Association of maternal autoimmune disease and early childhood infections with offspring autism spectrum disorder: A population-based cohort study
Funding information: Financial Markets Foundation for Children; National Health and Medical Research Council, Grant/Award Number: APP1197940
Abstract
The aim of this study was to examine potential synergistic effects between maternal autoimmune disease and early childhood infections and their association with autism spectrum disorder (ASD) in offspring. Both exposures have been associated with increased risk of ASD in previous studies, but potential synergistic effects remain underexplored. We conducted a population-based cohort study of singleton children born at term gestation (37–41 weeks) in New South Wales, Australia from January 2002 to December 2008. Maternal autoimmune diagnoses and childhood infections before age 2 years were identified from linked maternal and child hospital admissions, and ASD diagnoses by age 9 years were identified from linked disability services data. Multivariable logistic regression assessed the association between each exposure and ASD and additive interaction between exposures, controlling for potential confounders. A total of 18,451 children exposed to maternal autoimmune disease were propensity score matched (1:2) to 36,902 unexposed children. Any maternal autoimmune disease (adjusted odds ratio (aOR) 1.25, 95% confidence interval (CI) 1.07–1.47) and any childhood infection before age 2 years (aOR 1.38, 95% CI 1.15–1.67) were each associated with ASD. However, there was no evidence of additive interaction between the two exposures (relative excess risk due to interaction [RERI] 0.128, 95% CI -0.418-0.675) resulting in increased odds of ASD in offspring. Future studies could examine potential interactions between other sources of maternal immune activation and childhood infection and impact on ASD and other neurodevelopmental disorders.
INTRODUCTION
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in communication and social interaction and repetitive patterns of behavior, interests, or activities that appear in early childhood (American Psychiatric Association, 2016). Prevalence estimates from registries and surveillance systems in Europe (Delobel-Ayoub et al., 2020) and North America (Maenner et al., 2021) range from 0.5% to 3.1%. ASD is highly heritable, but its etiology is complex and comprises genetic, epigenetic, and environmental factors (Lord et al., 2020).
Activation of the maternal immune system during pregnancy is a potential risk factor for the development of ASD in the child (Bilbo et al., 2018;Han et al., 2021; Sotgiu et al., 2020). The maternal and fetal immune systems interface at the placenta and maternal immune dysregulation modifies the fetal immune system, including microglia, key players in neuronal development and synaptic pruning (Han et al., 2021; Sotgiu et al., 2020). Autoimmune diseases, characterized by an abnormal immune response to self (Somers, 2020), are a potential source of maternal immune activation. Maternal autoimmune diseases, including type 1 diabetes (Xiang et al., 2018), rheumatoid arthritis (Rom et al., 2018) and autoimmune thyroid disease (Chen et al., 2016), have been associated with ASD in observational studies. Exposure to maternal immune activation is hypothesized to prime offspring microglia, leading to abnormal responses to immune stimulation in childhood or adolescence that affect neurodevelopment, described as a “two-hit” model (Estes & McAllister, 2016; Perry & Holmes, 2014; Savino et al., 2020). Childhood infections, particularly CNS infections, are one such immune stimulus and have been associated with ASD (Atladóttir et al., 2010; Karlsson et al., 2022; Sabourin et al., 2019). Based on this two-hit model, a synergistic effect would be expected between childhood infection and prenatal exposure to maternal immune activation, such as autoimmune disease.
Evidence of a two-hit model has been found in animal studies of autism (Bilbo et al., 2018; Ji-Xu & Vincent, 2020) and in human studies of schizophrenia (Blomström et al., 2016; Maynard, Sikich, Lieberman, & LaMantia, 2001). However, to our knowledge, no previous studies have examined potential synergistic effects between maternal autoimmune disease and early childhood infections on the risk of ASD in humans. This study aims to address this gap in the literature by examining (1) the association between maternal autoimmune disease and child ASD by age 9 years, (2) the association between early childhood infections and child ASD by age 9 years, and (3) potential synergistic effects between the two study exposures.
METHODS
Study population
All children born in New South Wales (NSW), Australia from January 2002 to December 2008 were identified from the NSW Perinatal Data Collection, which includes data on all births ≥20 weeks gestation or ≥ 400 gm at public and private hospitals and homebirths in the state. Birth data for children in the cohort were linked to hospital admissions, ambulatory mental health encounters, disability services, and death records. The study cohort was restricted to singleton children born at term (37–41 weeks gestation) without major congenital conditions who survived to their 9th birthday. Birth data for the cohort were also linked to mothers' hospital admission records to identify maternal health conditions.
Hospital admissions were identified from the NSW Admitted Patients Data Collection, a dataset including all admissions to public and private hospitals in the state. Data are longitudinally linked and include up to 51 relevant diagnoses recorded for each admission coded using the International Classification of Disease, Tenth Revision Australian Modification (ICD-10 AM). All maternal admissions data from July 2001 to June 2020 and all child admissions from birth to age 9 years were included in the study.
Ambulatory mental health records were identified from the NSW Mental Health Ambulatory Data Collection, a dataset of all public mental health services for nonadmitted patients, including day programs, psychiatric outpatient visits, and outreach services. Each encounter includes one related ICD-10 AM diagnosis, if relevant, and all child encounters from birth to age 9 years were included in the study.
Disability services data were identified from the NSW Family and Community Services Disability Dataset, including basic information from all publicly funded providers of services for people with disabilities including accommodation support, therapy, early intervention services, case management, employment, and advocacy. Providers are required to regularly report standardized data on all individuals receiving services and all child services from birth or July 2003 to age 9 years were included in the study.
Autism spectrum disorder
Diagnosis with autism spectrum disorder (ASD) by age 9 years was the primary outcome in this study, defined as one or more of the following: (1) hospital admissions with ICD10-AM code F84, (2) ambulatory mental health encounter with ICD10-AM code F84, (3) disability service identifying the “autism” disability group, based on DSM IV criteria (Reppermund et al., 2017).
Maternal autoimmune disease
The primary exposure of interest in this study was any maternal autoimmune disease. A list of ICD-10 AM codes for 35 of the most prevalent autoimmune conditions among reproductive age women developed for previous studies (Nielsen et al., 2021) was used to identify autoimmune disease in linked hospital admissions (Table S1). Children whose mothers had any hospitalization with a relevant diagnosis code at any time were considered exposed to maternal autoimmune disease.
Early childhood infections
Hospitalization for infection during the first 2 years of life, referred to as early childhood infections, was the secondary exposure of interest. A list of relevant ICD-10 AM codes for infection was developed based on previous studies (Miller et al., 2020), grouping diagnosis codes into six groups–bacterial, viral, gastrointestinal, upper respiratory, lower respiratory, and skin infections (Table S1). Children with at least one hospitalization with a relevant diagnosis code following discharge from hospital at birth and before their second birthday were considered exposed.
Propensity score matching
Propensity score matching was used to control for potential confounding due to differences in characteristics between mothers with and without autoimmune conditions. Propensity scores were calculated using a logistic regression model including maternal age, country of birth, socioeconomic disadvantage, place of residence, parity, and smoking during pregnancy from birth data and maternal mental health diagnoses from hospital admissions (Table S2). Child sex was not included as a variable in propensity score matching to allow for assessment of potential interaction by child sex. Each child exposed to maternal autoimmune disease was optimally matched to two unexposed children born in the same six-month period based on the logit of their propensity score (caliper = 0.2 times the Standard Deviation) (Austin, 2011). The balance of covariates in the matched cohort was confirmed by calculating absolute standardized difference measures, with differences <0.1 considered sufficiently balanced.
Statistical analysis
Logistic regression modeling was used to examine the association between both maternal autoimmune disease and child ASD diagnosis by age 9 years. Odds ratios (OR) and 95% confidence intervals (CI) were calculated for any maternal autoimmune disease and specific conditions with at least five exposed cases. All models were additionally adjusted for child sex. Similarly, logistic regression modeling was used to examine the association between early childhood infections and child ASD diagnosis by age 9 years. Any infection, total infections (ordinal variable: none, 1, 2, 3+), and six specific infection types were considered as exposures. Each infection type was considered a separate dichotomous exposure and individuals with other infection types were considered unexposed rather than being excluded, given the matched design. Since propensity score matching was not used to balance potential confounding variables by exposure to early childhood infection, the relevant models were directly adjusted for those variables. Models were also adjusted for the child's total length of stay in hospital before age 2 to control for the effect of hospitalization (Atladóttir et al., 2010).
Assessing interaction
Potential synergistic effects between maternal autoimmune disease and early childhood infections on child ASD were assessed by calculating three measures of additive interaction - the relative excess risk from interaction (RERI), the attributable proportion (AP), and the synergy index (SI), using the rare outcome assumption and calculating confidence intervals using the delta method (Hosmer & Lemeshow, 1992; Knol & VanderWeele, 2012). Together these measures assess whether the effect of the two study exposures together is greater (RERI > 0, AP >0%, S > 1), equal (RERI = 0, AP = 0%, S = 1), or less (RERI < 0, AP < 0%, S < 1) than the sum of the two exposures separately. While additive interaction was of primary interest, multiplicative interaction was also assessed between the two exposures, as well as between each exposure and child sex by including relevant interaction terms in regression modeling (p value <0.05). Interaction was assessed for any maternal autoimmune disease and any early childhood infection, as well for specific autoimmune conditions and infection types that were associated with ASD in the single exposure analyses.
Sensitivity analyses
Four sensitivity analyses were conducted to examine the robustness of the main study results. First, to avoid including future autoimmune diagnoses, logistic regression models were repeated restricting the definition of maternal autoimmune disease to include only admissions before or within 180 days of the child's delivery. Second, to account for effects of birth complications, logistic regression models were repeated on a subset of the study cohort without severe neonatal morbidity events (Lain et al., 2012). Third, to account for potential heterogeneity in ASD identification, logistic regression models were repeated with the outcome identified from disability services data alone, the most complete single data source available in this study. Finally, to control for the effects of other maternal inflammatory exposures, logistic regression models were repeated adjusting for maternal infections during pregnancy and maternal asthma.
All analyses were performed using SAS 9.4 software (SAS Institute, Cary, North Carolina, USA). Ethics approval for the study was obtained from the NSW Population and Health Services Research Ethics Committee.
RESULTS
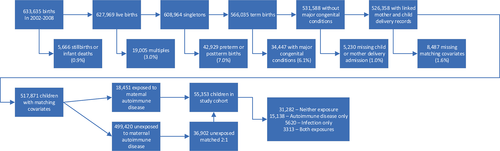
A total of 663,635 children were born in NSW between 2002 and 2008, of which 517,871 (78%) met inclusion criteria, including 18,451 children (3.6%) exposed to maternal autoimmune disease and 36,902 matched unexposed children (Figure 1). The final study cohort included 55,353 individuals, of which 15,138 (27%) were exposed to maternal autoimmune disease alone, 5620 (10%) to early childhood infection alone, 3313 to both exposures (6%), and 31,282 to neither exposure (57%). The maternal characteristics of the full population and the final study cohort are shown in Table 1. The propensity score matching process was successful in balancing all included characteristics (absolute standardized difference < 0.1). In the matched cohort, 655 children (1.2% cumulative incidence) were diagnosed with ASD by age 9 years, including 526 males (1.9% cumulative incidence) and 129 females (0.5% cumulative incidence).
| Full population (N = 517,871) | Propensity score matched sample (N = 55,353)a | |||||
|---|---|---|---|---|---|---|
| Autoimmune diagnosis (n = 18,451) | No autoimmune diagnosis (n = 499,420) | Absolute standardized difference | Autoimmune diagnosis (n = 18,451) | No autoimmune diagnosis (n = 36,902) | Absolute standardized difference | |
| No. (%) | No. (%) | No. (%) | No. (%) | |||
| Age at birth | 0.056 | 0.003 | ||||
| < 20 years old | 563 (3.1) | 19,042 (3.8) | 563 (3.1) | 1136 (3.1) | ||
| 20–24 | 2421 (13.1) | 71,040 (14.2) | 2421 (13.1) | 4867 (13.2) | ||
| 25–29 | 4991 (27.1) | 137,878 (27.6) | 4991 (27.1) | 9984 (27.1) | ||
| 30–34 | 6407 (34.7) | 167,639 (33.6) | 6407 (34.7) | 12,821 (34.7) | ||
| 35–39 | 3366 (18.2) | 86,632 (17.4) | 3366 (18.2) | 6722 (18.2) | ||
| ≥ 40 years old | 703 (3.8) | 17,189 (3.4) | 703 (3.8) | 1372 (3.7) | ||
| Country of birth | 0.150 | 0.004 | ||||
| Australia/New Zealand | 14,392 (78.0) | 357,102 (71.5) | 14,392 (78.0) | 28,847 (78.2) | ||
| Other | 4059 (22.0) | 142,318 (28.5) | 4059 (22.0) | 8055 (21.8) | ||
| Parity | 0.037 | 0.001 | ||||
| First birth | 7249 (39.3) | 205,277 (41.1) | 7249 (39.3) | 14,484 (39.3) | ||
| Prior birth | 11,202 (60.7) | 294,143 (58.9) | 11,202 (60.7) | 22,418 (60.8) | ||
| Mental health diagnosis | 0.226 | 0.002 | ||||
| Yes | 3679 (19.9) | 58,690 (11.8) | 3679 (19.9) | 7323 (19.8) | ||
| No | 14,772 (80.1) | 440,730 (88.3) | 14,772 (80.1) | 29,579 (80.2) | ||
| Smoking in pregnancy | 0.021 | 0.003 | ||||
| Yes | 2658 (14.4) | 68,236 (13.7) | 2658 (14.4) | 5279 (14.3) | ||
| No | 15,793 (85.6) | 431,184 (86.3) | 15,793 (85.6) | 31,623 (85.7) | ||
| Socioeconomic disadvantage | 0.033 | 0.002 | ||||
| Quintile 1–most disadvantage | 3945 (21.4) | 112,430 (22.5) | 3945 (21.4) | 7901 (21.4) | ||
| Quintile 2 | 4312 (23.4) | 120,131 (24.1) | 4312 (23.4) | 8650 (23.4) | ||
| Quintile 3 | 3671 (19.9) | 95,254 (19.1) | 3671 (19.9) | 7348 (19.9) | ||
| Quintile 4 | 2888 (15.7) | 75,895 (15.2) | 2888 (15.7) | 5734 (15.5) | ||
| Quintile 5 - least disadvantage | 3635 (19.7) | 95,710 (19.2) | 3635 (19.7) | 7269 (19.7) | ||
| Place of residence | 0.007 | 0.004 | ||||
| Major city | 14,274 (77.4) | 385,184 (77.1) | 14,274 (77.4) | 28,607 (77.5) | ||
| Inner regional | 3197 (17.3) | 86,172 (17.3) | 3197 (17.3) | 6380 (17.3) | ||
| Outer regional | 888 (4.8) | 24,802 (5.0) | 888 (4.8) | 1745 (4.7) | ||
| Remote/very remote | 92 (0.5) | 3262 (0.7) | 92 (0.5) | 170 (0.5) | ||
- a Sample created by 2:1 optimized matching (caliper = 0.2 × Standard Deviation of Logit of Propensity score).
Crude and adjusted ORs for the association between maternal autoimmune disease and child ASD are shown in Table 2. Exposure to any autoimmune condition was associated with increased odds of ASD (OR 1.25, 95% CI 1.07–1.47). Twelve specific conditions were examined including type 1 diabetes (T1D) (OR 1.46, 95% CI 0.92–2.31), multiple sclerosis (MS) (OR 1.70, 95% CI 0.88–3.30), and rheumatoid arthritis (RA) (OR 2.00, 95% CI 0.89–4.48); however, none were associated with ASD in the adjusted models. There was no evidence of multiplicative interaction between maternal autoimmune disease and child sex (p = 0.209).
| Maternal autoimmune condition by body system | ASD cases | Cumulative incidence | Crude ORb, 95% CI | Adjusted ORa, 95% CI | ||
|---|---|---|---|---|---|---|
| Exposed | Unexposed | Exposed | Unexposed | |||
| Any autoimmune condition | 250 | 405 | 1.35% | 1.10% | 1.24 (1.06–1.45) | 1.25 (1.07–1.47) |
| Neurological | ||||||
| Multiple sclerosis | 16 | 20 | 1.67% | 1.04% | 1.61 (0.83–3.12) | 1.70 (0.88–3.30) |
| Endocrine | ||||||
| Graves' disease | 36 | 72 | 1.33% | 1.33% | 1.00 (0.67–1.50) | 1.01 (0.68–1.52) |
| Autoimmune thyroiditis | 21 | 33 | 1.41% | 1.11% | 1.28 (0.74–2.21) | 1.33 (0.77–2.31) |
| Type 1 diabetes | 32 | 44 | 1.68% | 1.15% | 1.46 (0.92–2.31) | 1.46 (0.92–2.31) |
| Gastrointestinal | ||||||
| Ulcerative colitis | 21 | 56 | 0.80% | 1.06% | 0.75 (0.45–1.24) | 0.77 (0.46–1.27) |
| Crohn's disease | 17 | 48 | 0.81% | 1.15% | 0.71 (0.41–1.23) | 0.70 (0.40–1.22) |
| Coeliac disease | 26 | 45 | 1.09% | 0.94% | 1.16 (0.71–1.88) | 1.18 (0.72–1.91) |
| Haemopoietic | ||||||
| Immune thrombocytopenic purpura | 11 | 14 | 1.57% | 1.00% | 1.58 (0.71–3.50) | 1.55 (0.70–3.46) |
| Musculoskeletal | ||||||
| Rheumatoid arthritis | 12 | 12 | 1.70% | 0.85% | 2.02 (0.90–4.51) | 2.00 (0.89–4.48) |
| Cardiovascular | ||||||
| Rheumatic fever/carditis | 7 | 17 | 1.17% | 1.42% | 0.82 (0.34–1.99) | 0.81 (0.34–1.98) |
| Cutaneous/mucous membranes | ||||||
| Psoriasis | 11 | 13 | 2.04% | 1.20% | 1.71 (0.76–3.84) | 1.67 (0.74–3.78) |
| Systemic disorders | ||||||
| Systemic lupus erythematosus | 11 | 18 | 1.42% | 1.16% | 1.23 (0.58–2.61) | 1.23 (0.58–2.63) |
- Abbreviations: ASD, autism spectrum disorder; OR, odds ratio.
- a Regression model additionally adjusted for child sex.
- b Cohort propensity score matched to control for year of birth, parity, maternal age, country of birth, maternal mental health conditions, socioeconomic disadvantage, place of residence, and smoking during pregnancy.
Crude and adjusted ORs for the association between early childhood infections and ASD are shown in Table 3. Any early childhood infection was associated with increased odds of ASD (OR 1.38, 95% CI 1.15–1.67), controlling for potential confounders and total length of stay. There was some cumulative effect of multiple infections, with children hospitalized for 3 or more infections having more than twice the odds of ASD (OR 2.20, 95% CI 1.29–3.76). In addition, both viral infections and upper respiratory infections were associated with increased odds of ASD diagnosis, with children hospitalized for an upper respiratory infection having almost twice the odds of an ASD diagnosis (OR 1.92, 95% CI 1.49–2.49). There was no evidence of multiplicative interaction between early childhood infection and child sex (p = 0.291).
| ASD cases | Cumulative incidence | Crude OR, 95% CI | Adjusted ORa, 95% CI | Adjusted ORb, 95% CI | |||
|---|---|---|---|---|---|---|---|
| Exposed | Unexposed | Exposed | Unexposed | ||||
| Any infection | 158 | 497 | 1.77% | 1.07% | 1.66 (1.39–1.99) | 1.44 (1.20–1.73) | 1.38 (1.15–1.67) |
| Infection count | |||||||
| 1 infection | 117 | 497 | 1.63% | 1.07% | 1.54 (1.25–1.88) | 1.37 (1.11–1.68) | 1.33 (1.08–1.64) |
| 2 infections | 25 | 497 | 1.90% | 1.07% | 1.79 (1.19–2.68) | 1.45 (0.96–2.18) | 1.37 (0.90–2.07) |
| 3 or more infections | 16 | 497 | 3.52% | 1.07% | 3.38 (2.04–5.60) | 2.51 (1.50–4.20) | 2.20 (1.29–3.76) |
| Infection typec | |||||||
| Bacterial | 6 | 649 | 2.80% | 1.18% | 2.42 (1.07–5.47) | 2.23 (0.98–5.07) | 1.89 (0.82–4.35) |
| Viral | 34 | 621 | 1.95% | 1.16% | 1.70 (1.20–2.41) | 1.55 (1.09–2.20) | 1.46 (1.03–2.09) |
| Gastrointestinal | 38 | 617 | 1.88% | 1.16% | 1.64 (1.18–2.28) | 1.43 (1.02–2.00) | 1.35 (0.96–1.89) |
| Upper respiratory | 69 | 586 | 2.48% | 1.11% | 2.25 (1.75–2.90) | 2.00 (1.55–2.59) | 1.92 (1.49–2.49) |
| Lower respiratory | 58 | 597 | 1.69% | 1.15% | 1.48 (1.13–1.95) | 1.20 (0.91–1.58) | 1.13 (0.86–1.49) |
| Skin | 5 | 650 | 1.22% | 1.18% | 1.03 (0.43–2.49) | 0.89 (0.36–2.16) | 0.81 (0.33–1.97) |
- Abbreviations: ASD, autism spectrum disorder; OR, odds ratio.
- a Regression model adjusted for child sex, year of birth, parity, maternal age, country of birth, maternal mental health conditions, socioeconomic disadvantage, place of residence, and smoking during pregnancy.
- b Additionally adjusted for total all-cause hospital length of stay.
- c Each infection type was considered separately. Unexposed cases refer to those without the specific infection type, but who may have experienced another type.
Potential additive interaction was assessed for three pairs of exposures associated with the outcome: any maternal autoimmune disease and any infection; any maternal autoimmune disease and viral infection, and any maternal autoimmune disease and upper respiratory infection (Table 4). While children exposed to any maternal autoimmune disease and any infection had a higher cumulative incidence rate than those exposed to either exposure alone, there was no evidence of additive interaction between these exposures (RERI 0.128, 95% CI-0.418-0.675; AP 7.5%, 95% CI-23.1-38.1%; SI 1.22, 95% CI-0.52–2.84). Similarly, there was no evidence of additive or multiplicative interaction between maternal autoimmune disease and specific infection types.
| Exposure profile | ASD cases /population | Cumulative incidence | Crude OR, 95% CI | Adjusted ORa, 95% CI | Adjusted ORb, 95% CI | Interaction statistics |
|---|---|---|---|---|---|---|
Any autoimmune = No Any Child Infection = No |
313/31,282 | 1.00% | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | RERI: 0.128 (−0.418, 0.675) AP: 7.5% (−23.1%, 38.1%) SI: 1.22 (0.52, 2.84) |
Any autoimmune = Yes Any Child Infection = No |
184/15,138 | 1.22% | 1.22 (1.01–1.46) | 1.23 (1.03–1.48) | 1.23 (1.03–1.48) | |
Any autoimmune = No Any child Infection = Yes |
92/5620 | 1.64% | 1.65 (1.30–2.08) | 1.41 (1.12–1.79) | 1.36 (1.07–1.72) | |
Any autoimmune = Yes Any child infection = Yes |
66/3313 | 1.99% | 2.01 (1.54–2.63) | 1.79 (1.37–2.35) | 1.72 (1.31–2.25) | |
Any autoimmune = No Child viral infection = No |
383/35,817 | 1.07% | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | RERI: −0.313 (−1.463, 0.837) AP: −20.0% (−102.7%, 62.7%) SI: 0.64 (0.11, 3.85) |
Any autoimmune = Yes Child viral infection = No |
238/17,796 | 1.34% | 1.25 (1.07–1.48) | 1.27 (1.08–1.49) | 1.27 (1.08–1.49) | |
Any autoimmune = No Child viral infection = Yes |
22/1085 | 2.03% | 1.92 (1.24–2.96) | 1.70 (1.10–2.64) | 1.61 (1.04–2.50) | |
Any autoimmune = Yes Child viral infection = Yes |
12/655 | 1.83% | 1.73 (0.97–3.08) | 1.66 (0.93–2.98) | 1.56 (0.87–2.81) | |
Any autoimmune = No Child URT infection = No |
366/35,128 | 1.04% | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | RERI: 0.486 (−0.621, 1.59) AP: 19.3% (−19.0%, 57.5%) SI: 1.47 (0.63, 3.43) |
Any autoimmune = Yes Child URT infection = No |
220/17,441 | 1.26% | 1.21 (1.03–1.44) | 1.23 (1.04–1.45) | 1.23 (1.04–1.45) | |
Any Autoimmune = No Child URT infection = Yes |
39/1774 | 2.20% | 2.14 (1.53–2.98) | 1.88 (1.35–2.64) | 1.81 (1.29–2.54) | |
Any Autoimmune = Yes Child URT infection = Yes |
30/1010 | 2.97% | 2.91 (1.99–4.24) | 2.65 (1.81–3.88) | 2.53 (1.72–3.71) |
- Abbreviations: AP, attributable proportion; ASD, autism spectrum disorder; OR, odds ratio; RERI, relative excess risk due to interaction; SI, synergy index; URT , upper respiratory tract.
- a Regression model adjusted for child sex, year of birth, parity, maternal age, country of birth, maternal mental health conditions, socioeconomic disadvantage, place of residence, and smoking during pregnancy.
- b Additionally adjusted for total all-cause hospital length of stay.
Results of the four sensitivity analyses are presented in Supplementary Table S3. Restricting maternal autoimmune exposure to diagnoses before or within 180 days of delivery, excluding children with severe neonatal morbidity events, identifying ASD from disability services data, or adjusting for maternal asthma and infections during pregnancy did not meaningfully change the results of the main analysis (Table S3).
DISCUSSION
Both maternal autoimmune disease and early childhood infections were associated with child ASD diagnosis by age 9 years in this large, propensity score matched cohort of children born in New South Wales, Australia. Children exposed to both exposures had the highest cumulative incidence of ASD, but there was no evidence of a synergistic effect of infection in early life among those with prenatal exposure to maternal autoimmune disease. These study results remained robust when adjusting for the timing of maternal autoimmune diagnoses, severe neonatal morbidity, maternal infections in pregnancy, and maternal asthma.
The association between maternal autoimmune disease and child ASD is consistent with the results of previous observational studies (Croen et al., 2019; Rom et al., 2018; Spann et al., 2019; Vinet et al., 2015; Xiang et al., 2018). A 2015 systematic review and meta-analysis of 10 studies reported an overall 34% increase in the odds of ASD among children exposed to maternal autoimmune disease (pooled OR 1.34, 95% CI 1.23–1.46) (Chen et al., 2016). Our study examined the association of 12 autoimmune conditions with offspring ASD, none of which reached statistical significance. However, the estimates for T1D, MS, and RA had lower confidence intervals close to one and were limited in precision due to small sample size. Previous cohort studies have reported an association between maternal T1D and child ASD (Chen et al., 2021; Xiang et al., 2018), and a previous meta-analysis have reported an association between maternal diabetes generally and child ASD (Xu et al., 2014). Similarly, a recent systematic review and meta-analysis found an association between rheumatoid arthritis and ASD (pooled OR 1.39, 95% CI 1.16–1.67) (Zhu et al., 2020). There is a need for additional meta-analyses of specific autoimmune conditions to address the limited sample size in individual cohort studies.
Our study found an association between infections in early life and later diagnosis with ASD. Previous cohort studies found children who experienced infections had an increased risk of ASD compared to children who did not experience infections in Denmark (Hazard Ratio 1.38, 95% CI 1.31–1.45) (Atladóttir et al., 2010) and the United States (OR 1.70, 95% CI 1.50–1.90) (Sabourin et al., 2019). Children with ASD are more likely to experience comorbidities and to be admitted to hospital than children without ASD (Alexeeff et al., 2017; Atladóttir et al., 2010; Brooks et al., 2021). However, we found that the association between any infection and ASD remained after adjusting for total hospital length of stay and neonatal morbidity, suggesting an association specific to infection. The direction of this association is uncertain; infections in early life may increase the risk of developing ASD, or children with ASD may have an increased risk of infection due to immune dysregulation.
Most children who are exposed to maternal autoimmune disease or who experience infections in early life do not develop ASD, suggesting a complex relationship between these exposures and offspring ASD. Even in murine models, there are inconsistent behavioral outcomes in mice with the same genetic background and maternal immune insults (Meyer, 2014). Multiple factors including intensity, timing, type of immune activation may modify the effects on child neurodevelopment. A recent review (Meyer, 2019) highlights potential factors promoting susceptibility, including high-intensity immune activation, maternal hypoferremia/anemia, gestational diabetes, stress, and gut dysbiosis, as well as potential factors promoting resilience: an efficient anti-inflammatory and antioxidant response, high maternal iron/zinc/vitamin D, and maternal availability of omega 3 fatty acids/choline. A better understanding of these factors is a priority to lessen the potential negative impacts of maternal autoimmune activation.
We did not find evidence of a synergistic effect between maternal autoimmune disease and early childhood infections, however that does not preclude the possibility of a two-hit model. A large cohort study in Sweden reported interactions between maternal infections and childhood infections that increased the risk of later psychosis (Blomström et al., 2016). Future studies should consider interaction between early childhood infections and other measures of maternal immune activation, including maternal infections, sources of chronic inflammation, or biomarkers of inflammation. Additionally, studies should examine whether maternal autoimmune disease and childhood infections are associated with specific features of ASD, as the different exposures may impact different neural pathways (Li et al., 2018).
This study has several strengths including the use of three population-based data sources to identify children with ASD: inpatient, outpatient, and disability services data. Data linkage also allowed for adjustment for potential confounders, including perinatal and sociodemographic factors. The associations between exposures and the outcome remained after multiple sensitivity analyses suggesting robust findings. To our knowledge, this is the first study to examine potential synergistic effects of maternal autoimmune disease and early childhood infections and associations with offspring ASD in a large cohort study.
The study also has limitations, most importantly that both study exposures were based on diagnosis codes documented in hospital admissions. There is likely some misclassification of exposure for children whose mothers had autoimmune conditions managed in other settings or those considered less relevant to the delivery admission, such as psoriasis (Nielsen et al., 2020). However, individuals experiencing severe symptoms or exacerbations during pregnancy are likely to have a diagnosis recorded. The study also did not capture childhood infections that did not require hospitalization. The study may not have identified all individuals with ASD in the cohort, particularly those not receiving publicly funded autism services; however, rates are similar to population rates (May & Williams, 2018).
CONCLUSIONS
In this cohort study, exposure to maternal autoimmune disease and early childhood infections were each associated with diagnosis of ASD by age 9 years. There was no evidence of synergistic effects between these exposures that would be consistent with a two-hit model of increased risk of ASD from infection among vulnerable individuals exposed to maternal autoimmune disease in utero. Future studies could examine potential interactions between other sources of maternal immune activation and childhood infection and impact on ASD.
ACKNOWLEDGMENTS
This research was based on routinely collected data from the New South Wales Ministry of Health and we thank the Ministry's Centre for Health Records Linkage for linking the datasets. This study was supported by a National Health and Medical Research Council (NHMRC) investigator grant (APP1197940) and NN was supported by the Financial Markets Foundation for Children. Open access publishing facilitated by The University of Sydney, as part of the Wiley - The University of Sydney agreement via the Council of Australian University Librarians.
CONFLICTS OF INTEREST
The authors have no additional conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.




