Vijay Nair (1941–2024): A Beacon of Wisdom
Graphical Abstract
Vijay Nair, one of India's most prominent organic chemists, passed away on April 12, 2024 at the age of 82. After a distinguished research career in the U.S. pharmaceutical industry, he returned to India to lead what was to become the National Institute for Interdisciplinary Science and Technology (NIIST). During this second phase of his career, he made fundamentally important contributions to the art of organic synthesis and mentored a number of organic chemists.
Vijay Nair (Figure 1), one of India's prominent organic chemists, passed away on 12th April 2024 at the age of 82 in Trivandrum, the capital of the south Indian state of Kerala. For the last three decades, he lived in the same city, working in the Council of Scientific and Industrial Research (CSIR) Institute originally known as the Regional Research Laboratory (RRL), which was later rechristened as the National Institute for Interdisciplinary Science and Technology (NIIST). He was fifty years old when he joined the up-and-coming and rather little-known RRL in 1990, after a distinguished research career in the pharmaceutical industry in the U.S. In the years to follow, Nair made fundamentally important contributions to the art of organic synthesis, led the institute, mentored a number of organic chemists and in the process transformed the landscape of organic chemistry in India.

Vijay Nair.
Born in 1941 in the picturesque mountainside village of Konni in Kerala, Vijay Nair had his early education in the nearby schools and completed his BSc degree in Chemistry at the Catholicate College in Pathanamthitta. His fascination with chemistry took him to Banaras Hindu University (BHU) in the north of the country, where he obtained his MSc and PhD working with Prof. R. H. Sahasrabudhey. He left India for further research and did another PhD with Prof. J. P. Kutney at the University of British Columbia on the synthesis of veratrine alkaloids. This was followed by a postdoctoral stay in the laboratory of Prof. Josef Fried at the University of Chicago where he investigated enzymatic resolution of steroids. He moved to the University of Toronto to work with Prof. Peter Yates and then to Prof. Gilbert Stork's laboratory at Columbia University. As he had attested on numerous occasions, Vijay Nair found his ‘guru’ in Prof. Stork. His stay at Columbia was the beginning of a life-long association which was to be punctuated by a future visit by Prof. Stork to Trivandrum in 1994 and periodic pilgrimages that Vijay Nair undertook from Trivandrum to New York to meet Prof. Stork. Working with him, Nair accomplished a stereocontrolled synthesis of Djerassi-Prelog lactonic acid.1 From Prof. Stork's laboratory, he joined the Lederle Laboratories (Division of American Cyanamid Company) in Pearl River, New York. His sixteen-year-long stay at the Lederle Laboratories can be described as the first stage of his career. The “Outstanding Scientist Award” that he received in 1981 from the American Cyanamid Company was a remarkable achievement. To his own admission, however, what Vijay Nair cherished the most during his time in New York were his frequent visits to Prof. Stork's lab and the continued involvement in the research that was going on there.
All through his long stint the Lederle Laboratories, Nair kept his passion for investigations in synthesis alive. In 1990, he was offered a senior position as the Deputy Director at the then Regional Research Laboratory, Trivandrum under the Council of Scientific and Industrial Research (CSIR) of the Indian Government. He accepted the offer and soon devoted himself to setting up a research group focused on organic synthesis. He used to recollect with fondness the generous support he received for many subsequent years from his former employer, the American Cyanamid Company, in the form of a research grant.
In RRL, Vijay Nair's laboratory undertook investigations in various areas of synthesis. In particular, he was fascinated by the versatility of o-quinones and their derivatives, as they can manifest a variety of reactivity profiles (carbo/heterodiene, carbo/heterodienophile etc.) as depicted by the example in Scheme 1a. His efforts over an extended period demonstrated that almost all the reactive sites in o-quinones and related compounds can be selectively functionalised by a careful choice of reaction partners and conditions. Several fascinating and useful reactions of o-quinones that involved cycloadditions and rearrangements were developed in his group mostly in the 1990s.2
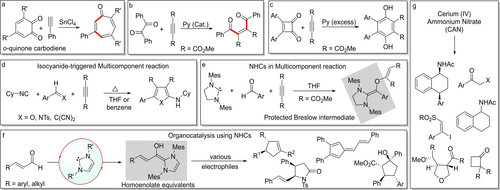
Snippets of the synthetic methods developed in Vijay Nair's laboratory.
Nair's interest in o-quinones stemmed from the unrealised potential of the carbodiene and the 1,2-dione subunits that they possessed, unlike their more illustrious cousins, p-quinones. The novel cycloaddition reactions of o-quinones that he developed indeed demonstrated the utility of the carbodiene fragment subsumed in o-quinones; however, these studies also revealed the high and unusual reactivity of the 1,2-dione unit. This eventually prompted Nair to explore the chemistry of 1,2-diones outside the o-quinonoid setting, in detail. As anticipated, the enhanced electrophilicity of the carbonyl groups made nucleophilic additions facile and rapid. However, a more intriguing feature emerged from these studies: that the C-C bond connecting the two carbonyls could be easily ruptured leading to the re-organisation of the 1,2-diones. Often, only very mild conditions were sufficed for these fragmentations, like the cut-and-paste type reactions in Scheme 1b and 1c.
The transformation depicted in Scheme 1b in fact is an intersection of two very fertile areas of investigations in Nair's research group, namely the chemistry of 1,2-diones and nucleophilic organocatalysis. Efforts in the latter area typically involved the union of two electrophiles, which would not generally react together, via the mediation of an aprotic nucleophilic organocatalyst. For example, catalytic amounts of pyridine were all that was needed for an excellent electrophile like dimethyl acetylene dicarboxylate (DMAD) to react with other common electrophiles such as aldehydes, N-sulfonylimines and Michael acceptors. This like-attracts-like type of addition is rationalised by invoking a zwitterionic intermediate formed via the addition of pyridine to DMAD.3
The zwitterions of this kind were known from the pioneering work of Prof. Rolf Husigen, and Nair drew inspiration from his illustrious contributions in the areas of 1,3- and 1,4-diploar cycloadditions. He maintained that the breadth and depth of Huisgen's contributions deserved more recognition and may have helped in sensitising the community to this end when he termed the triphenylphosphine-azodicarboxylate zwitterion (intermediate in the Mitsunobu reaction) as the “Huisgen zwitterion” for the first time. When the discovery of novel reactions of this zwitterion were made in the group, Nair was joyous mainly for finding a fitting tribute to Huisgen than for the usual excitement he showed for such transformations.4
Multicomponent reactions (MCRs) constitute another area of synthesis where Vijay Nair made outstanding contributions. His work on MCRs pivoted around the idea that the original nucleophile responsible for the above zwitterion formation may on occasions participate in the reaction. Such successful three-component reactions required a judicious combination of a nucleophile and two compatible electrophiles, that react selectively and sequentially. Pyridine, (iso)quinoline, dimethoxycarbene, isocyanides and even N-heterocyclic carbenes (NHCs) were successfully employed as nucleophiles in a wide variety of novel MCRs that allowed access to a diverse array of products. The isocyanide-DMAD zwitterion, originally described by Winterfeldt, was tamed in Nair's lab to undergo smooth cyclisation reactions with intercepting agents such as aldehydes, imines, quinones, and dicyanoalkenes (Scheme 1d). Taken together, the isocyanide-based reactions developed by Nair form a major group of MCRs that are distinct from the classical Passerini-Ugi type MCRs.5
Vijay Nair deemed the participation of NHCs in MCRs as highly remarkable for two major reasons. Firstly, they demonstrated utility of NHCs as nucleophilic one-carbon synthons in addition to their well-known applications as catalysts and ligands. Secondly, the three-component reaction of NHC, aldehydes and DMAD afforded a product that resulted from the covalent trapping of the purported Breslow intermediate. The structure of the Breslow intermediate-DMAD adduct, confirmed by single-crystal X-ray diffraction, was indirect but strong evidence in support of Breslow's original 1958 proposition (Scheme 1e).6
Notwithstanding the above significance, the most important contribution to NHC chemistry from Nair's group perhaps were not NHC-MCRs, but their catalytic applications. In particular, the 2006 report of the stereoselective cyclopentene formation in the addition of NHC-homoenolates to chalcones via the intermediacy of bicyclic β-lactones attracted widespread attention and led to a flurry of activity in the area. Through his efforts in subsequent years, the versatility and utility of NHC-homoenolates were expanded considerably (Scheme 1f).7
Work in Vijay Nair's group also established the utility and versatility of cerium(IV) ammonium nitrate (CAN) as an oxidant. A plethora of practical and convenient oxidations that showcased CAN's tremendous range of oxidative power were developed through investigations over a period of a decade and a half (Scheme 1g).8
Vijay Nair served as the Director of the Institute, CSIR-NIIST, during the period 1997-2001. He led with far-sightedness, inclusivity and exemplary pragmatism that set in motion the growth of a modest laboratory in the southern corner of India to become an internationally visible institute. At the dawn of the new century, Nair found himself free from administrative responsibilities and continued his research in the next two decades to make much of his notable contributions. He was recognised and supported by CSIR in the form of an Emeritus Scientist award and by the Department of Science and Technology (DST) via the Raja Ramanna Fellowship. He was also honoured as a Fellow of the Indian Academy of Sciences and the Chemical Research Society of India (CRSI) awarded him their Silver Medal in 2006 and later the Gold Medal for Lifetime Contributions in 2017.
Vijay Nair was a prolific and popular mentor. In his three-decade-long career in CSIR-NIIST, he supervised 53 PhD scholars and more than 200 Masters students, dozens of post-doctoral fellows, and Junior Scientists. He guided his disciples gently, and all of them fondly remember the unbound creative freedom, care and continued support that they enjoyed. Regular group dinners, attended also by his wife Dr. Sita Nair, a senior paediatrician in Trivandrum, and daughters Dr. Vineeta Nair (paediatrician) and Dr. Neelima Nair (organic chemist), were occasions of enjoyment. Alumni of the Nair group are now recognisable as organic chemists, academicians, industry managers, and entrepreneurs all around the globe. Friends and colleagues loved and respected Nair for his warmth and wisdom, and he was often consulted for advice on professional as well as personal matters.
Vijay Nair will be remembered not just for his valuable contributions to the field of organic synthesis, but for transforming a large number of raw, young minds into well-trained organic chemists and global citizens. And, he will be missed too.





