Influence of fullerenes on the thermal and flame-retardant properties of polymeric materials
ABSTRACT
At present, the application of fullerene in polymer materials has become an attractive issue. Fullerene can enhance the thermal and flame-retardant properties of polymers due to its high capacity to trap free radicals. Fullerene also has good synergistic effect with inorganic metal flame retardant, intumescent flame retardant, brominated flame retardant (BFR), clay, carbon nanotubes, graphene oxide, and so on. In this review, the impact and mechanism of fullerene and its derivatives on the thermal and flame-retardant properties of polymeric materials are discussed. And the prospect of fullerene in flame-retardant polymer composites is also briefly introduced by analyzing the research progress in the recent years. © 2019 Wiley Periodicals, Inc. J. Appl. Polym. Sci. 2020, 137, 47538.
INTRODUCTION
Fullerene, as the third allotrope of elemental carbon after diamond and graphite, is a big family including C60, C70, C78, C82, C84, C90, C96, and so on, and the most important member in which is C60. In 1996, the Nobel Prize in Chemistry was awarded to Kroto, Curl, and Smalley for their astonishing discovery of C60 in 1985.1 The fullerenes were synthesized in large scale using arc vaporization of graphite in 1990.2
Since the discovery of fullerenes, researchers have performed the most vigorous investigations of C60, because C60 has the highest stability. The typical fullerene, C60, is a sphere with 12 pentagons and 20 hexagons. Each carbon atom in C60 is attached to other atoms by sp2 hybridization. Only 60 atoms are likely to form such highly symmetrical geometry. Because of its soccer-ball-like structure, C60 is also named buckminsterfullerene to play tribute to the geodesic dome architect, Buckminster Fuller.
The particular geometry of fullerenes, full of π-electrons on the surface, leads to plenty fascinating properties, such as special optical properties, lubrication performance, catalytic performance, superconductivity, biocompatibility, oxidation resistance, and other excellent performance. Therefore, it has great research and application value in the fields of solar cells,3, 4 catalysts,5, 6 gas storage,7, 8 superconducting materials,9-11 biomedicine,12-14 cosmetics,15, 16 and so on.
In 1991, Krusic et al.17 reported on the super high-capacity radical capture ability of C60 for the first time. A C60 molecule has 30 CC bonds that can capture more than 34 free radicals; thus it is also called a radical sponge. Since then, the study on the influence of C60 on the thermal and flame-retardant properties of polymeric materials has gradually begun.
THE INFLUENCE OF C60 ON THE THERMAL STABILITY OF POLYMERS
The investigation of the improvement of thermal properties of polymer/fullerene nanocomposites has be one of the most interesting research projects for the special impacts of fullerene (C60) on the thermal stability of polymer/fullerene composites.
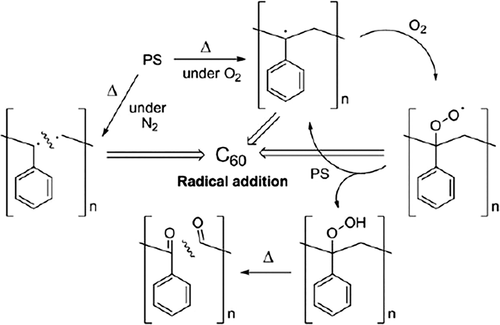
C60 was added to polyvinyl chloride (PVC), poly(methyl methacrylate) (PMMA), and polystyrene (PS) using solution blending method to study the impact of C60 on the thermal stability of polymers by Troitskii et al.18-23 It was found that C60 can slow down the autocatalytic thermal degradation of PVC in the existence of HCl, but under the condition of removing HCl, C60 has little influence on the thermal degradation rate of PVC. Therefore, it is believed that C60 can enhance the thermal stability of PVC by reacting with macromolecular radicals and chlorine radicals generated during thermal degradation process of PVC to form lower active substance. The degradation of PMMA and PS was investigated under oxygen by thermogravimetric method at given temperatures and the detailed data were listed in Table 1. Pure PMMA decomposes quickly in thermal-oxidative environment, but an induction period of up to 70 min occurred after adding 0.2 mol % of C60. It was thought that the retarding effect of C60 on the thermo-oxidative degradation of PMMA was resulted from the interaction of C60 with macromolecular radicals and oxygen-containing radicals with production of more stable species. The less active compounds inhibited the depolymerization of PMMA, thereby increasing the thermal stability of PMMA. The thermal properties of PS/C60 were consistent with PMMA/C60, both the thermal stability and oxidation induction period were improved. This increase in thermal performance was derived from the fact that C60 captured the alkyl radicals and oxygen-containing radicals generated during the degradation of PS at high temperature to form lower active compounds and inhibited the degradation of PS.
| Polymer | C60 content (mol %) | Given temperature (oC) | Induction period (min) |
|---|---|---|---|
| PMMA | 0 | 282 | ~0 |
| PMMA | 0.2 | 282 | ~70 |
| PS | 0 | 238 | ~10 |
| PS | 0.04 | 238 | ~40 |
Zuev et al.24 have investigated the impact of C60 on the thermal behavior and thermo-degradation of poly-n-alkyl acrylates (PAA-n) and poly-n-alkyl methacrylates (PMA-n) by thermogravimetry in dynamical conditions and pyrolysis/gas chromatography in isothermal conditions at 400–650 °C. It was shown that the addition of C60 could improve the thermal stability of PAA-n and PMA-n. However, for PAA-n, the recorded temperature of the maximum weight loss was slightly increased by the introduction of C60, as there were two thermal degradation pathways for PAA-n (random break of main chain and nonfree-radical degradation of side chain). C60 was just effective for the former. Meanwhile, the main degradation process of PMA-n was a radical pathway. The effect of C60 was more obvious in the thermal behavior of PMA-n; in fact, the enhancement of the Tmax was about 20 °C. Figure 1 represented the influence of fullerene presence on the yield (wt %) of the degradation products coming from radical pathway in PAA-n samples. By comparing the types and yields of the pyrolysis products of PAA-n, it can be found that the content of olefin and ethanol produced by the nonfree-radical pathway in the degradation products of PAA-n containing C60 significantly increased, whereas the content of the degradation products produced by the free-radical path decreased. The effect of C60 on the thermal degradation of PMA-n was more obvious. The analytical results of PMA-n thermal degradation at 550 °C were reported in Table 2. Compared with the system without C60, the monomer content in the degradation products decreased by 2–3 times after the addition of C60, meanwhile, the content of polyolefin and methacrylate increased by 2–3 times. The suggestion was made that C60 could change the decomposition mechanism from a radical mechanism to a nonradical pathway. C60 acted as an efficient free-radical catcher preventing the unzipping of the polymer backbone and favoring the nonfree-radical pathway.
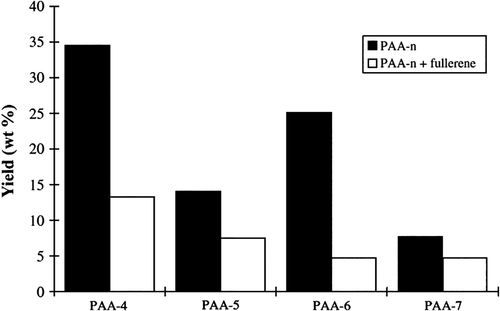
| Light | Olefin | Aldehyde | Alcohol | Methacrylic acid | Monomer | |
|---|---|---|---|---|---|---|
| PMA-4 | 0.6 | 8.6 | 0.2 | 0.1 | 7.5 | 82.9 |
| PMA-5 | 1.7 | 20.1 | 0.5 | 0.6 | 15.2 | 62.0 |
| PMA-6 | 1.7 | 17.4 | 0.3 | 0.7 | 10.6 | 69.2 |
| PMA-7 | 2.1 | 24.8 | 0.5 | 0.5 | 13.3 | 58.7 |
| FPMA-4a | 2.0 | 36.8 | 0.5 | 0.3 | 34.5 | 25.9 |
| FPMA-5a | 5.7 | 40.2 | 0.7 | 1.3 | 30.8 | 21.3 |
| FPMA-6a | 5.3 | 35.8 | 0.5 | 1.8 | 22.8 | 33.8 |
| FPMA-7a | 8.0 | 44.4 | 0.6 | 0.9 | 26.2 | 20.0 |
- a Mixture of PMA-n with fullerene.
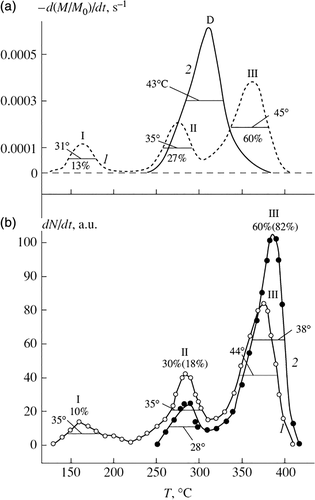
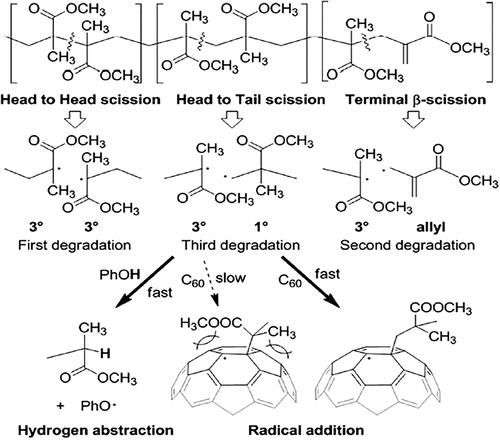
Shibaev et al.25, 26 have investigated the effect of C60 on the thermal and thermo-oxidative stability of PMMA using TGA and mass-spectrometric thermal analysis (MTA). The temperature variation of the yield thermal degradation products from free-radical PMMA was shown in Figure 2. Heating a sample of PMMA in N2 was accompanied by three peaks in TGA: peak I (~165 °C) was assigned to the degradation initiated by the chain defects of the head-to-head type (H-H bonds); peak II (~270 °C) was attributed to the degradation caused by the unsaturated terminal groups; and the most intense peak III (~360 °C) was explained by the random degradation of the PMMA backbone. After adding a small amount of C60, peak I disappeared and peak II significantly reduced in intensity, from which it could be concluded that C60 acted as a trap of free radicals formed as a result of PMMA degradation in the regions of peaks I and II. In air atmosphere, for all C60-containing samples, the curve of the weight loss was ~30–60 °C higher as compared to that for pure PMMA. It was suggested that the effect of C60 upon the thermo-oxidative degradation of PMMA may proceed by two pathways. The first involved the attachment of PMMA radicals to C60 molecules, whereby fullerene competed with oxygen and partly displaced this oxidizing agent from reaction with PMMA. The second pathway was related to a possible direct interaction of C60 with oxygen, which led to a deficit in oxygen for the PMMA oxidation and to the yield of unoxidized products of thermo-oxidative degradation of PMMA from the system.
It is well known that the dispersion of nanomaterials in matrix is very important. To improve the dispersion of C60 in polymers, a variety of chemical reactions were used to graft C60 onto different polymers. In addition, organic group modification is another effective method to improve the compatibility of C60 with polymers.
Tang et al.27, 28 introduced 2,5-dimethyl-2,5 (tert-butylperoxy) hexane peroxide (DHBP) into linear low-density polyethylene (LLDPE)/C60 and PP/C60 systems. In the presence of DHBP, in situ interfacial reaction between C60 and the matrix came up by a free-radical mechanism during melt mixing. The dispersion of C60 was enhanced due to the chemical linking between C60 and the matrix. The detailed data for thermo-oxidative stability and mechanical properties of PP and PP/C60 nanocomposites were summarized in Table 3. Compared with pure sample, the thermal stability of polymer was enhanced after the introduction of C60 and DHBP. The initial degradation temperature was determined by the unreacted C60 in the composite, so the effect of C60 and DHBP was not as good as C60 alone. However, due to the increased interaction between C60 and the matrix, the addition of DHBP could significantly increase the tensile, bending, and impact strength of the polymer/C60 composite. The initial decomposition temperature (T5%) and Tmax in air atmosphere after adding 1.2 wt % C60 and 0.5 wt % DHBP in PP were increased from 263 °C and 313 °C to 277 °C and 369 °C, respectively. Meanwhile, the tensile strength, Young's modulus, flexural strength, flexural modulus, and impact strength of composite enhanced from 36.5 MPa, 820 MPa, 50.3 MPa, 1044 MPa, and 7.0 kJ m−2 to 38.0 MPa, 928 MPa, 54.8 MPa, 1178 MPa, and 10.3 kJ m−2, respectively.
| Samples | T5 wt %a (°C) | T10 wt %a (°C) | Tmaxb (°C) | TS (MPa) | YM (MPa) | EB (%) | FS (MPa) | FM (MPa) | IS (kJ m−2) |
|---|---|---|---|---|---|---|---|---|---|
| PP | 263 | 273 | 313 | 36.5 | 820 | 678 | 50.3 | 1044 | 7.0 |
| 010D | 256 | 266 | 310 | 36.0 | 904 | 430 | 46.4 | 1033 | 6.0 |
| 01C | 270 | 281 | 332 | 36.5 | 991 | 693 | 47.6 | 999 | 7.3 |
| 01C010D | 257 | 270 | 316 | 37.6 | 1138 | 450 | 54.3 | 1180 | 8.4 |
| 05C | 277 | 286 | 346 | 36.1 | 761 | 647 | 47.9 | 980 | 7.5 |
| 05C010D | 269 | 281 | 337 | 37.9 | 843 | 525 | 54.9 | 1314 | 8.8 |
| 05C030D | 267 | 279 | 332 | 37.7 | 723 | 503 | 53.9 | 1173 | 8.3 |
| 12C | 301 | 317 | 390 | 36.1 | 654 | 675 | 50.7 | 1131 | 8.1 |
| 12C010D | 289 | 306 | 360 | 37.4 | 796 | 473 | 52.3 | 1204 | 85. |
| 12C030D | 280 | 294 | 358 | 38.2 | 795 | 175 | 53.6 | 1222 | 9.0 |
| 12C050Dc | 277 | 289 | 369 | 38.0 | 928 | 180 | 54.8 | 1178 | 10.3 |
- TS: tensile strength; YM: Young's modulus; EB: elongation-at-break; FS: flexural strength; FM: flexural modulus; IS: impact strength.
- a T5 wt % and T10 wt % were the temperatures at which 5 and 10 wt % weight loss occurred, respectively.
- b Tmax was the temperature at which the maximum weight loss rate occurred.
- c 12C050D meant that the sample contains 1.2 wt % C60 and 0.5 wt % DHBP, so are the other samples.
Cataldo et al.29, 30 grafted C60 onto polyisoprene chains by γ-irradiation in n-hexane, toluene, or decalin. The electronic absorption spectroscopy confirmed that C60 indeed reacted with polyisoprene macroradicals produced by γ radiolysis using addition reaction. The TGA results showed that the initial decomposition temperature of the irradiated samples decreased, which may be due to the residual solvent in the samples. From the DTG data, it could be found that the pure polyisoprene had the largest thermal weight loss rate of 7.87 mg min−1 at 380 °C, while which of the polyisoprene/C60 nanocomposite was only about 4 mg min−1 at the same temperature, indicating that the addition of C60 retarded the thermal degradation rate of polymer and increased the thermal stability of polymer.
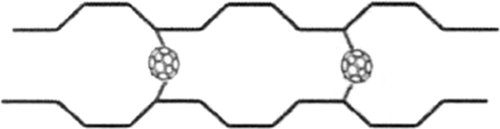
Liu et al.31 prepared HDPE/ethylene-acetate copolymer (EVA)/C60 nanocomposite by melt blending. C60 can capture macromolecular radicals produced via irradiation, forming a unique crosslinked network structure (Scheme 1), in which C60 acted as a “bridge.” The more C60 was added, the more gels obtained after irradiation, further proving the occurrence of crosslinking reaction and the “bridge” model. It is this special crosslinking structure that increased the dynamic modulus, glass-transition temperature (T g), and mechanical properties of composite.
Katiyar et al.32 studied the impact of C60 on the thermal properties of poly(methyl methacrylate) and poly(n-butyl methacrylate). It was found that the thermal stability of C60 containing poly(methyl methacrylate) (F-MMA) and poly(n-butyl methacrylate) (F-BMA) were better than that of pure samples, and the enhancement increased as the content of C60 increased. After adding C60, the T g of the composites moved to higher temperature because C60 hindered the motion of the molecular chain. The rise of the initial decomposition temperature was attributed to the fact that C60 captured the macromolecular free radicals generated during the degradation process, which prevented the matrix from depolymerizing into monomers and exhibited as an effective stabilizer of the poly(alkyl methacrylate)s (Scheme 2).
Moreover, C60 was also covalently linked to atactic polypropylene,33 PVC,34 polyvinyl carbazole,35 poly-p-methyl styrene,36 and epoxy resin (EP)37 to improve the thermal properties of polymers.
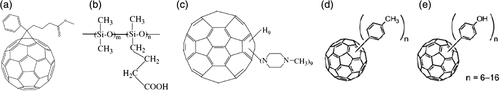
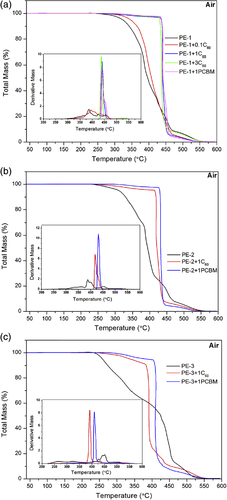
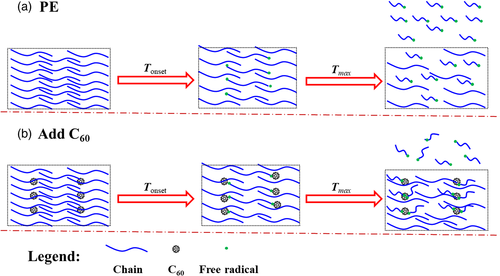
Except for C60, the impact of its derivatives on the thermal properties of polymers has also been investigated. Paulo et al.38-41 systematically studied the effect of C60 and 6,6-phenyl-C61-butyric acid methyl ester [PCBM, as shown in Scheme 3(a)] on the thermal stability of PE, PMMA, and PS. The detailed data for thermo-oxidative stability (air atmosphere) of PE, PMMA, PS, and their corresponding nanocomposites were listed in Table 4. When studying the influences of C60 and PCBM on the thermal performance of PE, three types of PEs were selected for comparison. From the TGA results, it could be found that the introduction of C60 and PCBM can effectively improve the thermal stability for all three PE under air atmosphere, C60 loading as low as 1.0 wt % could make T5% of all three PE increase about 100 °C, illustrating that C60 has a general applicability in improving the thermo-oxidative stability of PE. The DTG curves (as shown in Figure 3) clearly showed that the degradation process of PE composites changed from multistep for pure PE to one-step process. This is resulted from the free-radical capturing effect of C60 and PCBM, which hindered the free-radical degradation process occurred at the low temperature, leading to the degradation to take place via nonradical mechanism at the high temperature, thereby increasing the thermo-oxidative stability of PE. Furthermore, researchers showed that the effect of PCBM was better than that of C60. This observation was probably due to the better dispersion of PCBM in PE. On the one hand, PCBM had an organic ligand which was more compatible with PE; on the other hand, compared to C60, PCBM had a lower melting temperature [T m(PCBM)~280 °C] and, therefore, in TGA test, PCBM could diffuse freely in the matrix at the temperature above 280 °C, resulting in better dispersion at a molecular level. Similarly to the results obtained with PE, PMMA-fullerene and PS-fullerene were more stable than pristine PMMA and PS, and PCBM showed a better thermal stabilization impact than C60 for same loading, especially in air atmosphere.
| Polymer | Nanoparticle | Additive (wt %) | T5% (oC) | ΔT5% (oC) |
|---|---|---|---|---|
| PE-1 | - | 0 | 323.1 | - |
| PE-1 | C60 | 1.0 | 436.9 | +113.8 |
| PE-1 | PCBM | 1.0 | 437.4 | +114.3 |
| PE-2 | - | 0 | 311.0 | - |
| PE-2 | C60 | 1.0 | 414.7 | +103.7 |
| PE-2 | PCBM | 1.0 | 429.4 | +118.4 |
| PE-3 | - | 0 | 258.9 | - |
| PE-3 | C60 | 1.0 | 338.4 | +79.5 |
| PE-3 | PCBM | 1.0 | 383.2 | +124.3 |
| PMMA | - | 0 | 296.5 | - |
| PMMA | C60 | 1.0 | 320.9 | +24.4 |
| PMMA | PCBM | 1.0 | 338.7 | +42.2 |
| PS | - | 0 | 332.7 | - |
| PS | C60 | 1.0 | 347.2 | +14.5 |
| PS | PCBM | 1.0 | 367.1 | +34.4 |
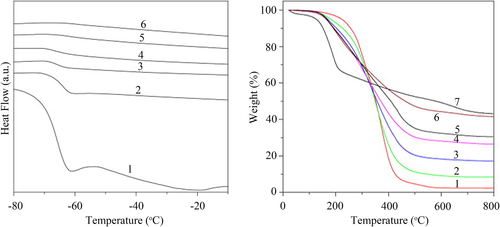
Ouyang et al.42-44 prepared supramolecular assembled nanocomposites by ionic interactions through dispersing the side chain carboxylated poly(dimethylsiloxane) [PSIX, as shown in Scheme 3(b)] and the 1-(4-methyl)-piperazinyl fullerene [MPF, as shown in Scheme 3(c)] in tetrahydrofuran (THF). The strong ionic interaction between MPF and PSIX was proved by Fourier infrared (FTIR) spectroscopy and X-ray photoelectron spectroscopy (XPS) analyses. The results of differential scanning calorimetry (DSC, as shown in Figure 4) indicated that the glass transition broadness of composites with MPF became much narrower in comparison with that of PSIX. This might be that the crosslinking caused by MPF improved the homogeneity of chain motion. As the content of MPF increased, the glass transition platform was disappeared, indicating that the highly crosslinked PSIX chains had been formed. From TGA results, it can be found that the thermal stability of PSIX/MPF composites was in-between those of PSIX and MPF, showing that the introduction of MPF could enhance the thermal stability of PSIX to a certain extent. However, PSIX was side-functionalized poly(dimethylsiloxane) (PDMS), which degraded through random depolymerization along the chain. But MPF mainly reacted with the carboxyl group in the side chains, resulting in an unsatisfactory effect on the thermal stability of the matrix. While for end-functionalized PDMS composites, the influence of MPF would be much better.
Kokubo et al.45 added four different structures of fullerenes: pure C60, multiarylated [60]fullerenes with tolyl (tolyl-C60), phenol groups (phenol-C60), and PCBM (as shown in Scheme 3) into PS and PMMA, respectively, to discuss the thermal properties of composites. The degradation temperatures of PS/fullerene and PMMA/fullerene nanocomposite films under air were summarized in Table 5. It was found that there was little effect in the thermal and thermo-oxidative stabilities for PS/tolyl-C60 and PS/phenol-C60 films. But pristine C60 and PCBM could significantly enhance the thermal properties of PS. Adding just 0.8 wt % PCBM could make the corresponding temperature when the mass loss was 10% increase up to 45 °C. The effect of phenol-C60 and PCBM, however, was much better than C60 in PMMA. It was believed that the degree of π-conjugation of fullerenes and their dispersion in polymers played key roles in these enhancements. When PS was heated under air, a relatively stable 2° benzyl radical would form because the hydrogen at the benzyl position was taken away by oxygen. The subsequent oxidation would generate peroxy radicals, which could induce auto-oxidation of PS and led to the polymer degradation at a relatively low temperature. The free-radical scavenging ability of fullerenes could inhibit the chain reaction in the thermo-oxidative degradation of PS (Scheme 4). There were 1° and 3° radicals in both thermal and thermo-oxidative degradation of PMMA. The steric impact of 3° radicals made its reaction with C60 slow, while phenol-C60 could capture 3° radicals with hydrogen atom. However, the difference in polarity led to easy agglomeration of phenol-C60 in PS and easy agglomeration of C60 in PMMA (Scheme 5). PCBM had amphiphilicity and high π-conjugation, so it worked well in both PS and PMMA. These results suggested that an appropriate combination of polymers and the additives took an important part in enhancing the thermal properties of polymers.
| Polymer | Additive (wt %) | Td90 (°C)b | |||
|---|---|---|---|---|---|
| C60c | Tolyl-C60c | Phenol-C60c | PCBMd | ||
| PS | 0 | 305 | |||
| PS | 0.1 | 330(+25) | - | - | - |
| PS | 0.2 | 337(+32) | 309(+4) | 306(+1) | - |
| PS | 0.4 | 352(+47) | 317(+12) | 306(+1) | - |
| PS | 0.8 | 349(+44) | 322(+17) | 304(−1) | 350(+45) |
| PMMA | 0 | 318 | |||
| PMMA | 0.1 | 319(+1) | - | - | - |
| PMMA | 0.2 | 319(+1) | 316(−2) | 331(+13) | - |
| PMMA | 0.4 | 329(+11) | 308(−10) | 333(+15) | - |
| PMMA | 0.8 | 329(+11) | 316(−2) | 332(+14) | 337(+19) |
- a The values in parentheses are differences of nanocomposite Td90 versus blank.
- b Td90 was defined in which 10 wt % loss occurred.
- c Toluene was used for the preparation of nanocomposite film.
- d CHCl3 was used for the preparation of nanocomposite film.
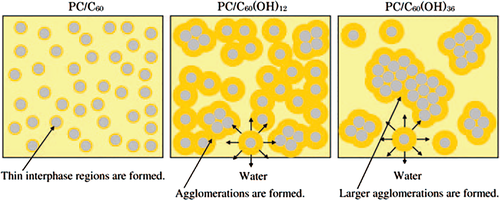
Saotome et al.46 added pristine C60 and poly-hydroxylated-fullerenes, C60(OH)12 and C60(OH)36 to polycarbonate (PC) by solution mixing. The transmittance of the films at 400–800 nm changed little with the addition of hydroxylated fullerenes, according to the UV–visible spectrum. DSC and TGA results listed in Table 6 showed that the T g of PC/C60(OH)12 film increased from 137 °C to 146 °C and the Tmax raised from 501 °C to 522 °C, indicating the improvement of thermal stability. The reason might be the formation of a rigid polymer phase region around C60(OH)12 by hydrogen bonding. After adding C60(OH)36, the Tmax decreased due to the partial hydrolysis of PC and agglomeration of C60(OH)36 particles. The deterioration of tensile properties might be caused by the agglomeration of particles as the starting point for cracks. The authors believed that there were two kinds of force between PC and fullerenes: (1) weak π–π stacking interaction between the unfunctionalized moieties of fullerene and the aromatic moieties of PC polymer and (2) strong dipole–dipole interaction between polar hydroxylated fullerenes and polar carbonate moieties of PC. The latter might improve the compatibility of composites. The improvement of thermal stability for PC/C60 composite was not obvious because of the weak π–π stacking effect. The strong dipole–dipole interaction between C60(OH)12 and PC made the thermal stability of composite increase. However, for PC/C60(OH)36 composite, the thermal stability was decreased. There were two explanations (1) it was easier to form large agglomerations for C60(OH)36 and (2) the releasable water in C60(OH)36 could cause partial hydrolysis of PC (Scheme 6).
| PC | PC/C60 | PC/C60(OH)12 | PC/C60(OH)36 | |
|---|---|---|---|---|
| T g (°C) | 137 | 135 | 146 | 135 |
| Tmax (°C) | 501 | 506 | 522 | 480 |
Zaharescu et al.47, 48 have compared the effects of different fullerene derivatives on the thermo-oxidative stability of HDPE, low-density polyethylene (LDPE), LLDPE, and isotactic polypropylene. It was also found that the effect of C60 derivatives was better than that of pristine C60.
In summary, fullerenes can increase the thermal properties of polymers by trapping free radicals, and generally, the effect of derivatives is better because of their better dispersion. Moreover, it is important to select an appropriate combination of polymers and the additives for enhancing the thermal stability of polymers.
THE INFLUENCE OF C60 ON THE FLAME RETARDANCY OF POLYMERS
The first time for C60 used in flame retardant was in 2001. Loutfy and Wexler49 reported that fullerenes could serve as ablative cooling and flame-retardant materials. The results showed that because of the high heat of sublimation and low rate of ablation, fullerene coating on the steel surface behaved outstanding ablative cooling in the temperature range of 600–1000°C, effectively protecting the steel interior and improving the flame-retardant performance.
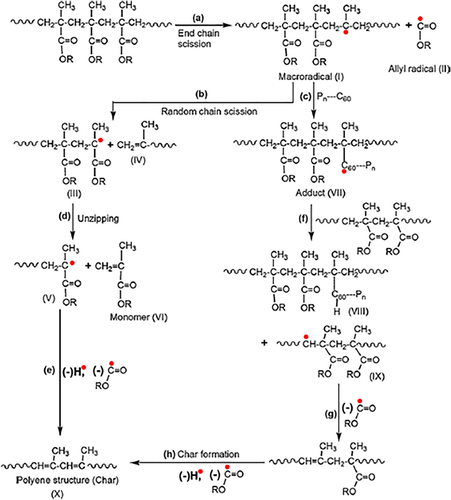
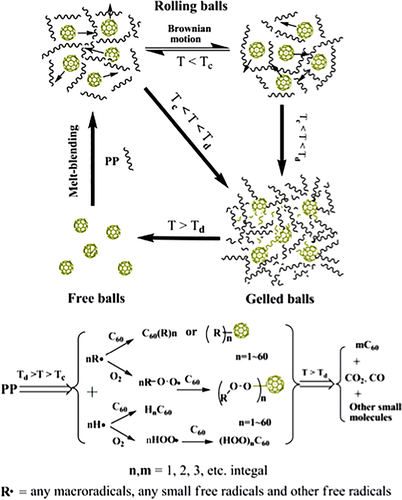
Our group takes the lead in applying fullerenes to the flame retardant of polymers. In 2008, Song et al.50, 51 prepared PP/C60 nanocomposites by melt blending and studied the effect of C60 on the thermal stability and flame retardancy of PP. It was found that C60 could disperse uniformly in PP, and the size was about 200 nm. Just a low loading of C60 could greatly enhance the thermal stability of PP under N2. The Tmax of the nanocomposites noticeably increased from 482 °C to 496 °C after adding 2.0 wt % C60. PP degraded by oxidation dehydrogenization in air, leading to the decrease in thermal stability. The initial decomposition temperature (Tonset) and the maximum degradation temperature (Tmax) of PP were about 263 °C and 338 °C, respectively, 155 °C and 144 °C lower than those in N2. But the introduction of C60 could make the thermo-oxidative degradation of PP to higher temperature. As for PP containing 1 wt % C60, its Tonset and Tmax were 15 °C and 54 °C higher than those for PP. Moreover, the Tonset and Tmax moved to higher temperatures as the loading of C60 increased. Furthermore, the addition of C60 could not only prolong the ignition time (tign) but also reduce the peak release rate (PHRR). After adding 1 wt % C60, the time to peak heat release rate (tPHRR) was about 78 s, which was 13 s higher than pure PP. At the same time, the PHRR was reduced by 42%, showing the enhancement of flame-retardant property of PP. The TGA and Cone calorimeter data for PP and its composites were listed in Table 7. The mechanism for C60 to improve the thermal stability and flame retardancy was mainly attributed to the ability of C60 to capture free radicals (Scheme 7). After melt blending, C60 still existed as crystallites and only a little C60 could freely move through Brownian motion. At a temperature about 260 °C, PP started to be oxidized and degraded, producing highly active free radicals like H• and HOO• and more stable radicals like macromolecular free radicals. And the C60 that could freely move would capture these free radicals, especially the more active radicals. Subsequently, the C60 as crystallites would release and participate in the free-radical addition reaction gradually. In particular, a single C60 can trap dozens of free radicals. Crosslink network would form as enough free radicals were captured by C60, resulting in a sharp increase in viscosity. On the one hand, the increased melt viscosity slowed down heat and oxygen exchange, leading to the increase in heat resistance. On the other hand, more energy and time were required for products generated by degradation of the underlying polymer to diffuse into the surface, delaying the combustion of polymer and reducing the heat release. However, the crosslink network would be destroyed as heated to higher temperature. Due to the excellent thermal stability of C60, most of the network would convert back into C60 molecule. The other part would be oxidized to carboxylic acid derivatives and small molecules such as CO2, CO, and other gas fuels. In summary, C60 indirectly improved the flame retardancy of polymer materials via capturing free radicals.
| Sample | C60 content (wt %) | Tonset (oC) | Tmax (oC) | tign (s) | tPHRR (s) | PHRR (kW m−2) | AHRR (kW m−2) | AMLR (g s−1) |
|---|---|---|---|---|---|---|---|---|
| PP | 0 | 264 | 338 | 24 | 65 | 1382 | 282 | 0.048 |
| PF1 | 0.5 | 271 | 364 | 37 | 76 | 930 | 234 | 0.037 |
| PF2 | 1 | 279 | 392 | 39 | 78 | 810 | 212 | 0.033 |
| PF3 | 2 | 284 | 399 | 38 | 82 | 750 | 186 | 0.028 |
- Tonset: temperature at which 5 wt % weight loss occurred; Tmax: temperature at which maximum weight loss occurred; tign: time to ignition; PHRR: peak heat release rate; AHRR: average heat release rate; tPHRR: time to PHRR; AMLR: average mass loss rate.
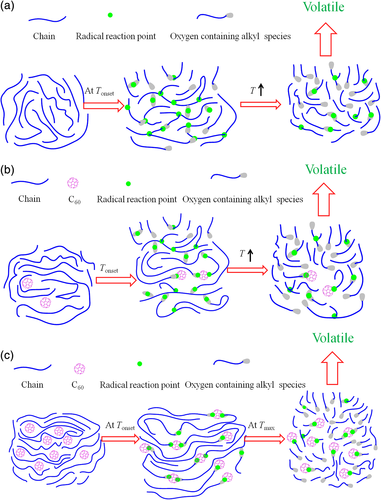
Similar conclusions were obtained in HDPE/C60 system by Zhao et al.52-55 C60 can trap macromolecular radicals generated during degradation to form a crosslinking network, enhancing the thermal and flame retardancy properties of HDPE. Compared with pure HDPE, the addition of 2.5 wt % C60 increased the Tonset in N2 and air by about 10 °C and 90 °C, respectively, the tign by 10 s and reduced the smoke density from 128.6 m2 kg−1 to 42 m2 kg−1. The schematic model in N2 was shown in Scheme 8. At low temperature (Tonset), the chain scission of HDPE was inhibited because of the production of alkyl-grafted C60, thereby increasing the thermal stability and ignition temperature of HDPE. As the temperature rose (Tmax), the structure of alkyl-grafted C60 and CC bonds was destroyed, resulting in more volatiles production and greater mass loss. Therefore, the influence of C60 on Tonset was greater than that of Tmax. The schematic model in air was presented in Scheme 9. The thermo-oxidative degradation of HDPE was mainly a process of radical chain scission: alkyl radicals and alkyl peroxide radicals could initiate the generation of a series of macromolecular radicals, which combined with each other to form the crosslink network. C60 could capture the alkyl peroxide radicals generated during the thermo-oxidative degradation of polymer. But this free-radical capturing effect is very sensitive to temperature. For HDPE/C60 composites, at the temperature above 240 °C, the free-radical trapping effect would occur. Alkyl radicals and alkyl peroxide radicals generated by polymer degradation reacted with C60 to become nonradical species, which retarded the hydrogen abstraction and chain scission. When the temperature reached to Tmax, more radicals were produced due to the breaking down of polymer chain and the chain scission could not be effectively suppressed. C60 is easier to trap alkyl peroxide radicals, and the degradation temperature in N2 was high enough to destroy the interaction between C60 and free radicals. Therefore, the effect of C60 on the improvement of thermal oxidative stability was more obvious than that of thermal stability.
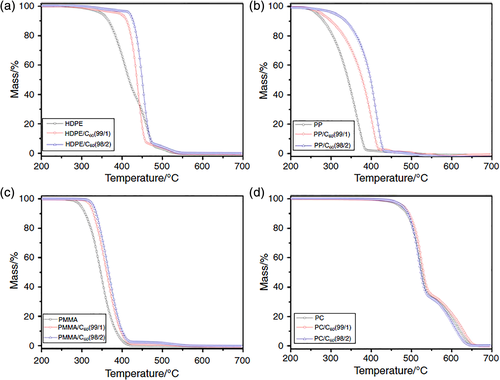
Zhao et al.55 also studied the effect of C60 on the thermal and flame-retardant properties of polymers with different degradation mechanisms and chemical structures, such as HDPE, PP, PMMA, and PC. Figure 5 represented the TG curves for HDPE/C60, PP/C60, PMMA/C60, and PC/C60 composites in air. It has been found that C60 could reduce the thermal degradation and flammability of polymers with free-radical degradation mechanism (HDPE, PP, and PMMA), especially to the thermal oxidative stability of these polymers. However, the presence of C60 made little influence on the thermal degradation of PC. The reason was that hydrolysis/alcoholysis and rearrangement of carbonate that were nonradical process play a leading part in the thermal decomposition of PC. It was further proved that the enhancement of thermal property and flame retardancy of polymer was resulted from the free-radical capturing effect of C60.
THE COOPERATION EFFECT OF C60 WITH OTHER FLAME RETARDANTS
Although C60 can improve the thermal and thermo-oxidative properties of polymers remarkably, it affects little in the flame-retardant performance of polymers when it uses alone. The cooperation of C60 and other flame retardants has gradually become one important aspect of the application of C60 in flame retardant.
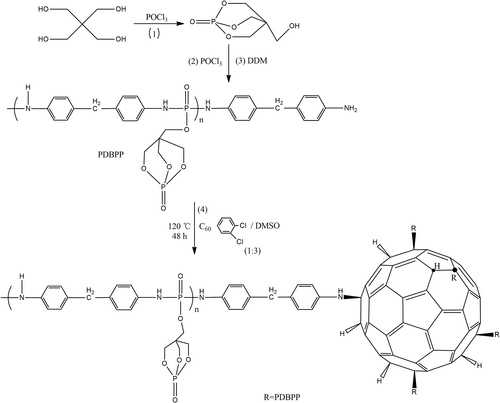
Song et al.56 has fabricated C60-decorated oligomeric intumescent flame-retardant poly (4,4-diaminodiphenylmethane-O-bicyclicpentaerythritol phosphate–phosphate) (PDBPP), C60-d-PDBPP via chemical grafting reaction. The synthetic route was presented in Scheme 10.
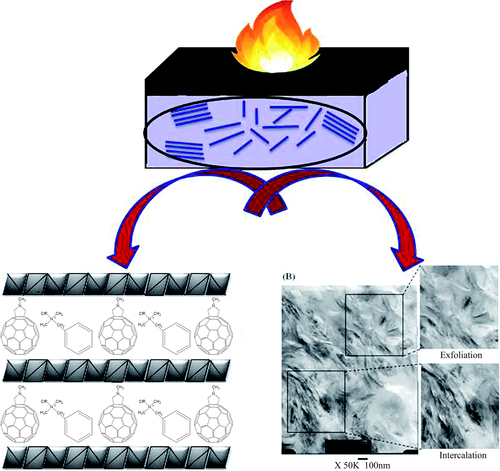
From the TGA results, it could be found that upon incorporating C60-d-PDBPP, thermal and thermo oxidation degradation of PP was considerably slowed down. In addition, this effect was more pronounced in air, and C60-d-PDBPP was much obvious than pure C60 in regard to improving the thermal oxidation degradation of PP. Pure PP began to thermal oxidative degradation at 264 °C (Tonset) and basically decomposed completely at 338 °C (Tmax). The Tonset and Tmax of PP containing 1 wt % C60-d-PDBPP were increased to 332 °C and 409 °C, respectively, which were about 68 °C and 71 °C higher than that of pristine PP. While in the case of PP containing 1 wt % pure C60, its Tonset and Tmax were 279 °C and 392 °C. It was same for the high activity to free radicals of C60-d-PDBPP and C60, but the ability to trap free macromolecular radicals of the former was weakened because of its large steric hindrance. In N2, the improvement of the thermal stability of PP just relied on the effect of capturing macromolecular radicals of C60-d-PDBPP, which was inferior to C60 due to its structure. However, the thermo-oxidative degradation of PP in air was mainly caused by oxygen. On the one hand, oxygen molecules were easily diffused into the melt due to their small size. Therefore, oxygen and its derived free radicals could be quickly captured by C60-d-PDBPP. On the other hand, PDBPP was more easily oxidized, consuming part of the oxygen and degraded into char than PP, which could protect PP in turn. Therefore, it was superior for C60-d-PDBPP in improving the thermo-oxidative stability of PP than C60. Moreover, the PHRR of PP was remarkably reduced and the time-to-ignition and time to PHRR were prolonged due to the addition of C60-d-PDBPP, which indicating that C60-d-PDBPP could confer super flame-retardant properties on PP. The better performance of C60-d-PDBPP on thermo-oxidative and flame-retardant properties on PP was attributed to the cooperation effect between the free-radical capturing of C60 and compact char layer formed by C60-d-PDBPP during combustion. Thus, dendrimer-like oligomeric intumescent flame retardant bridged with fullerene (C60-d-PDBPP) can be a more super flame retardant than pure C60 for composites.
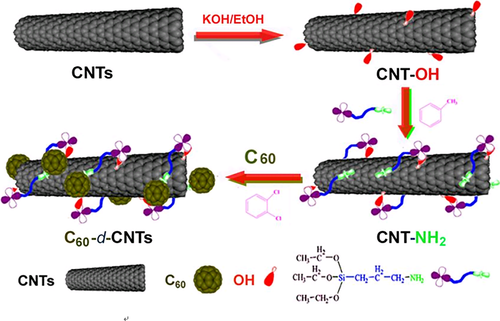
Song et al.57 also investigated the effect of covalently functionalized carbon nanotubes (CNTs) decorated with C60 (C60-d-CNT, as shown in Scheme 11) on thermal and flame-retardant properties of PP. Compared with pristine C60 and CNTs, the dispersity of C60-d-CNT was much better. Although there were some aggregates, single CNT could also be observed. The impact of C60-d-CNT on the improving oxidation resistance of PP was also superior to pure C60 and CNTs. After adding 1 wt % C60-d-CNT, the Tonset and Tmax in air were improved by 68 °C and 75 °C, respectively. While adding the same amount of CNTs, these two values were increased by 55 °C and 72 °C. The Tonset and Tmax of PP/C60-1 wt % were enhanced by 15 °C and 54 °C. In addition, the tign and tPHRR of PP/C60-d-CNT were significantly greater than those of PP/CNTs, suggesting the better flame-retardant effect of C60-d-CNT. These improvements were attributed to the better dispersion of C60-d-CNT and the lower critical content to form network for C60-d-CNT. In summary, the combination of free-radical capture effect of C60, shielding effect of the network formed by CNTs and the ceramic structure generated by the decomposition of silane coupling agent led to the further improvement of flame-retardant properties.
Subsequently, C60 decorated graphene oxide (GO) (Scheme 12) was synthesized successfully and used for flame retardant of PP58 and HDPE.59 Similarly, the dispersion of C60 decorated GO was better than that of pristine C60 and GO because of hybridization. The good cooperation effect of radical capturing effect of C60 and the protection of GO could remarkably improve the thermal and mechanical properties of polymers.
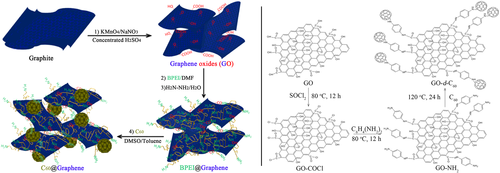
To overcome the shortcoming of brominated flame retardants (BFRs), releasing large of smoke and heat during combustion, Guo et al.60 introduced C60 into HDPE/BFR (decabromodiphenyl oxide/Sb2O3, BFR in short) system. For PE/BFR composites, bromine free radicals produced during thermal degradation or ignition would capture the chain radicals in the condensed phase, which was beneficial to the crosslinking reaction of PE. As temperature increased, the bromine free radicals would enter the gas phase and trap alkyl radicals in gas phase, improving the flame-retardant performance and releasing a large amount of smoke. For PE/BFR/C60 composites, at lower temperature, C60 was in preference to react with bromine free radical and excess bromine radicals or C60 continued to trap chain radicals in condensed phase inducing the decrease of flame-retardant performance at this temperature. As temperature increased, the chemical bonds between bromine radicals and C60 would break, bromine radicals entered into gas phase capturing free radicals in gas phase and C60 in condensed phase trapped free radicals in condensed phase, resulting in the increase of flame-retardant properties. Therefore, when the content of C60 is large enough, the flame-retardant performance can be improved both at the early and the late of combustion. From thermo-gravimetry and cone calorimeter, it was found that the higher addition of C60 enhanced the thermal and thermo-oxidative properties of HDPE/BFR composites. Moreover, the addition of C60 could significantly reduce the PHRR and the average specific extinction area. The effects of C60 on the rheological behaviors and pyrolysis products were also investigated. It was shown that C60 could trap alkyl radicals, chain radicals, and bromine radicals in the condensed phase, making the thermo-oxidative degradation terminate and the release of heat and smoke decrease.
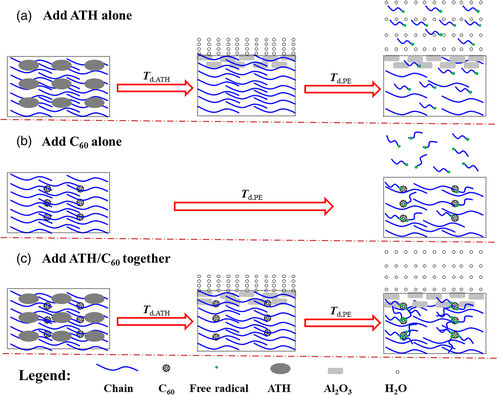
The traditional inorganic flame retardant, aluminum hydroxide (ATH), is environmentally friendly, but the low flame-retardant efficiency of ATH limits its application. A very high loading content is required to achieve satisfactory flame-retardant property, which leads to deterioration in the mechanical properties. Cooperated with C60, the total loading content of the flame retardant can reduce significantly.61, 62 In SBS/ATH/C60 system, the total content of the flame retardant (56 mass%) was lower than that in SBS/ATH system (60mass%) when UL-94 reached V-0 level. Moreover, the addition of C60 reduced the ATH content from 160 to 120phr in PE/ATH system on the premise of V-0 level in UL-94 test. The mechanism of synergy between ATH and C60 was presented in Scheme 13. A crosslinking network would form due to the free radicals capturing by C60 at the beginning of degradation, which inhibited the free radicals volatilize into gaseous phase. However, as temperature increased, the matrix decomposed violently, forming many free radicals and small molecules. And the crosslinking network could no longer prevent them from entering into gaseous phase, failing to achieve good flame-retardant effect. When heated, ATH would decompose to Al2O3, generating a cover layer that blocked the exchange of heat and substance in and out of the matrix. As C60 cooperated with ATH, the protecting layer hindered the release of free radicals, allowing C60 to have more time to capture free radicals and making the matrix more difficult to burn.
Benzalkoniumchloride-N-methyl pryrrolidine-fullerene (BEN-(C60-O)) were designed and synthesized by Tsai et al.,63 which could increase the flame retardancy of EP when cooperated with the functionalization of montmorillonite type clay (CL88). The introduction of BEN-(C60-O) enlarged the layer space of montmorillonite, made the EP/BEN-(C60-O)-CL88 composite achieve V1 rating in the UL-94 vertical burning testing and the LOI value reach 30. There were two reasons for these improvements. On the one hand, the better dispersion of CL88 lengthened the path of air into the matrix. On the other hand, a large number of CC groups in C60-O could capture the free radicals formed during combustion. Furthermore, the addition of BEN-(C60-O) reduced peak heat release rate and total heat release from 599.03 kW m−2 and 92.01 MJ m−2 to 567.77 kW m−2 and 86.45 MJ m−2, respectively. The significant decrease in THR demonstrated that the amount of organic matter being burned reduced, showing the better flame-retardant properties (Scheme 14).
CONCLUSIONS
Based on the high ability to trap free radicals, the addition of C60 can improve the thermal and flame-retardant properties of polymers by trapping free radicals generated during degradation. It has shown that C60 has higher activity for oxygen-containing free radicals, so the improvement of the thermo-oxidative stability of polymer is larger than that of the thermal stability. At initial combustion, the polymer is in an oxygen-containing environment. As combustion continues, oxygen is consumed and the polymer is in anaerobic environment. Consequently, C60 is more effective at initial combustion, which is in accordance with experimental results. Generally, the effect of fullerene derivatives is better than pure C60 because of its better compatibility with polymers. Although C60 can improve the thermal and thermo-oxidative properties of polymers remarkably, it affects little in the flame-retardant performance of polymers when it uses alone. The cooperation of C60 and other flame retardants has gradually become one important aspect of the application of C60 in flame retardant. And it has proved that C60 has positive effect with inorganic metal flame retardant, intumescent flame retardant, BFR, clay, carbon nanotubes, graphene oxide, and so on. It can be a good choice to use C60 or its derivatives in combination with other flame retardants.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundations of China (No. 51673173, 51703197) and the Major Industrial Projects of Ningbo City (No. 2015B11005).
Biographies

Yanqun Pan is a doctorate student of the Key Laboratory of Macromolecular Synthesis and Functionalization, Zhejiang University. She studied at the Department of Polymer Science and Engineering, Zhejiang University, from September 2010 to June 2014, and obtained her bachelor’s degree. Now, she continued her study on flame-retardant polymers with nanofillers under the supervision of Prof. Zhengping Fang.

Zhenghong Guo is an associate professor of the School of Biological and Chemical Engineering of Ningbo Institute of Technology, Zhejiang University. She obtained Ph.D. in Polymer Chemistry and Physics from Zhejiang University in 2007 under the supervision of Prof. Zhengping Fang. Now, she focuses on the study of polymer blends and composites and eco-friendly flame-retardant polymer materials.

Shiya Ran is an associate professor of the School of Biological and Chemical Engineering of Ningbo Institute of Technology, Zhejiang University. She got her Ph.D. degree in Department of Polymer Science and Engineering at Zhejiang University in 2016. She focuses her research on the modification of polymeric materials via blending and compounding, carbon nanomaterials, and rare earth compounds flame-retardant polymers.

Zhengping Fang is a professor and Dean of the School of Biological and Chemical Engineering of Ningbo Institute of Technology, Zhejiang University. He obtained his B.S. degree in Chemistry in 1983 and Master degree in Polymer Chemistry and Physics in 1986 from Fudan University of China. He has been engaged in research on polymer blends and composites and eco-friendly flame-retardant polymer materials for tens of years.




