Sorption of vapors in methanol soaked and in thermally annealed Matrimid® films
Abstract
Matrimid® shows well separated sub-glass transitions and substantial entrapment of dichloromethane (DCM) in the solution-cast films. The entrapped DCM was removed by soaking the polymer in the excess of liquid methanol followed either by thermal annealing (vacuum at 200°C) or by the desorption at mild conditions. These physical treatments influenced the sorption kinetics and even the sorption isotherms for vapors of DCM, methanol (MeOH) and acetonitrile (MeCN) at 35.0°C. The sorption isotherms for the thermally annealed Matrimid® were shifted to those for the methanol-soaked Matrimid® upon its exposure to sufficiently saturated vapors. The physical treatments changed the β sub-glass transition of the polymer, and did not cause changes in the chemical composition and microstructure as sensed using ATR-FTIR and SEM, respectively. Relaxation of the annealed Matrimid® was achieved by its soaking in liquid MeOH and by the exposure to vapors, their threshold saturation followed the same order as diffusivity: MeOH > MeCN > DCM. Although methanol appears the least effective agent to induce the relaxation of Matrimid®, it diffuses in and desorbs from the polymer readily. Liquid MeOH and its vapor thus appear the media of choice for causing the relaxation and removal of entrapped solvents from Matrimid®.
1 INTRODUCTION
Matrimid® is a glassy polyimide that is soluble in numerous easy-to-handle solvents and widely used for the preparation of separation membranes.1-8 Matrimid® membranes show penetrant induced plasticization (e.g., carbon dioxide), non-Fickian diffusion (e.g., alcohols), and aging. The polymer also shows well detectable sub-glass transitions (β, γ).3, 9 These transitions presumably originate from the motions of localized segments of the macromolecule possessing rigid segments9-14 and are influenced by sorbed volatile compounds.3, 15-17
Since Matrimid® shows solvent entrapment, several thermal annealing protocols have been proposed in the literature to remove the solvents.3, 8, 9, 18-22 One of the common thermal annealing protocols involves heating of Matrimid® to 200°C in air or in vacuum, we have chosen the latter in this study. This polymer was also reported to be thermally stable in nitrogen and argon atmospheres at this temperature.23-26 Heating of Matrimid® in nitrogen (250 and 350°C) was shown to induce frequency shifts for the CNC axial stretch at around 1390 cm−1 using diffuse reflectance Fourier transform infrared spectroscopy (measured at the elevated temperature), which indicated the closer proximity of the polymer chains, the possible cross-linking via the formation of the charge transfer complexes was discussed.3 Electrostatic interactions due to the annealing at 250°C were found less significant than the van der Waals forces for BTDA-DAPI (poly(3,3′-4,4′-benzophenone tetracarboxylic–dianhydride diaminophenylindane, which corresponds to the composition of Matrimid®) using fluorescence spectroscopy.20
Besides the removal of the entrapped solvent, setting of the physical state of the polymer is of interest. This task is similar to the long-known methanol (MeOH) treatment of physical aging of poly[(trimethylsilyl)propyne], PTMSP. This glassy high free volume polymer is repeatedly rejuvenated to a state showing high gas permeabilities upon its storing in liquid MeOH and followed by its evaporation.27 The rejuvenation of the physical structure and the removal of entrapped solvents by their soaking in liquid MeOH was observed also for a series of polymers of intrinsic microporosity—PIMs.28-37 In analogy, the enhancement of the separation characteristics of the carbonized Matrimid® membranes by their soaking in liquid MeOH were reported.22 Not only liquid MeOH but also its vapor was shown to rejuvenate the physical structure of the polymers of intrinsic microporosity, reduce aging, and to remove entrapped volatile compounds.31, 33, 38, 39 Clearly, while heating can be applied to remove entrapped volatile compounds (casting solvents, contaminants), the thermal stability of the surrounding materials (support, sealing, housing, etc.) in more complex items, such as composite membranes and membrane modules, must be ensured. Moreover, the exposure of polymer membranes to MeOH vapor rather than to liquid is preferred in applications33 as this can reduce the delamination of layers of composite membranes. The removal of the entrapped solvent by a soaking agent most likely involves the relaxation of the polymer chains. Thermal annealing of glassy polymers and their exposure to low-molecular-weight compounds can influence the sorption properties of the polymer, while these characteristics have scarcely been reported.40
The exposure of Matrimid® films to MeOH liquid or vapor potentially enables to remove entrapped volatile compounds. We thus aim to quantify the degree of the removal of the entrapped compounds at a mild temperature compared with the thermal annealing, the rate and degree of the MeOH removal at the mild temperature, and differences in the characteristics of the polymer glass due to the different sample history. While dichloromethane (DCM) is a common solvent for Matrimid®, MeCN is studied here as a potential alternative to MeOH as a soaking agent.
2 MATERIALS AND METHODS
2.1 Film preparation, chemicals
Film from Matrimid® 9725 (a micropulverized version of Matrimid® 521841, Huntsman) was prepared by casting its 1.5 wt% solution in dichloromethane onto a glass Petri dish with a cover. Solvent evaporated within 4 days under ambient conditions. The film (124 ± 7) μm was then submerged in an excess of liquid MeOH (1000 g of MeOH per 1 g of Matrimid®) for 4 h. Afterwards, the sample was either exposed to (i) vacuum (<100 mbar) at 200°C for 24 h; (ii) to vacuum (<0.1 mbar) at 35.0°C for 70 h, or (iii) to vacuum (<0.1 mbar) at 35.0°C for 70 h and then to vacuum (<100 mbar) at 80°C for 2 h. The above choice of physical treatments covers thermal annealing and soaking in MeOH under commonly used and easy to reproduce conditions.
One sample was used for all sorption experiments (see below) using the above physical treatments, its mass after the ad (i) treatment remained constant (37.3 ± 0.2 mg) thorough the experimental series involving ×15 annealing at 200°C and ×25 soaking in liquid MeOH. Importantly, mass of the sample after the ad (ii) and ad (iii) treatments equaled, to within the experimental uncertainty, that after the ad (i) treatment, which indicates the removal of the entrapped solvents by the soaking in MeOH and no detectable weight loss of the polymer.
Methanol (MeOH, 99.93 wt%, Lachner), acetonitrile (MeCN, 99.96 wt%, Lachner), dichloromethane (DCM, 99.92 wt%, Lachner) and liquid nitrogen (Siad) were used without further purification, purities are according to the Certificates of Analysis.
2.2 Vapor sorption microgravimetry
Vapor sorption experiments and vacuuming at 35.0°C were performed using a sorption apparatus30, 42, 43 utilizing a quartz spring microbalance and a vacuum pump (<0.01 Pa, Leybold D4B). Prior to the experiments, the studied liquids were inserted into the apparatus and cleared from inert gases prior to the measurement using a series of three cycles involving evaporation, cryogenic condensation, and vacuuming. During the sorption measurements, the Matrimid® film was exposed either to vacuum or to the vapor of the studied component (without air or other inert gases) at a chosen pressure and 35.0 ± 0.1°C, mass of the sample was monitored over time. Combined uncertainty (cover factor 2) of the sorption uptakes were 12 mg/g (sample exposed to vapor) and 5 mg/g (sample exposed to vacuum). A vacuum oven and a vacuum pump (Binder VDL, Pfeiffer HiScroll 12) were used for the thermal annealing of the samples, laboratory balance (Ohaus DV215CD) was used.
2.3 Vapor sorption isotherms
2.4 Sorption kinetics
2.5 Dynamic mechanical analysis
Dynamic mechanical analysis (DMA) tests were conducted using a commercial device DMA 850 (T.A. Instruments, USA) with the clamps of 10 mm initial sample length. Sample dimensions were measured using a frame micrometer and a caliper. The measurements were done under nitrogen atmosphere in the tensile mode with the force amplitude of 200 mN, and the static force (pre-tension) of 250 mN. Frequency was 1 Hz, the temperature increased with the ramp of 3°C/min from −150 to +400°C or to the specimen failure. The components of the complex modulus, that is, the storage modulus (E′) and the loss modulus (E″), and the loss factor (tan δ = E″/E′) were evaluated from the experimental data.
2.6 ATR-FTIR spectra
Vibrational spectra were collected for Matrimid® films subjected to the physical treatments ad (i) and (ii) in the section Film preparation, chemicals using Fourier Transform Infrared Spectrometry in the attenuated total reflection mode (ATR-FTIR). The spectra were recorded using a Nicolet iS50 FTIR spectrometer (ThermoScientific) equipped with an outside diamond ATR crystal. A total of 256 scans were collected for each spectrum with 2 cm−1 spectral resolution at ambient conditions, spectra were recorded at five individual spots for each film and averaged.
2.7 Scanning electron microscopy
Scanning electron microscopy (SEM) micrographs of the cryogenic (liquid nitrogen) breaks of the Matrimid® films subjected to the physical treatments ad (i) and (ii) in the section Film preparation, chemicals were captured using a SEM (TESCAN VEGA 3 LMU). The samples were coated with 5 nm of gold prior to the scanning.
3 RESULTS AND DISCUSSION
3.1 Sorption of DCM, MeOH, MeCN vapors in Matrimid®
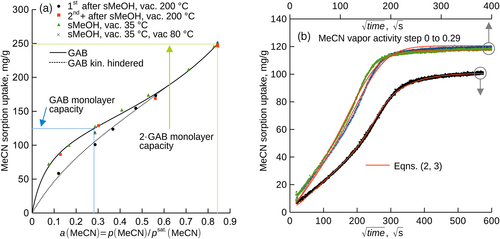
Sorption isotherms for DCM, MeOH, MeCN vapors in Matrimid® showed sigmoidal shapes and were substantially influenced by the history of the specimen. The isotherms for the thermally annealed Matrimid® without its preceding exposure to studied vapors, also first measurement after the annealing at 200°C, differed markedly from those for the specimen previously exposed to the vapors, or the specimen from which methanol introduced in MeOH soaking evaporated under mild conditions (35°C, 35 and then 80°C). Sorption uptakes for the annealed polymer appeared lower and were converted by the studied compounds to those of the soaked polymer at unequal effectivity (DCM > MeCN > MeOH, Figure 1, Figure 2a). Although MeOH is a common soaking liquid for a number of polymers, it just suffices in case of Matrimid® as the MeOH vapor activity of a ≥ 0.84 was needed to achieve full relaxation (activity equals one for the liquid).
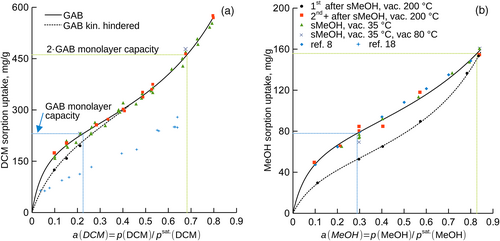
Our sorption isotherm for the sorption of DCM vapor in relaxed Matrimid® showed systematically higher uptakes than reported in the literature18 (Figure 1a) while that for MeOH vapor agreed quantitatively to that reported in the literature8 (Figure 1b). We are not aware of an apparent reason for the discrepancy as comparable polymer, physical treatments, and methods (macroscopic observations) were used.
All observed sorption isotherms were of sigmoidal shapes and followed the GAB model, Equation (1), which enabled to identify the completion of the first and second sorption layer on the isotherm; parameters of Equation (1) are listed in Table 1. In all cases, annealing at 200°C reduced parameter h, that is, sorbate molecules attached less firmly to the sorption centers of the polymer. This complies with the less steep initial increase of the respective sorption isotherms (Figure 1, Figure 2a). MeOH vapor caused the relaxation of the thermally annealed Matrimid® much less effectively than MeCN and DCM in terms of the threshold vapor activity needed to merge the isotherms. The apparent monolayer capacity for DCM and MeCN increased due to the annealing in vacuum at 200°C while this quantity decreased for MeOH. The apparent monolayer capacity expressed in mole-based units ranged 0.93–2.38 mol(sorbate)/mol(mer units), the apparent specific surface area ranged 222–833 m2/g depending on the physical treatment and sorptive. The rather wide intervals of these quantities presumably originate in the substantial changes of the physical state of the polymer due to its thermal history or specific interactions with the sorptives.
| Compound, parameters | 1st after sMeOH, vac. 200°C in ad (i), that is, thermally annealed Matrimid® | Other sample histories: 2nd + after sMeOH in ad (i), ad (ii), that is, relaxed Matrimid® |
|---|---|---|
| DCM, (vm, h, f), vmm, Asp | (271 mg/g, 10.1, 0.697), 1.76 mol/mol, 508 m2/g | (231 mg/g, 23.5, 0.761), 1.50 mol/mol, 433 m2/g |
| MeOH, (vm, h, f), vmm, Asp | (53.8 mg/g, 10.1, 0.797), 0.93 mol/mol, 222 m2/g | (77.9 mg/g, 20.2, 0.630), 1.34 mol/mol, 321 m2/g |
| MeCN, (vm, h, f), vmm, Asp | (177 mg/g, 6.38, 0.495), 2.38 mol/mol, 833 m2/g | (124 mg/g, 22.7, 0.616), 1.68 mol/mol, 588 m2/g |
- Abbreviations: DCM, dichloromethane; GAB, Guggenheim-Anderson-de Boer.
- Note: Physical treatments are according to section Film preparation, chemicals, the first and the consequent exposures to vapors are distinguished for the treatment ad (i). sMeOH abbreviates soaking in liquid MeOH, vmm, stands for vm expressed in mole-based units (mol of sorbate per mol of mer units), apparent specific surface area is Asp = σ vm/(M·NA), in which σ is cross-sectional area estimated according to Hill75 using parameters from the database,76 M is molar mass of the sorbate, NA is Avogadro number.
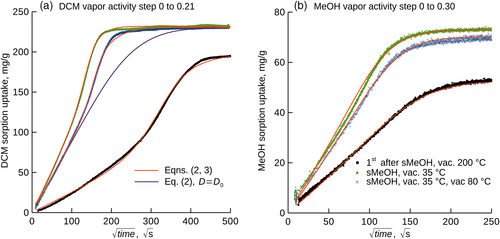
The use of the GAB model enabled to study the sorption kinetics for all tested compounds under comparable conditions, that is for the vapor activity steps (i) from zero to that corresponding to the completion of the first sorption layer (Figures 2b and 3), and (ii) from the vapor activity corresponding to the first layer to that corresponding to the second layer (Figures S1, S2, and S3). The kinetics for the protocol ad (i) showed sigmoidal shapes and were well parameterized with the semi-empirical model described by Equations (2 and 3), the initial part of the kinetics followed Equation (2), see Figure 3a and Figure S4. Clearly, the studied physical treatments of Matrimid® substantially influenced diffusivity (Table 2) as well as the uptakes at the apparent sorption equilibrium. The highest initial diffusivity, D0, see Equation (3), was observed for MeOH in all cases. Since already the exposure of the film to vacuum at 80°C influenced the diffusivity, kinetic hindrance of the polymer chains and/or decay of the free volumes likely occurred, to some extent, already at this temperature. The lowest influence of the additional annealing at 80°C on diffusivity was observed for MeCN. The kinetics for the protocol ad (ii) showed sigmoidal shapes and reached equilibrium uptakes that were equal regardless the preceding physical treatment of Matrimid®, which indicates relaxation of the polymer to a comparable physical state. Higher annealing temperature led to slower sorption, which coincides with the earlier observations20 and implies reduction of the fractional free volume.
| Compound, vector of parameters | 1st after sMeOH, vac. 200°C, ad (i), that is, thermally annealed Matrimid® | sMeOH, vac. 35.0°C, ad (ii), that is, relaxed Matrimid® | sMeOH, vac. 35.0°C, vac. 80°C, ad (iii) |
|---|---|---|---|
| DCM, (D0, DE, T, k) | (0.442 × 10−14 m2/s, 1.76 × 10−14 m2/s, 110 × 103 s, 36.1 × 10−6 1/s) | (5.82 × 10−14 m2/s, 9.36 × 10−14 m2/s, 20 × 103 s, 203 × 10−6 1/s) | (4.44 × 10−14 m2/s, 4.77 × 10−14 m2/s, 25 × 103 s, 213 × 10−6 1/s) |
| MeOH, (D0, DE, T, k) | (7.52 × 10−14 m2/s, 3.92 × 10−14 m2/s, 6.0 × 103 s, 78.3 × 10−6 1/s) | (14.7 × 10−14 m2/s, 6.03 × 10−14 m2/s, 6.0 × 103 s, 197 × 10−6 1/s) | (10.1 × 10−14 m2/s, 9.67 × 10−14 m2/s, 7.8 × 103 s, 68.7 × 10−6 1/s) |
| MeCN, (D0, DE, T, k) | (1.42 × 10−14 m2/s, 1.49 × 10−14 m2/s, 65 × 103 s, 68.0 × 10−6 1/s) | (4.70 × 10−14 m2/s, 8.60 × 10−14 m2/s, 15 × 103 s, 77.0 × 10−6 1/s) | (3.78 × 10−14 m2/s, 7.89 × 10−14 m2/s, 20 × 103 s, 66.5 × 10−6 1/s) |
- Abbreviation: DCM, dichloromethane.
The desorption kinetics for DCM, MeCN, and MeOH from the Matrimid® film previously subjected to soaking in MeOH and desorption in vacuum at 35.0°C, see ad (ii) in section Film preparation, chemicals, are shown in Figure 4. The kinetics are responses to the vapor activity steps from that corresponding to the complete sorption monolayer according to the GAB model to zero, and did not follow Fick's second law. Importantly, while 92.3% of the initial MeOH uptake desorbed after 250 × 103 s in vacuum at 35.0°C, only 80.0% of MeCN and 71.9% DCM desorbed under the same conditions (Figure 4).
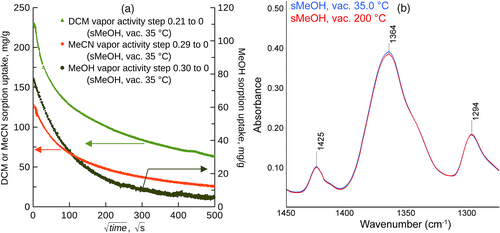
The removal of the entrapped DCM in Matrimid® by its exposure to MeOH vapor was tested for the film after the treatment ad (ii) in Film preparation, chemicals, which was previously equilibrated with the vapor of DCM (a = 0.43) and then exposed to vacuum at 35.0°C for about 1 h. After four steps each involving the equilibration of the sample with MeOH vapor (a = 0.84, 35.0°C, 55 g of MeOH vapor per gram of Matrimid®) and about 1 h of vacuuming, the sample was exposed to vacuum for 70 h at 35.0°C, yielding the sorption uptake of 6 mg/g which equals that observed after the MeOH desorption test (Figure 4 and Figure S5). Sufficiently saturated MeOH vapor (tested for a = 0.84) thus removed the entrapped solvents similarly as soaking in liquid MeOH.
3.2 DMA of Matrimid® subjected to physical treatments
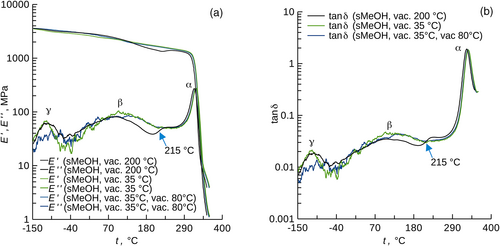
The physical treatments of the Matrimid® films (see section Film preparation, chemicals) not only influenced the sorption characteristics but also the β sub-glass transition (Figure 5; storage and loss moduli compared well to those in the literature9, 48). This transition was linked to small scale motions of the repeat segment9, 14 for rigid materials, that is to the hindrance of the thermal motion. The annealing of the film at 200°C in vacuum thus caused a partial hindrance of the thermal motions of the polymer (see the inflection point at 215°C in Figure 5). No such partial conversion was reported for Matrimid® annealed at 330°C in a nitrogen purge for 30 min.9 Thus, while thermal annealing of Matrimid® in vacuum at 200°C did not lead to a highest possible hindrance of the polymer chains, it enabled to repeatedly remove entrapped volatile compounds from the polymer to the uptakes lower or equal to those reached by the desorption under mild conditions (in vacuum at 35.0°C, in vacuum subsequently at 35.0 and 80°C). It can thus be hypothesized that there exists some annealing temperature >200°C and likely <335°C, for which soaking of the annealed Matrimid® in liquid MeOH does not lead to its conversion to the (completely) relaxed state. Soaking in liquid MeCN could then be applied but penalized by the more pronounced entrapment. Similar phenomena can be expected to occur due to the thermal annealing of other glassy polymers. However, Matrimid® has the favorable property that it shows discernible sub-glass transitions while the β transition is sensitive to the thermal history of the sample.
3.3 ATR-FTIR spectra
Vibrational spectra for two films subjected to the physical treatments ad (i) and (ii) in section Film preparation, chemicals showed minor differences (Figure 4b and Figure S6). In particular, no wavenumber shifts and hardly discernible intensity changes were observed for the characteristic bands of Matrimid®77-79 at 1779 and 1715 cm−1 (antisymmetric and symmetric stretching CO vibration, respectively) indicating imide rings, 1672 cm−1 (amide I), 1364 cm−1 (νas CN), 1092 cm−1 (ν CNC) and 719 cm−1 (δoop CNC). The band at 1672 cm−1 and those above 3500 cm−1 presumably correspond to the traces of precursors or intermediates.80 Positions of some of the characteristic bands differed from the ones assigned in the literature,77, 79 namely the bands observed here at 1715, 1092, and 719 cm−1. The shift of the band at around 1364 cm−1 was reported to indicate closer proximity of the chains and discussed with regard to the possible cross-linking via the formation of the charge transfer complexes at 250 and 350°C.3 No frequency shift of this band was detected in this work at ambient conditions (Figure 4b), which confirms the earlier interpretation20 that van der Waals rather than electrostatic interactions are involved in the thermal annealing of Matrimid® and likely also in the β transition. Thus, if charge-transfer complexes occur at 200°C, they decay upon cooling.
3.4 Scanning electron microscopy
SEM micrographs of the cryogenic breaks of the Matrimid® films subjected to the physical treatments ad (i) and (ii) in the section Film preparation, chemicals showed patterns typical for amorphous solids, no substantial difference due to the physical treatment was discerned (Figure S7).
4 CONCLUSIONS
Soaking of Matrimid® in liquid methanol (MeOH) followed either by thermal annealing (in this study vacuum at 200°C) or by the desorption at mild conditions (in this study vacuum at 35.0°C, optionally followed by vacuum at 80°C) led to the quantitative removal of the entrapped volatile compounds, in this work dichloromethane (DCM), acetonitrile (MeCN), MeOH. Since Matrimid® is a membrane forming polyimide soluble in DCM, the removal of the entrapped solvent and the setting of a favorable physical state of the polymer are of vital interest. We show that the thermal history of the sample not only leads to substantial differences of sorption kinetics for the studied compounds, but also to differences in the apparent equilibrium sorption uptakes. The latter leads to the conclusion that liquid MeOH is the least but sufficiently effective agent converting Matrimid® annealed at a high temperature (here 200°C) to the relaxed state; MeCN and DCM (solvent) are more effective. At the same time, MeOH shows substantially lower entrapment in the polymer than MeCN, which makes MeOH the soaking liquid of choice. Not only that, the exposure of Matrimid® to the highly saturated MeOH vapor (tested for a = 0.84) has the same effect (removal of entrapped DCM, conversion to the relaxed physical state) as soaking in the liquid MeOH.
Thermal annealing of Matrimid® caused changes in its β sub-glass transition, which invokes changes in the small-scale motions of the repeat segments of the polymer presumably due to inter- or intra-chain interactions (DMA). These interactions are noncovalent (ATR-FTIR), do not influence the microstructure (SEM), and their existence is likely related to the changes in the diffusivity and sorption isotherms for MeOH, MeCN, and DCM in Matrimid®. Overall, changes in the sorption uptakes and sorption kinetics in glassy polymers due to their different history have scarcely been reported in the literature, we did so for Matrimid® and comment on the related material changes. This contributes to the highly practical topic of the removal of entrapped solvents from glassy polymers.
AUTHOR CONTRIBUTIONS
Ondrej Vopicka: Conceptualization (lead); formal analysis (lead); investigation (lead); methodology (lead); visualization (lead); writing – original draft (lead). Marcela Dendisová: Investigation (equal); visualization (equal); writing – review and editing (equal). Petr Sysel: Conceptualization (equal); formal analysis (equal); writing – review and editing (equal). Zdeněk Hrdlička: Conceptualization (equal); investigation (equal); methodology (equal); writing – review and editing (equal).
FUNDING INFORMATION
Open Access costs were covered by the University of Chemistry and Technology, Prague. No other funding was received.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.