.35 | [0.85, 3.37] | 1.70 | 1.53 |
| AD8 conservative cut-off | AD8 conservative cut-off | ||||||||||
| B | SE | 95% CI for odds ratio | Odds ratio | z | B | SE | 95% CI for odds ratio | Odds ratio | z | ||
| Step 1: | Nagelkerke R2 = 0.02, AIC = 217.05 | Step 1: | Nagelkerke R2 = 0.02, AIC = 217.06 | ||||||||
| Sex at birth | 0.53 | 0.36 | [0.86, 3.50] | 1.70 | 1.49 | Sex at birth | 0.53 | 0.36 | [0.86, 3.50] | 1.70 | 1.49 |
| T2 age | 0.02 | 0.02 | [0.98, 1.06] | 1.02 | 1.02 | T2 age | 0.02 | 0.02 | [0.98, 1.06] | 1.02 | 1.02 |
| Step 2: | Nagelkerke R2 = 0.05, AIC = 215.96 Model comparison: χ2(1) = 3.09, p = 0.079 |
Step 2: | Nagelkerke R2 = 0.04, AIC = 217.06 Model comparison: χ2(1) = 1.99, p = 0.16 |
||||||||
| ACB total score | 0.14 | 0.081 | [0.98, 1.35] | 1.15 | 1.78 | ACB ≥3 | 0.53 | 0.37 | [0.81, 3.51] | 1.70 | 1.43 |
- Note: Sex assigned at birth was coded as follows: 0 = assigned male sex at birth, 1 = assigned female sex at birth female. ACB ≥3 was coded as follows: 0 = No (i.e., ACB <3), 1 = Yes (i.e., ACB ≥3). Bolded values are significant at p < 0.05.
- Abbreviations: AD8 = Eight-item Interview to Differentiate Aging and Dementia; PRMQ = Prospective and Retrospective Memory Questionnaire; T1, Time 1; T2, Time 2.
ACB Scale results
Hierarchical regression modeling with ACB characterized using the ACB Scale similarly indicated that after accounting for the significant effects of age and sex assigned at birth, total ACB (β = 0.15, t = 3.03, Δp = 0.003, ΔR2 = 0.02) as well as ACB ≥3 (β = 0.16, t = 3.39, Δp = 0.0008, ΔR2 = 0.03) predicted greater concurrent self-reported memory complaints (Table 3).
| Study 1B | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PRMQ total score | PRMQ total score | ||||||||||
| B | SE | 95% CI | 𝛽 | t | B | SE | 95% CI | 𝛽 | t | ||
| Step 1: | R2 = 0.057, F(2,379) = 12.57, p = 0.000005 | Step 1: | R2 = 0.057, F(2,379) = 12.57, p = 0.000005 | ||||||||
| Sex at birth | 0.31 | 0.09 | [0.14, 0.49] | 0.36 | 3.51 | Sex at birth | 0.31 | 0.09 | [0.14, 0.49] | 0.36 | 3.51 |
| T1 age | −0.02 | 0.005 | [−0.03, −0.005] | −0.15 | −3.07 | T1 age | −0.02 | 0.005 | [−0.03, −0.005] | −0.15 | −3.07 |
| Step 2: | ΔR2 = 0.02, ΔF(3378) = 9.15, Δp = 0.003 | Step 2: | ΔR2 = 0.03, ΔF(3378) = 11.49, Δp = 0.0008 | ||||||||
| ACB total score | 0.07 | 0.02 | [0.03, 0.12] | 0.15 | 3.03 | ACB 2012 ≥3 | 0.35 | 0.12 | [0.15, 0.57] | 0.16 | 3.39 |
| Study 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AD8 traditional cut-off | AD8 traditional cut-off | ||||||||||
| B | SE | 95% CI for odds ratio | Odds ratio | z | B | SE | 95% CI for odds ratio | Odds ratio | z | ||
| Step 1: | Nagelkerke R2 = 0.02, AIC = 241.83 | Step 1: | Nagelkerke R2 = 0.02, AIC = 241.83 | ||||||||
| Sex at birth | 0.55 | 0.33 | [0.91, 3.34] | 1.73 | 1.66 | Sex at birth | 0.55 | 0.33 | [0.91, 3.34] | 1.73 | 1.66 |
| T2 age | 0.009 | 0.02 | [0.98, 1.04] | 1.01 | 0.51 | T2 age | 0.009 | 0.02 | [0.98, 1.04] | 1.01 | 0.51 |
| Step 2: | Nagelkerke R2 = 0.05, AIC = 240.12 Model comparison: χ2(1) = 3.70, p = 0.054 |
Step 2: | Nagelkerke R2 = 0.03, AIC = 243.03 Model comparison: χ2(1) = 0.79, p = 0.37 |
||||||||
| ACB total score | 0.19 | 0.10 | [1.00, 1.46] | 1.21 | 1.93 | ACB ≥3 | 0.34 | 0.38 | [0.66, 2.95] | 1.41 | 0.90 |
| AD8 conservative cut-off | AD8 conservative cut-off | ||||||||||
| B | SE | 95% CI for odds ratio | Odds ratio | z | B | SE | 95% CI for odds ratio | Odds ratio | z | ||
| Step 1: | Nagelkerke R2 = 0.02, AIC = 217.05 | Step 1: | Nagelkerke R2 = 0.02, AIC = 217.05 | ||||||||
| Sex at birth | 0.53 | 0.36 | [0.86, 3.50] | 1.70 | 1.49 | Sex at birth | 0.53 | 0.36 | [0.86, 3.50] | 1.70 | 1.49 |
| T2 age | 0.02 | 0.02 | [0.98, 1.06] | 1.02 | 1.02 | T2 age | 0.02 | 0.02 | [0.98, 1.06] | 1.02 | 1.02 |
| Step 2: | Nagelkerke R2 = 0.03, AIC = 217.98 Model comparison: χ2(1) = 1.07, p = 0.30 |
Step 2: | Nagelkerke R2 = 0.02, AIC = 218.96 Model comparison: χ2(1) = 0.96, p = 0.76 |
||||||||
| ACB total score | 0.11 | 0.10 | [0.91, 1.36] | 1.11 | 1.05 | ACB ≥3 | 0.13 | 0.42 | [0.49, 2.51] | 1.14 | 0.31 |
- Note: Sex assigned at birth was coded as follows: 0 = assigned male sex at birth, 1 = assigned female sex at birth female. ACB ≥3 was coded as follows: 0 = No (i.e., ACB <3), 1 = Yes (i.e., ACB ≥3). Bolded values are significant at p < 0.05.
- Abbreviations: AD8 = Eight-item Interview to Differentiate Aging and Dementia; PRMQ, Prospective and Retrospective Memory Questionnaire; T1, Time 1; T2, Time 2.
Study 2 results: Associations of ACB at T1 with self-reported cognitive decline at T2
CALS results
After controlling for potential effects of age at T2 and sex assigned at birth, a logistic regression model with ACB total score showed that ACB predicted the AD8 ≥1 cut-off at T2 (Odds ratio = 1.19, 95% CI = 1.02, 1.39, z = 2.22, Nagelkerke R2 = 0.06, p < 0.05; Table 2). No other logistic regression model was significant: after controlling for potential effects of age at T1 and sex assigned at birth, ACB total score did not significantly predict AD8 ≥2 cut-off at T2 nor did ACB ≥3 significantly predict either AD8 ≥1 or AD8 ≥2 cut-off at T2.
ACB Scale results
After controlling for potential effects of age at T2 and sex assigned at birth, neither the logistic regression model with the ACB total score nor the ACB ≥3 significantly predicted the AD8 ≥1 cut-off at T2 (Table 3). However, the model with ACB total score approached significance (Odds ratio = 1.21, 95% CI = 1.00, 1.46, z = 1.93, Nagelkerke R2 = 0.05, p = 0.054), χ2(1) = 3.70, p = 0.054). Neither ACB total score nor ACB ≥3 significantly predicted AD8 ≥2 at T2.
DISCUSSION
That autistic children and adolescents grow up to be adults is a trivial statement, yet to date, research on autism has predominantly examined child and adolescent samples, while studies of adulthood, particularly middle and older adulthood, are limited. Thus, as a field, we know little about aging and cognition in autistic adults (Mason et al., 2022). To help address this critical knowledge gap, the current study examined one factor—AC medication exposure—that may particularly impact autistic adults, their cognition, and their aging-related cognitive trajectories.
This is the first study to characterize AC medication use and examine associations between these medications and self-reported cognitive complaints in autistic adults. We hypothesized that AC medication use would be common among middle-aged and older autistic adults. This hypothesis was confirmed: based on the ACB Scale and CALS, 48.2% and 62.9% of autistic adults in the current study, respectively, were taking at least one AC medication. Further, based on the ACB Scale and the CALS, 20.5%–26.5%, respectively, of autistic adults reported clinically meaningful levels of AC medication use (ACB score ≥3).
In the broader aging literature, the prevalence of AC medication use varies, depending on the scale(s) used to quantify AC potency, the country in which participants reside, and specific characteristics of the samples investigated (e.g., whether the study is of community-dwelling older adults, hospitalized older adults, or older adults residing in a memory care facility; or whether participants do or do not have a diagnosis of an aging-related cognitive disorder such as dementia). Additionally, differences in rates of AC medication use across studies are likely attributable, in part, to the time period during which data were collected, as AC medication use among older adults has increased over the past several decades (Grossi et al., 2020).
Estimates in a 2009 study, using an earlier version of the ACB Scale used in the current study (Boustani et al., 2008), found that ~20%–50% of U.S. adults >65 years old took at least one AC medication (Campbell et al., 2009). A UK population-based study of older adults aged ≥64 years indicated that 49.6% of the sample between 1990 and 1993 and 64.3% of the sample between 2008 and 2011 were taking at least one medication with anticholinergic effects (Grossi et al., 2020). Ramos et al. (2022) characterized AC medication use in a sample of 512 Spanish adults (aged 50–96 years) with subjective memory complaints, who were grouped according to those with and without cognitive decline, as determined by cognitive testing. ACB was characterized using the CALS and an earlier version of the ACB Scale (Boustani et al., 2008) than that used in the present study. The mean total ACB score based on the CALS was 2.1 (SD = 1.9) in the group with cognitive decline, and 1.6 (SD = 1.7) in the group without cognitive decline, and ACB ≥3 was found in 37.20% of the cognitive decline group and in 24.71% of the group without cognitive decline. For comparison, in the current study, the mean total ACB score based on the CALS was 1.8 (SD = 2.2), and ACB ≥3 was found in 26.5% of autistic adults. Importantly, the participants in the current study were younger on average (M = 52.0, SD = 9.1) than those in either the group with (M = 74.7, SD = 7.9) or the group without cognitive decline (M = 68.1, SD = 9.1) investigated by Ramos and colleagues.
In addition to finding that AC medication use is prevalent among autistic adults, we further found that higher levels of AC medication use were associated with concurrent self-reported prospective and retrospective memory problems. Based on the CALS, which provides a more updated, comprehensive list of AC medications relative to the ACB Scale, AC medication use was also associated with self-reported cognitive decline at 2-year follow-up.
Subjective memory impairment has been suggested to be the earliest clinical manifestation of dementia (Jessen et al., 2006), and among adults aged ≥65 years, subjective memory complaints predict future dementia at 4-year follow-up (Waldorff et al., 2012). Self-reported cognitive decline is correlated with performance-based metrics of cognitive decline (i.e., declines in word learning) (Passler et al., 2021) and is associated with disorders of cognition, including MCI and dementia (Kasai et al., 2021). Thus, future studies should examine whether autistic adults with subjective memory complaints and/or self-reported cognitive decline are at elevated risk for the development of disorders of cognition, including MCI, dementia, and AD, and whether such a potential elevated risk is associated with AC medication use.
AC medication use has been associated with subjective cognitive decline among community-dwelling adults (aged 47–91 years; M = 65.2 (8.6) at risk for AD (based on APOE ε4 carrier status and/or family history of dementia) (Margolis et al., 2021). Among adults ≥65 years with subjective cognitive decline, high ACB (≥3) is associated with a more than four-fold increased risk for dementia development (Naharci et al., 2017). Thus, the results reported here indicate that studies of aging and cognition in autism should take into consideration the potential impacts of AC medication use on self-report or performance-based measures of cognition.
There was a single model that was significant for Study 2: when characterizing ACB based on the CALS, the total ACB score was associated with the self-reported measure of cognitive decline (AD8) cut-off of ≥1 after controlling for age and sex assigned at birth. In contrast, for Study 1B, all models reached statistical significance. The sample of Study 2 (N = 197) is smaller relative to the sample of Study 1B (N = 382). Thus, Study 2 may have been underpowered to detect effects. No a priori power analysis was computed, and we do not report a post hoc power analysis given known problems with post-hoc power calculations (Althouse, 2021; Goodman & Berlin, 1994; Hoenig & Heisey, 2001). Thus, the examination of AC medication and associations with retrospective self-reported cognitive decline in a larger sample of autistic adults is an important direction for future research.
Importantly, extant studies on AC medication use and its associations with cognitive functioning in non-autistic individuals typically characterize samples older than the sample of autistic adults reported here. The mean age of autistic adults in the current study was 52.0 years (SD = 9.1 years); in contrast, most research has characterized AC medication use and its cognitive effects in samples of participants aged ≥65 years. In comparing participants in the current Study 1A sample aged <65 (n = 368) with those ≥65 years (n = 47), there were no significant differences in the two age groups for AC medication prevalence on any of the metrics used to characterize the full Study 1A sample (N = 415) (Table S6). Thus, the substantial levels of AC medication use and its associations with subjective cognitive complaints in this comparatively younger sample indicate that consideration of AC medication use—including consideration of use at younger ages than typically examined—may be important in this population, which may be at elevated risk for AC medication exposure (e.g., antipsychotic medication exposure (Mandell et al., 2008)) earlier in the lifespan.
The strengths of the current study include the characterization of AC medication use in a large sample of middle-aged and older autistic adults. AC medication characterization was robust, using two scales. One of the two scales was also relatively more conservative: the ACB Scale, developed in 2012, does not include many AC medications reported in the sample given that these medications were not yet developed or in use at the time of the scale's publication.
The results reported here must be viewed in light of the limitations of the current study. The study's sample does not include autistic persons with co-occurring intellectual disabilities (ID). Autistic individuals with co-occurring ID have high rates of polypharmacy, including psychotropic polypharmacy rates (Esbensen et al., 2009; Tsakanikos et al., 2007; Vohra et al., 2016), which suggests that exposure to AC medications among these autistic persons may be even higher than rates reported here. Understanding the prevalence of AC medication and its potential impacts on cognition among these individuals is a needed area of research.
Another limitation of the current study is that the data reported were self-report, including self-reported cognitive decline and memory problems. Future studies should conduct medical record reviews and consider examining AC/ACB effects on performance-based measures of cognition (e.g., working memory, attention, verbal fluency) in addition to subjective cognitive complaints. Given that current medication use was captured via self-report, it is possible that participants did not list all of the medications they were taking. Moderating this concern, however, was our use of conservative inclusionary/exclusionary criteria, including only those participants whose reported medications were complete and unambiguous, while excluding those individuals for whom medication use was unclear, either because they indicated they were taking too many medications to list or because the medications listed were not named (e.g., participants indicated they were taking antihistamines or antidepressants but did not provide either the generic or brand name of the medication). Thus, it is likely that, if anything, the prevalence of AC medication use is underestimated in the current sample, indicating that the risk could be even greater. Also moderating concerns regarding the use of self-report medication data, the high rates of psychotropic medications in the current study are consistent with those in other studies of autistic adults (Lake et al., 2012; Vohra et al., 2016), including studies using large databases (Houghton et al., 2017; Vohra et al., 2016).
In the current study, cognitive decline was operationalized via a self-report retrospective measure collected at a single timepoint. Longitudinal studies that examine change over time (i.e., change across two or more time points) in self-reported memory and other cognitive challenges are needed. The present study did not specifically query additional details of participants' current medication use, such as the dosage and frequency of each medication, adherence to each medication, or the length of time participants had been using each medication. Future studies examining frequency, dosage, adherence, and medication history would allow for analyses that also examine possible dose-dependent effects and/or cumulative AC medication exposure as these relate to cognitive outcomes.
AC medications may impact the transition from normative trajectories of aging to mild cognitive impairment (MCI) (Campbell et al., 2018), and among older persons (>70 years) with normal cognitive functioning, those taking more potent anticholinergic medications (medications with an ACB score ≥2) are at increased risk for the development of various markers of cognitive impairment at follow-up 6 years later (Campbell et al., 2010). Promisingly, however, deprescribing AC medications may reduce adverse outcomes associated with AC medication use in older adults (Carrière et al., 2009; Lupu et al., 2021), although more research is needed concerning how deprescribing impacts cognitive functioning and how best to implement deprescribing (Soiza et al., 2021).
Given the results reported here, alongside emerging evidence that middle-aged and older autistic adults demonstrate elevated rates of self-reported cognitive decline (Klein et al., 2023) and delirium or dementia (Hand et al., 2020), including early-onset dementia and other cognitive disorders (Vivanti et al., 2021), AC medication use may be an important modifiable risk factor in aging-related outcomes among autistic adults. To promote healthy aging, autistic adults may benefit from prescribers' careful attention to and consideration of recommendations concerning prescribing practices as these relate to AC medications (Institute of Medicine, 2015). These practices include minimizing the use of certain AC medications in older persons, where possible, in order to reduce the risks conferred by these medications (2019 American Geriatrics Society Beers Criteria® Update Expert Panel, 2019).
Although we did not ask for additional details concerning participants' current medication use, some participants provided this information unprompted, including the reason they were taking each medicine they reported. Many reported taking some of the most commonly found AC medications in the sample (i.e., antihistamines, benzodiazepines, and antipsychotic medications) for insomnia. Given that autistic adults experience greater sleep-related problems compared to non-autistic adults (Morgan et al., 2020), and among middle-aged and older autistic adults, sleep difficulties are common and associated with increased anxious symptomatology (Charlton et al., 2023), it may be particularly important to understand why autistic adults are prescribed or are taking AC medications in order to identify alternative interventions where possible. Additionally, many of the high-potency AC medications frequently reported in the sample are over-the-counter medications (e.g., diphenhydramine and doxylamine succinate). Thus, providers may wish to provide guidance concerning the potential harms of common over-the-counter medications so that autistic adults are able to make informed decisions about their use of these medicines (Institute of Medicine, 2015).
Cognitive effects represent one type of adverse effect associated with AC medication exposure. There are other adverse effects of AC medications in aging persons, however, including elevated risk for falls (Stewart et al., 2021; Wong et al., 2023) and mortality (Ali et al., 2020; Graves-Morris et al., 2020; Ruxton et al., 2015). Thus, in addition to probing the cognitive-related effects of AC medication use, future work should also investigate other health-related adverse effects associated with AC medications in autistic persons. The identification of such effects and ways to minimize them may hold promise for improving aging-related quality of life across cognitive and physical health domains in autistic adults.
CONCLUSIONS
The current study revealed that a large proportion of middle-age and older autistic adults take at least one AC medication, and a substantial minority take clinically-relevant levels of AC medication. Use of these medications was in turn associated with both greater contemporaneous subjective memory complaints and self-reported cognitive decline based on retrospective reporting. This study provides preliminary evidence for an association between relatively high AC medication usage and cognitive challenges in autistic adults.
ACKNOWLEDGMENTS
We wish to express our gratitude to the autistic adults in SPARK, as well as the SPARK clinical sites, and SPARK staff. We appreciate obtaining access to recruit participants through SPARK Research Match on SFARI Base.
FUNDING INFORMATION
This research was supported by start-up funds from the George Washington University to G.L.W. Support for writing this manuscript was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (under grant R21HD106164) to G.L.W. Support for writing this manuscript was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (under grants R21HD100997; R21HD106164) to N.R.L. Support for writing this manuscript was provided by the National Institute of Mental Health (under grant K01MH129622) to G.A.M.
ETHICS STATEMENT
The authors assert that all procedures contributing to this work adhere to the ethical standards of the relevant national and institutional committees and are in accordance with the Declaration of Helsinki.
Open Research
DATA AVAILABILITY STATEMENT
Approved researchers can apply for access to the de-identified phenotypic SPARK dataset at https://base.sfari.org.




