Measures of tonic and phasic activity of the locus coeruleus—norepinephrine system in children with autism spectrum disorder: An event-related potential and pupillometry study
Funding information: National Institutes of Health, Grant/Award Number: R21-MH114095
Abstract
A growing body of research suggests that locus coeruleus-norepinephrine (LC-NE) system may function differently in individuals with autism spectrum disorder (ASD). Understanding the dynamics of both tonic (resting pupil diameter) and phasic (pupil dilation response [PDR] and event-related potential [ERP]) indices may provide meaningful insights about the nature of LC-NE function in ASD. Twenty-four children with ASD and 27 age- and nonverbal-IQ matched typically developing (TD) children completed two experiments: (1) a resting eye-tracking task to measure tonic pupil diameter, and (2) a three-stimulus oddball paradigm to measure phasic responsivity using PDR and ERP. Consistent with prior reports, our results indicate that children with ASD exhibit increased tonic (resting pupil diameter) and reduced phasic (PDR and ERP) activity of the LC-NE system compared to their TD peers. For both groups, decreased phasic responsivity was associated with increased resting pupil diameter. Lastly, tonic and phasic LC-NE indices were primarily related to measures of attention-deficit/hyperactivity disorder (ADHD), and not ASD, symptomatology. These findings expand our understanding of neurophysiological differences present in ASD and demonstrate that aberrant LC-NE activation may be associated with atypical arousal and decreased responsivity to behaviorally-relevant information in ASD.
INTRODUCTION
Autism spectrum disorder (ASD) is a pervasive neurodevelopmental disability characterized by impairments in social communication and interaction as well as the presence of restricted and repetitive behaviors (APA, 2013). In addition to these core diagnostic features, individuals with ASD often show secondary or associated symptoms including, but not limited to, atypical attention (Keehn et al., 2013), sleep problems (Elrod & Hood, 2015), and anxiety (Vasa et al., 2020). To account for the wide range of both core and associated symptoms, a large body of research has shown diffuse structural and functional deviations of cortical and subcortical neural circuits in ASD (Amaral et al., 2008; Ecker et al., 2022; Geschwind & Levitt, 2007; Muller, 2007). However, more recently, London (2018) suggested that such widespread neurostructural and neurofunctional differences in ASD may be the result of atypical neuromodulation. Specifically, the locus coeruleus-norepinephrine (LC-NE) system, which projections broadly to all areas of cortex including the limbic system as well as to thalamic and brainstem nuclei and the cerebellum (Szabadi, 2013), mediates behavior associated with several ASD symptom domains, and may, in part, explain the distributed nature of brain differences observed in the condition (London, 2018). Therefore, it is critical to understand differences in LC-NE activity that may be present in ASD (Bast et al., 2018).
The locus coeruleus (LC)—a small nucleus located in the brain stem—is the primary source of NE (Berridge & Waterhouse, 2003; Samuels & Szabadi, 2008b). The LC-NE system consists of tonic and phasic activities (Berridge & Waterhouse, 2003; Poe et al., 2020); tonic activity regulates the sleep–wake cycle (Hayat et al., 2020) with lower tonic activity associated with drowsiness (Bitsios et al., 2006), whereas elevated activity accompanies increased attention (Sara, 2009) and arousal (Carter et al., 2010). Phasic LC-NE activity occurs in response to salient sensory information or to novel or behaviorally-relevant stimuli (Vazey et al., 2018; Zhao et al., 2019). To describe the association between tonic and phasic activities and task performance, Aston-Jones and Cohen (2005) put forward the adaptive gain theory, which proposes that the LC-NE system operates in two modes: phasic and tonic. The phasic mode occurs at intermediate levels of tonic LC activation, during which phasic LC responses are the most robust, and task performance reaches its peak. The tonic mode is accompanied by elevated tonic LC activation, reduced phasic responsivity, and disengagement from the current task and exploration of alternatives.
The size and location of the LC a bilateral “tube-like” structure in dorsal pons adjacent to the fourth ventricle; 17–33 mm2 (German et al., 1988; Liebe et al., 2020) have prevented systematic non-invasive structural and functional analysis. Instead, eye-tracking (see Joshi & Gold, 2020, for review) and electrophysiological (Nieuwenhuis, 2011; Pfeffer et al., 2022) methods have been shown to provide indirect measurements of tonic and phasic LC-NE activities. Under passive task conditions pupil size is associated with activity of LC neurons (e.g., Joshi et al., 2016; Murphy, O'Connell, et al., 2014b), suggesting that resting pupil diameter is an indirect measure of tonic LC-NE activity. Furthermore, phasic changes in pupil size are elicited by electrical stimulation of the LC (Joshi et al., 2016; Liu et al., 2017) and by behaviorally-relevant stimuli (Gilzenrat et al., 2010; LoTemplio et al., 2021; Murphy, O'Connell, et al., 2014b). In addition, the event-related potential (ERP) P3 component, which is elicited by low frequency and/or intrinsically important stimuli usually in the context of an oddball-like paradigm, is influenced by lesions to the LC (Pineda et al., 1989), may be elicited by optogenetic stimulation of the LC (Vazey et al., 2018), and associated with pupil dilation response (Murphy et al., 2011; although, see Muckschel et al., 2017, for evidence that against the LC-P3 hypothesis). Although pupil diameter and neurophysiological measures, such as the P3 component, are influence by multiple different neuromodulatory systems, together the evidence suggests that they may provide proxy measurements of LC-NE activity.
Early evidence of atypical modulation of the LC-NE system in ASD was provided by studies demonstrating increased plasma levels of NE (Lam et al., 2006; although see Kubota et al., 2020; Martchek et al., 2006 for evidence of equivalent LC-NE cell counts and transporter binding). Indirect measurement of the LC-NE system using pupillometry has also provided evidence of atypical tonic and phasic activities in ASD (Arora et al., 2021; de Vries et al., 2021). However, pupil dilation response (PDR) has been largely focused on responses to social stimuli or luminance changes, which may restrict the explanatory power of these findings as they are related to the phasic activity of the LC-NE system (de Vries et al., 2021). Furthermore, while electrophysiological differences, specifically reduced amplitude of the P3 ERP component measured during oddball paradigms, have been well documented in ASD (Cui et al., 2017), decreased P3 amplitude in individuals with ASD has not been interpreted in the framework of the LC-NE system.
Although these findings suggest that LC-NE function may be atypical in ASD, pupillary measures (Bellato et al., 2020; Wainstein et al., 2017) also indicate that LC-NE dysfunction may present in children with attention-deficit/hyperactivity disorder (ADHD). Given the high co-occurrence of ASD and ADHD (Brookman-Frazee et al., 2018; Lai et al., 2019; Ying Rong et al., 2021) and the shared genetic association between the conditions (Cristino et al., 2014; Geschwind, 2011; Rommelse et al., 2010), it is critical to understand whether LC-NE disruption in children with ASD is associated with the presence of ADHD symptoms. Prior research has shown that pupillary response metrics differ across children with either ASD or ADHD (Boxhoorn et al., 2020) and that ASD, but not ADHD, symptoms were associated with increased baseline pupil size (Bast et al., 2021). Thus, atypical LC-NE system may be present in both ASD and ADHD, however, it remains unclear how tonic and phasic activation contributes uniquely to symptoms across conditions.
Thus, given converging evidence of atypical LC-NE function in ASD, the current study employed a multi-method approach to examine the ASD-related differences in tonic and phasic activities, and the associations between these measures in both TD children and children with ASD. Additionally, based on prior research linking LC-NE activity and ASD (Anderson et al., 2013; Bast et al., 2021) and ADHD (Shirama et al., 2020) symptomatology as well as considerable co-occurrence of these two conditions (Ying Rong et al., 2021), the current study investigated the relationship between tonic and phasic LC-NE indices and both ASD and ADHD symptomatology. We hypothesized that children with ASD would evidence increased tonic and decreased phasic LC-NE activation compared to their TD peers, and that these differences would be associated with greater ASD and ADHD symptomatology.
METHODS
Participants
A total of 24 children with ASD and 27 age- and nonverbal-IQ matched TD children participated in the study (see Table 1). ASD diagnoses were confirmed using the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2; Lord et al., 2012), the Social Communication Questionnaire (SCQ; Rutter et al., 2003), and expert clinical judgment according to DSM-5 criteria (APA, 2013). Children with genetic conditions associated with increased prevalence of ASD (e.g., Fragile-X syndrome, tuberous sclerosis) were excluded from the ASD group. Children in the TD group had no reported family history of ASD and were confirmed to be free of clinically significant ASD-related symptoms using the Social Responsiveness Scale, Second Edition (SRS-2; Constantino & Gruber, 2012). Additionally, for all the participants, ADHD symptoms were measured using the Conners 3 – Parent Short Form (C3-P/S; Conners, 2008). Informed assent and consent were obtained from all participants and their caregivers in accordance with the Purdue University Institutional Review Board (#1510016668).
| ASD | TD | Statistic | p-value | ||
|---|---|---|---|---|---|
| N (male:female) | 24 (21:3) | 27 (20:7) | χ = 1.81 | 0.18 | |
| Age (years) | 11.6 (1.5); 8.7–14.6 | 11.3 (1.7); 8.9–15.0 | t = 0.63 | 0.53 | |
| Verbal IQ | 100 (15); 71–126 | 111 (11); 95–135 | t = −3.12 | 0.003 | |
| Nonverbal IQ | 105 (18); 59–136 | 112 (15); 87–136 | t = −1.55 | 0.13 | |
| SCQ | 20 (6); 9–37 | - | - | - | |
| ADOS-2 | Social Affect | 10 (4); 4–18 | - | - | - |
| Restricted and Repetitive Behaviors | 2 (1); 0–5 | - | - | - | |
| Total | 13 (4); 10–21 | - | - | - | |
| C3-P/S | Inattention | 72 (13); 50–90 | 49 (8); 40–65 | t = 7.96 | < 0.001 |
| Hyperactivity/Impulsivity | 72 (15); 46–90 | 49 (10); 40–76 | t = 6.50 | < 0.001 | |
- Note: IQ was determined using the Wechsler abbreviated scale of intelligence, second edition (Wechsler, 2011). ASD symptoms were determined using the autism diagnostic observation schedule, second edition (ADOS-2; 32) and the Social Communication Questionnaire (SCQ; Rutter et al., 2003). ADHD symtoms determined using the Conners 3—Parent Short Form (C3-P/S; Conners, 2008). Mean (SD); range.
Experimental paradigms
This study consisted of two experiments: a resting eye-tracking task to measure tonic pupil diameter, and a three-stimulus oddball paradigm to measure phasic PDR and P3 amplitude (see Supplementary Materials for apparatus).
Resting eye-tracking task
Participants were seated approximately 60 cm from the display and completed 3, 2-min blocks of resting eye tracking. A black central fixation crosshair (1.4 visual angle) was presented on a gray background and participants were instructed to relax, remain as still as possible, and look at the crosshair. Background illumination of the room was fixed (450 lux).
Oddball paradigm
The procedure and stimuli were similar to prior three-stimulus visual oddball paradigms (Comerchero & Polich, 1999; Polich & Comerchero, 2003; Stige et al., 2007). A visual, rather than auditory, oddball paradigm was selected as the task was part of a larger study examining visual attention in ASD. The standard was a small circle, the target was a large circle, and the nontarget was a square. At a viewing distance of 60 cm, the diameter of the standard and target circles was ~2.9° and 4.6° visual angle, respectively. The nontarget square was 4.6° × 4.6° visual angle. Stimuli were drawn in isoluminant blue on a gray background to reduce luminance-related changes in pupil diameter.
Each trial began with the stimulus presented alone for a duration of 150 ms. Next, a blank gray screen was presented for a 1500 ms inter-stimulus interval (Figure 1). Stimuli were presented randomly with at least two standards presented before each target or nontarget stimulus. Prior to beginning the experiment, participants were instructed to respond via button press each time a large circle (i.e., target) appeared and to maintain fixation in the center of the screen. The experiment consisted of 780 trials, divided into three blocks of 260 trials (200 standard, 30 target, and 30 nontarget).
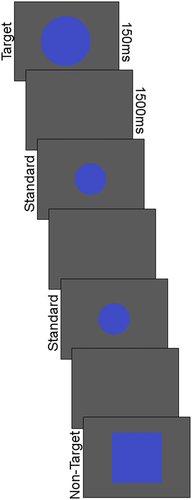
Analyses
Resting eye-tracking task
Results from the resting eye-tracking task have been presented for a subset of the participants (ASD = 17; TD = 20; Keehn et al., 2013). A procedure similar to that described by Steiner and Barry (2011) was used to calculate pupil diameter as pupil size is recorded as arbitrary units (i.e., pixels). Prior to data collection, a series of artificial pupils (2–10 mm; at 1 mm intervals) where placed at multiple fixed distances (550–700 mm; at 50 mm intervals) from the eye tracker, using multiple thresholds. Artificial pupil diameter (in pixels) was recorded at each distance for each false pupil. These measurements were entered into a multiple linear regression and coefficients from this analysis were used to predict absolute pupil size from the raw pupil data based on distance from the eye tracker.
The distance between the eye tracker and the forehead of the participant was used to monitor participant movement. Specifically, the root mean square of the first temporal derivative of the distance measurement was used as a metric of overall head movement; groups did not differ in overall movement, t(47) = 1.18, p = 0.24; d = 0.33. Periods in which the eye tracker did not record pupil diameter were considered artifacts and excluded (e.g., blinks, saccades). Furthermore, instances in which the pupil size exceeded 1.5x interquartile range (based on individual-level data) were considered outliers and were removed. Lastly, 200 ms before and after periods of missing or excluded data were removed. Excluded data were corrected using linear interpolation. Groups differed in the percentage of usable data prior to linear interpretation, ASD: 79% (11%); TD: 88% (9%), t(47) = 3.22, p = 0.002. Lastly, groups did differ in eye-movement measures during resting task (i.e., looking percentage, fixation duration, saccade amplitude; all p-values <0.05); however, these measures were not significantly associated with pupil size for either group (see Supplementary Materials, for more details).
Oddball paradigm
Behavioral measures
Percentage correct for each stimulus type as well as median response time (RT), standard deviation of RT (SD RT), and coefficient variance of RT (CV RT) for correct target trials were measured.
Eye tracking
Data were segmented into epochs 50 ms prior to and 1650 ms after stimulus onset. Similar to the resting pupil analysis, periods in which the eye tracker did not record pupil diameter were considered artifacts and excluded (e.g., blinks, saccades), outliers were removed, and data 50 ms before and after periods of missing or excluded data were removed. The remaining missing data were linearly interpolated. Next, trials with more than 50% of data missing for either the baseline period (−50 to 0 ms) or trial period (0 to 1650 ms) were removed from the analyses. Lastly, trials with less than 50% of looking occurring within an area of interest (AOI) surrounding the central stimulus were also excluded. Groups differed in the percentage of usable data, standard: ASD: 54% (21%); TD: 82% (11%), t(30.03) = 5.62, p < 0.001; target: ASD: 53% (19%); TD: 80% (14%), t(46) = 5.54, p < 0.001; nontarget: ASD: 53% (23%); TD: 84% (11%), t(29.44) = 5.92, p < 0.001. Finally, the percentage change from baseline was calculated. Baseline was defined as the average pupil diameter during the period prior to stimulus onset (−50 to 0 ms; LoTemplio et al., 2021). Average baseline pupil diameter was subtracted from each timepoint from the trial period (0 to 1650 ms) and then divided by mean baseline pupil to compute PDR for each trial and then multiplied by 100 to convert to a percentage. For each participant, averages for each stimulus type were then calculated, and the mean PDR was calculated for a time window of 1000 to 1600 ms.
Event-related potential
EEG data were processed using MATLAB-based toolbox EEGLAB (Delorme et al., 2011). Raw data were filtered (1–50 Hz), bad channels were removed, non-stereotyped artifacts were manually rejected (e.g., large movements), and independent component analysis (ICA) was completed. Components reflecting blinks, saccades, muscle contractions, and bad channels were then identified using SemiAutomatic Selection of Independent Components for Artifact correction in the EEG (SASICA; Chaumon et al., 2015) and removed. Bad channels were replaced using spherical interpolation, and data were referenced to the average reference. Using EEGLAB plug-in ERPLAP (Lopez-Calderon & Luck, 2014), artifact-corrected data were segmented into epochs 100 ms prior to and 1000 ms after the onset of the stimulus for each stimulus type (standard, target, nontarget), baseline corrected, and epochs containing residual artifacts were rejected. Groups differed in the number of bad channels replaced (ASD: 7 [8]; TD: 2 [3]), t(49) = 2.58, p = 0.01, and the percentage of usable data (standard: ASD: 53% [8%]; TD: 61% [13%], t (49) = −2.58, p = 0.01; target: ASD: 50% [13%]; TD: 60% [15%], t (49) = −2.57, p = 0.01; nontarget: ASD: 56% [0.09]; TD: 63% [0.12], t (49) = −2.30 p = 0.03). The number of independent components removed did not differ between groups (ASD: 10 [4]; TD: 8 [3]), t(49) = 1.61, p = 0.11. The P3 ERP component was extracted from midline frontal (Fz: 4, 11, 16, 19), central (Cz: 7, 54, 105, 124), and posterior (Pz: 61, 66, 71, 76) regions of interest (ROI; Figure 4) in the 400–600 ms time window (Stige et al., 2007).
Statistical analyses
A difference in tonic LC-NE activity was tested using an independent-samples t-test for resting pupil diameter. For the oddball paradigm, accuracy was analyzed using a mixed-model repeated measures ANOVA with between-subject factor, group (ASD, TD) and within-subjects factor, stimulus type (standard, target, nontarget). As behavioral responses were only required for target trials, independent-samples t tests were used to examine median RT, SD RT, and CV RT for correct target responses. To examine PDR-related phasic LC-NE differences, mean PDR for each individual was entered into a mixed-model repeated measures ANOVA with between-subjects factor group (ASD, TD) and within-subjects factor of stimulus type (standard, target, nontarget). To investigate ERP-related phasic differences, mean P3 amplitude for each individual was analyzed using a mixed-model repeated measures ANOVA with between-subjects factor group (ASD, TD) and within-subjects factors of stimulus type (standard, target, nontarget) and ROI (frontal, central, posterior). For all analyses, when Mauchly's test indicated that the assumption of sphericity had been violated, degrees of freedom were corrected using Greenhouse–Geisser estimates of sphericity. Pearson's correlations were used to examine the associations between resting pupil diameter, PDR, P3 ERP amplitude. Spearman's correlations were used to investigate the relationship between these indices and ASD and ADHD symptomatology.
RESULTS
Resting eye-tracking task
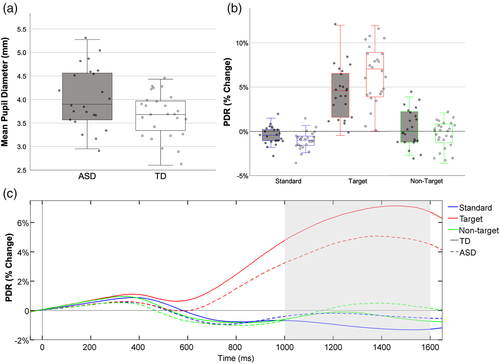
Two participants (1 ASD, 1 TD) were excluded for the resting pupil analysis as they had less than 25% usable data. As illustrated in Figure 3a, compared to their TD peers, children with ASD showed significantly larger resting pupil diameter, t(47) = 2.27, p = 0.03; d = 0.64.
Oddball paradigm
Behavioral
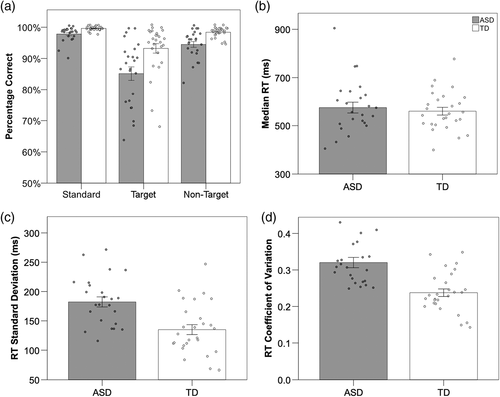
For percentage of correct responses, there was a significant main effect of stimulus type, F(1.07, 52.47) = 39.47, p < 0.001, ηp2 = 0.45, as accuracy for standard and nontarget conditions did not differ significantly from each other, but were significantly higher than accuracy for the target condition (standard–nontarget: t[50] = 6.14, p < 0.001; nontarget–target: t[50] = 4.96, p < 0.001). Children with ASD were less accurate compared to their TD peers, F(1, 49) = 23.01, p < 0.001, ηp2 = 0.32. Additionally, there was a significant interaction between group and stimulus type, F(1.07, 52.47) = 0.02, p = 0.047, ηp2 = 0.01 (Figure 2); children with ASD were less accurate across all trial types (see Table S2). There was no significant difference between groups for median RT; however, the ASD group showed significantly larger RT SD and CV RT compared to the TD group (Table S3).
Eye tracking
Three participants (2 ASD, 1 TD) were excluded from PDR analysis as they had less than 25% usable data. There was a significant main effect of stimulus type, F(1.30, 59.93) = 158.28, p < 0.001, ηp2 = 0.78 (target > non-target > standard; all p < 0.05). Groups did not differ significantly, F(1, 46) = 0.44, p = 0.51, ηp2 = 0.01; however, there was a significant interaction between stimulus type and group, F(1.30, 59.93) = 9.61, p = 0.001, ηp2 = 0.17. As illustrated by Figure 3, children with ASD showed significantly increased PDR in response to the standard stimuli, t(46) = 2.58, p = 0.01; d = 0.75, and decreased PDR in response to the target stimuli, t(46) = −2.31, p = 0.03; d = 0.11, compared with their TD peers. Groups did not significantly differ in their PDR in response to the nontarget stimuli, t(46) = 1.14, p = 0.26; d = 0.33.
Event-related potential
As presented in Table 2, there were significant main effects of stimulus type and ROI for P3 amplitude. Additionally, there were significant interactions between group and ROI, stimulus type and ROI, and group, stimulus type, and ROI. There was no significant main effect of group and no significant interaction between group and stimulus type (see Figure S1 for grand average ERPs for both groups).
| df | F | Effect size (ηp2) | p-value | |
|---|---|---|---|---|
| Group | (1, 49) | 0.40 | 0.01 | 0.53 |
| Type | (1.78, 87.29) | 40.34 | 0.45 | < 0.001 |
| ROI | (1.45, 71.27) | 127.24 | 0.72 | < 0.001 |
| Group × Type | (1.78, 87.29) | 2.12 | 0.04 | 0.12 |
| Group × ROI | (1.45, 71.27) | 5.99 | 0.11 | 0.009 |
| Type × ROI | (2.41, 117.97) | 74.09 | 0.60 | < 0.001 |
| Group × Type × ROI | (2.41, 117.97) | 7.09 | 0.13 | 0.001 |
To further examine the three-way interaction between group, stimulus type, and ROI, separate ANOVAs for each stimulus type that included between-subject factor group and within-subjects factor ROI were conducted. For the standard condition, there was a significant main effect of ROI, F(1.41, 68.92) = 40.62, p < 0.001, ηp2 = 0.45, but there was no significant main effect of group, nor a significant interaction between group and ROI (all p-values > 0.22).
For the target condition (Figure 4), there was a significant main effect of ROI, F(1.47, 71.90) = 122.01, p < 0.001, ηp2 = 0.71, but no significant main effect of group, F(1, 49) = 0.21, p = 0.65, ηp2 = 0.00. However, there was a significant interaction between group and ROI, F(1.47, 71.90) = 8.80, p = 0.001, ηp2 = 0.15. Follow-up independent-samples t tests showed that, compared to the TD group, there was a marginally significant decrease in amplitude for the ASD group at the posterior ROI, t(49) = −1.97, p = 0.05; d = 0.56, and a significant increase in amplitude at the frontal ROI, t(49) = 4.05, p < 0.001; d = 1.14; no group difference was present at the central ROI, t(49) = −1.66, p = 0.10; d = 0.47.
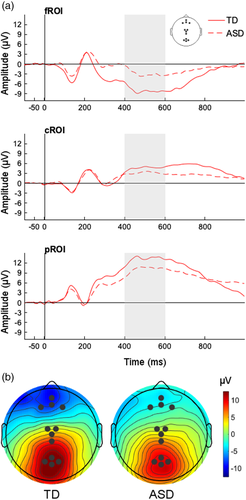
For the nontarget stimulus, there was a significant main effect of ROI, F(1.42, 69.64) = 109.28, p < 0.001, ηp2 = 0.69, but no significant main effect of group, F(1, 49) = 1.89, p = 0.18, ηp2 = 0.04, or interaction between group by ROI, F(1.42, 69.64) = 3.20, p = 0.063, ηp2 = 0.06.
Amount of usable data
Although groups were age-, IQ-, and sex-matched, they did differ on the percentage of usable data for resting pupil, PDR, and P3 ERP. As outlined by Miller and Chapman (2001), group differences on the covariate violate a critical assumption of the analysis of covariance (ANCOVA). Therefore, we did not include ANCOVA using missing data as a covariate. If missing data were associated larger pupil sizes or reduced PDR and ERP (as seen in ASD) then there would likely be strong associations across all participants and within the ASD group specifically. Correlations between resting pupil diameter and percentage of usable data was neither significant within ASD, r(23) = 0.15, p = 0.51, nor TD group, r(26) = 0.08, p = 0.70. Likewise, there was no significant correlation between mean of usable ERP across stimulus types and ERP amplitude to standard, target, or nontarget stimulus either in TD or in ASD group (see Table S7). The number of bad channels, which also differed across groups, was not correlated with ERP amplitude to standard, target, or nontarget stimuli for either the TD or the ASD group (all p-values >0.05) with the exception standard posterior ERP in ASD, r(23) = 0.45, p = 0.03. The mean of usable data across all stimulus types was only significantly correlated with PDR to standard stimulus in TD group, r(26) = 0.39, p = 0.048, whereas the mean was not correlated with PDR to other conditions in the TD group, target: r(26) = −0.14, p = 0.51; nontarget: r(26) = 0.09, p = 0.64, and was not correlated with PDR to any stimulus type in the ASD group, standard: r(22) = 0.17, p = 0.44; target: r(22) = −0.22, p = 0.33; nontarget: r(22) = −0.27, p = 0.23. Although percentage of usable PDR was correlated with PDR to standard stimulus in the TD group, such correlation was not significant in the ASD group, suggesting that missing data are not likely associated with between-group differences in the target condition. In sum, the amount of usable pupil and ERP data were not associated with the primary dependent measures (i.e., resting pupil size, target PDR, and P3 amplitude) within each group, suggesting that increased missing data in the ASD did not contribute significantly to differences in pupil diameter or ERP amplitude.
Correlations between tonic and phasic LC-NE measures and ASD and ADHD symptomatology
Association between tonic and phasic LC-NE measures
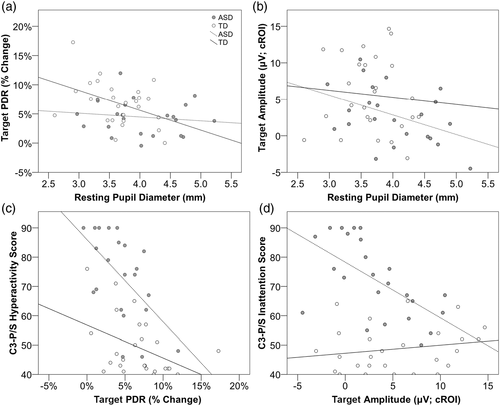
For all participants, larger resting pupil diameter was associated with reduced PDR as well as central P3 amplitude to target and nontarget stimuli (Table 3). Separate correlations for each group showed significant negative correlations between resting pupil diameter and target PDR for the TD, but not the ASD, group. Whereas for P3 amplitude, increased resting pupil diameter was significantly associated with reduced central P3 amplitude for the ASD, but not the TD, group (Figure 4a,b).
| Target | Non-target | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PDR | fROI | cROI | pROI | PDR | fROI | cROI | pROI | ||
| Resting pupil diameter | Combined | −0.36* (0.01) | 0.18 (0.21) | −0.31* (0.03) | −0.12 (0.43) | −0.17 (0.25) | −0.00 (0.99) | −0.29* (0.04) | 0.04 (0.79) |
| ASD | −0.19 (0.41) | −0.13 (0.57) | −0.43* (0.04) | −0.05 (0.83) | −0.30 (0.20) | −0.24 (0.28) | −0.39 (0.07) | −0.07 (0.76) | |
| TD | −0.42* (0.04) | 0.18 (0.37) | −0.09 (0.68) | 0.01 (0.97) | −0.11 (0.61) | −0.04 (0.84) | −0.18 (0.39) | 0.07 (0.74) | |
- Note: r-value (p-value); **p < 0.01, *p < 0.05.
Phasic ERP-PDR correlations for responses to target and nontarget conditions showed significant associations between target PDR and frontal ERP responses; greater PDR responses were related to larger (i.e., more negative) frontal target ERP amplitudes for combined groups and the TD group (Table 4). Additionally, for the TD group, nontarget PDR was directed related to central ERP amplitudes.
| Combined (n = 48) | ASD (n = 22) | TD (n = 26) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Frontal | Central | Posterior | Frontal | Central | Posterior | Frontal | Central | Posterior | |
| Target PDR | −0.38** (0.008) | 0.28 (0.056) | 0.27 (0.069) | −0.01 (0.964) | 0.25 (0.256) | 0.04 (0.854) | −0.42* (0.034) | 0.20 (0.329) | 0.27 (0.188) |
| Nontarget PDR | 0.03 (0.819) | 0.26 (0.075) | −0.09 (0.564) | −0.03 (0.885) | 0.14 (0.531) | −0.22 (0.330) | −0.06 (0.758) | 0.41* (0.039) | 0.06 (0.784) |
- Note: r-value (p-value); **p < 0.01, *p < 0.05.
Association between LC-NE indices and ASD and ADHD symptom measures
For the ASD group, ADOS-2 social affect, Restricted and Repetitive Behaviors (RRB), and Total scores were not significantly correlated with tonic LC-NE indices (Table S5). For phasic PDR and ERP measures, only central nontarget ERP amplitude was associated with increased RRB scores, r(23) = 0.41, p = 0.049.
Correlational analyses for ADHD symptom measures showed correlations between tonic and phasic indices and C3-P/S Inattention and Hyperactivity/Impulsivity subscales (Table S6). Larger pupil size (i.e., increased tonic activation) was associated with greater inattentive symptoms for combined groups (but not within separate groups). Reduced target-related PDR was associated with greater hyperactivity symptoms in combined groups as well as within both ASD and TD groups individually (Figure 5c). Likewise, reduced frontal negativity and decreased posterior P3 amplitude was related to increased inattentive (Figure 5d) and hyperactivity symptoms across both groups.
DISCUSSION
The present study examined indirect measures of tonic and phasic LC-NE activities in children with ASD compared with their TD peers, the association between these indices, and their relation to ASD and ADHD symptomatology. To our knowledge, the current study is the first to investigate both tonic and phasic LC-NE activities using pupillometry and ERP in the same cohort of children with ASD. Consistent with prior reports, we confirmed that children with ASD showed increased tonic LC-NE activity as evidenced by larger resting pupil diameter and decreased phasic activity as measured by both task-related PDR and ERP amplitude. These tonic and phasic indices of LC-NE activity were negatively correlated, such that those with larger resting pupil diameter evidenced reduced phasic responsivity as measured by both PDR and ERP. Lastly, although both ASD and ADHD symptom measures were related to indirect indices of LC-NE activity, more robust associations were present for differences in inattention and hyperactivity. Together, these findings broaden our understanding of the neuromodulatory framework associated with ASD.
Tonic LC-NE activity
In agreement with prior reports (Anderson et al., 2013; Anderson & Colombo, 2009; Bast et al., 2021; DiCriscio & Troiani, 2021), children with ASD showed increased resting pupil diameter compared to their TD peers. However, it should be noted that previous studies (e.g., Granovetter et al., 2020) have reported equivalent resting pupil diameter in ASD and TD individuals. These conflicting findings may result from methodological differences, as the current study measured pupil diameter during task-free, resting-state, whereas, for example, Granovetter and colleagues (Granovetter et al., 2020) measured the pupil diameter prior to the start of each task block. Nevertheless, findings from the present study are consistent with previous findings that employed a similar approach (Anderson et al., 2013; Anderson & Colombo, 2009; DiCriscio & Troiani, 2021) and add to a growing body of research that suggests that children with ASD exhibit elevated resting pupil diameter. Together, these findings suggest that individuals with ASD may operate in a persistent tonic mode of LC-NE function (Aston-Jones & Cohen, 2005), which is associated with decreased task engagement, increased distractibility, and, as discussed below, reduced phasic LC responsivity. Furthermore, as LC activation directly and indirectly influences autonomic nervous system (ANS; Samuels & Szabadi, 2008a), elevated tonic LC-NE activity in ASD may broaden our understanding of ASD symptoms related to ANS dysfunction, and its neural mechanisms, such as atypical attentional processes (Keehn et al., 2013), sleep difficulties (Elrod & Hood, 2015), anxiety (Vasa et al., 2020), and differences in sensory responsivity (Bouret & Sara, 2002).
Phasic LC-NE activity
In agreement with previous PDR (Granovetter et al., 2020) and ERP (Cui et al., 2017) studies, results from the present report also demonstrated decreased phasic responsivity in ASD, as evidenced by reduced target-related PDR and ERP amplitude in children with ASD compared to their TD peers. Together, these findings suggest reduced phasic activation of the LC-NE system in ASD. Pupil dilation response results from the present study do contradict outcome of a recent meta-analysis of PDR in ASD, which showed differences in latency but not amplitude of PDR in ASD (de Vries et al., 2021). As noted by the authors, these studies varied considerably with regard to the stimuli used (e.g., simple luminance change to faces) and task requirements (e.g., passive viewing vs. behavioral response required). Recently, Bast et al., (2021) demonstrated that manipulations to task requirements (i.e., task utility) differentially affect children with ASD, suggesting that inconsistent evidence of tonic and phasic pupil indices may be due to study-specific differences in task demands.
Consistent with our hypothesis and previous research (Cui et al., 2017), the ASD group showed reduced posterior P3(b) amplitudes relative to the TD group for target stimuli. Additionally, the ASD group also displayed a robust decrease in frontal ERP amplitudes for both the target and nontarget stimuli. This negative component has been investigated using oddball paradigms with inconsistent naming including negative central (Nc; Courchesne, 1977), late negativity (LN; Gumenyuk et al., 2004), late difference negativity (LDN; Ceponiene et al., 2004), or re-orienting negativity (RON; Schröger & Wolff, 1998; Wetzel & Schröger, 2014). Prior research has also shown that in addition to the P3 component, children with ASD also exhibit consistent reductions in Nc amplitudes (Courchesne et al., 1984; Courchesne et al., 1985; Salmond et al., 2007).
The Nc component is present in infancy, sensitive to stimulus probably, elicited by novel information, and may be related to an orienting response that occurs prior to sustained attention (Riggins & Scott, 2020). The Nc component has been hypothesized to be associated with a general arousal system that involves enhanced processing throughout the cortex via NE (Reynolds et al., 2010). Although theoretical links between the LC and electrophysiological responses have focused primarily on the P3 (Nieuwenhuis et al., 2005), this component is not present early in development (Riggins & Scott, 2020). Relatedly, spontaneous (tonic) and phasic LC activation of the LC-NE system as well as conduction velocity of LC axons undergo developmental changes during postnatal development (Nakamura et al., 1987). Moreover, modular organization of the LC indicate that subpopulations of neurons segregate into separate distinct networks (Chandler et al., 2019), which may have different developmental schedules. Thus, electrophysiological indices of phasic LC-NE activity may vary across development. Together, PDR and ERP P3/Nc results suggest that phasic responsivity to behaviorally-relevant target stimuli is reduced in ASD.
Finally, compared with their TD peers, children with ASD displayed increased PDR to standard stimuli. This finding may reflect a difficulty filtering repeated, non-rewarding stimuli in ASD (Murphy, Foxe, et al., 2014a; Sokhadze et al., 2017). Predictive coding accounts have been used to explain these differences in responding to repeated sensory input in individuals with ASD (e.g., Lawson et al., 2014; Pellicano & Burr, 2012; Sinha et al., 2014; Van de Cruys et al., 2014), and the LC-NE system has been hypothesized to play an important role in predictive coding (Yu & Dayan, 2005). Specifically, because the precision of prediction errors is set too high (and inflexibly) in ASD, repeated stimuli may still result in large prediction errors and be deemed important (Van de Cruys et al., 2014). Therefore, increased pupillary response to repetitive standard stimuli may reflect a failure to filter redundant, unnecessary stimuli in children with ASD, which may result from persistently elevated tonic LC activity.
Associations between tonic and phasic indices
Consistent with our hypothesis, we confirmed that tonic LC-NE activity, as indexed by resting pupil diameter, was associated with phasic LC-NE activity as measured by PDR and ERP amplitude to task-relevant target stimuli across all participants. For TD children, large resting pupil diameter was associated with decreased PDR, whereas for children with ASD, resting pupil was related to decreased central P3 amplitude. Thus, for both groups elevated tonic LC-NE activation was associated with measures of reduced phasic LC-NE activity.
Additionally, across all participants and within the TD group, phasic eye tracking, and ERP indices were significantly related. Specifically, for the target condition, greater phasic responsiveness as measured by increased PDR was associated with larger Nc (but not P3) amplitude. As discussed above, although previous theoretical (Nieuwenhuis et al., 2005) and empirical research (Murphy et al., 2011) has linked between the P3 and the LC-NE system, this work has focused on adults. The findings from the present study suggest that in children the Nc may also reflect phasic LC-NE activation.
Associations between LC-NE indices and ASD and ADHD symptoms
Despite converging evidence that an atypical LC-NE system may underlie potential mechanisms of ASD symptoms (see London, 2018, for review), results of the present study did not find an association between resting pupil diameter or target-related ERP/PDR and ASD symptomatology. Rather, increased ERP amplitude to nontarget stimuli was associated with greater RRB scores on the ADOS-2 in children with ASD. This finding is in agreement with Gomot and colleagues (Gomot et al., 2008), who showed that increased activation to novel, nontarget stimuli was associated with elevated ASD symptomatology. Although preliminary, the results suggest that increased sensitivity to novel information may be related to greater rigidity and less flexibly adapting to change (Gomot & Wicker, 2012).
In addition to ASD symptoms, ADHD symptomatology was also linked to tonic and phasic indices of LC-NE activity. Specifically, for both groups combined increased tonic LC activity was related to greater inattention, whereas reduced phasic activity, as measured by both PDR and ERP, was associated with increased inattention and impulsivity scores. Prior research has shown that inter-individual variability in LC-NE activity contribute to differences in attentional functions (e.g., sustained attention; Unsworth & Robison, 2017). Furthermore, previous pupillometry studies have demonstrated increased tonic (Shirama et al., 2020) and decreased phasic (Shirama et al., 2020; Wainstein et al., 2017) LC-NE activity in individuals with ADHD. Thus, differences LC-NE activity may not be specific to ASD, but rather reflect the genetic overlap between ASD and these other disorders (e.g., ADHD) (Cristino et al., 2014; Geschwind, 2011). Future studies may wish to examine LC-NE activity and associated autonomic and cognitive processes in a transdiagnostic sample of individuals with ASD, ADHD, and ASD + ADHD to see whether indirect measures of LC-NE function are associated with phenotypic characteristics across neurotypical and clinical populations (e.g., Bast et al., 2021; Boxhoorn et al., 2020).
LIMITATIONS
There are a number of limitations associated with the present study. First, although several studies have linked fluctuations in pupil diameter to LC-NE activation (Joshi et al., 2016), pupil size may not accurately reflect LC activation in real-time (Megemont et al., 2022) and other neuromodulatory systems have been shown to covary with pupil dilation (Cazettes et al., 2021; Joshi, 2021; Reimer et al., 2016). Thus, differences in tonic and phasic pupillary measures observed in ASD may be influenced by other neuromodulators (e.g., acetylcholine, serotonin), and future work should determine whether atypical pupil dilation in ASD is specific to the LC-NE system. Additionally, the amount of usable pupillometry and ERP data differed across groups; however, LC-NE indices were not associated with the percentage of data loss, suggesting that elevated tonic and reduced phasic LC-NE measures in ASD were not due to differences in data quality. Finally, correlational analyses were not corrected for multiple comparisons, and, thus, should be considered exploratory in nature.
CONCLUSION
ASD is associated with substantial variability within two core diagnostic domains as well as a significant number of diverse co-occurring symptoms. This phenotypic heterogeneity may result, in part, from atypical neuromodulation. Findings from the current study highlight that children with ASD exhibit atypical increased tonic and reduced phasic LC-NE activity as indirectly indexed by both pupillometry and electrophysiology. These findings add to a growing body of research that suggests that LC-NE dysfunction may be present in ASD. Understanding into the mechanism(s) underlying these differences in LC-NE activation, and how the LC-NE system may contribute to the development of the heterogeneous ASD phenotype is critical for informing future translational research, including developing and selecting treatment options, monitoring treatment response, and, perhaps, as a future neuro-behavioral marker.
ACKNOWLEDGMENTS
Special thanks to the children and families who generously participated. We would also like to acknowledge Sophia Bergman for her assistance with data acquisition. All authors report no biomedical financial interests or potential conflicts of interest. Research was supported by NIH R21-MH114095 (Brandon Keehn).
ETHICS STATEMENT
All study procedures were reviewed and approved by the institutional review board of Purdue University. Written informed assent and consent were obtained from all participants and their caregivers.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




