Ferroptosis Inducing Co(III) Polypyridine Sulfasalazine Complex for Therapeutically Enhanced Anticancer Therapy
Graphical Abstract
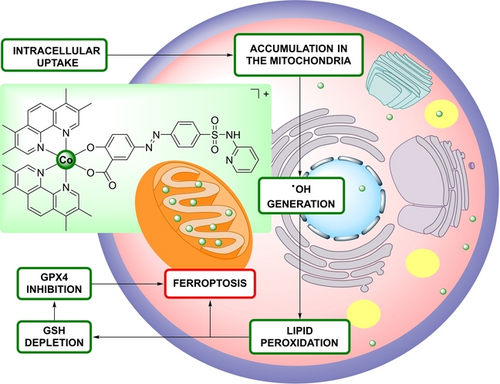
Targeting ferroptosis provides a novel avenue to overcome the limitations of apoptotic anticancer treatments. The Co(III) polypyridine sulfasalazine complex described here acts as a potent ferroptosis inducer for anticancer therapy. This complex selectively accumulates in mitochondria, generating reactive oxygen species, initiating lipid peroxidation, depleting glutathione, inhibiting glutathione peroxidase 4, and ultimately driving ferroptosis.
Abstract
Despite significant improvements in the treatment of cancerous tumors in the last decades, cancer remains one of the deadliest diseases worldwide. To overcome the shortcomings of currently applied chemotherapeutic treatments, much research efforts have been devoted towards the development of ferroptosis inducing anticancer agents. Ferroptosis is a newly described form of regulated, non-apoptotic cell death that is associated with high potential inside the clinics. Herein, the chemical synthesis and biological evaluation of a Co(III) polypyridine sulfasalazine complex as a ferroptosis inducer is reported. Upon entering the cancerous cells, the metal complex primarily accumulated in the mitochondria, triggering the production of hydroxy radicals and lipid peroxides, ultimately causing cell death by ferroptosis. The compound demonstrated to eradicate various monolayer cancer cells as well as colon carcinoma multicellular tumor spheroids. To the best of our knowledge this study reports on the first example of a Co(III) complex that is capable of inducing ferroptosis.
Introduction
Cancer is one of the leading causes of death worldwide. Recent statistics from the World Health Organization have revealed that approximately 10 million individuals died from cancer only in the year 2020. Projections suggest a significant increase in annual new cancer diagnoses up to 29.5 million along with a rise in cancer-related deaths to 16.4 million by the year 2040.1 The vast majority of clinically applied chemotherapeutic agents interact in cancer cells upon induction of apoptosis. Despite their frequent clinical application, these compounds are typically associated with poor therapeutic efficiencies and high tendencies to drug resistance.2 To overcome these limitations, there is a need for the development of new types of chemotherapeutic agents that interact through different mechanisms.
Among the most promising anticancer mechanisms, much research efforts have been devoted to ferroptosis inducing compounds.3 Ferroptosis is a distinct type of programmed cell death that is characterized by the iron catalyzed production and accumulation of lipid peroxides (LPO). Lipid peroxidation occurs when oxidants, like reactive oxygen species (ROS), attack polyunsaturated fatty acids.4 The hydroxyl radical (⋅OH) is a highly reactive and mobile ROS that is especially impactful on lipids. ⋅OH radicals can be formed in biological systems using the Fenton or Haber-Weiss reactions from hydrogen peroxide or superoxide.5 Notably, ferroptosis can also be triggered by intrinsic pathways, involving the inhibition or exhaustion of glutathione peroxidase 4 (GPX4), or extrinsic pathways, involving the inhibition of the cystine/glutamate transporter.6 Once the glutathione-dependent antioxidant defenses are overexerted, lipid peroxidation becomes unstrained and leads eventually to cell death by ferroptosis.7 Based on this mechanistic insight, ferroptotic cell death could be induced by a chemical compound that produces ⋅OH radicals or inhibits/depletes the antioxidant defenses system, resulting both in unstrained intracellular lipid peroxidation. While several molecular organic agents as well as nanoparticle formulations have been reported as ferroptosis inducers,8 molecular metal complexes as ferroptosis triggering compounds remain highly rare (overview of previously reported ferroptosis inducing metal complexes: Figure S1).9 To the best of our knowledge, there are no reports on Co complexes as ferroptosis inducing agents in the literature.
Herein, the chemical synthesis and biological evaluation of a Co(III) polypyridine sulfasalazine complex (3, Figure 1) as a ferroptosis inducer is reported. The metal complex was found to be broadly active against various cancerous cell lines with a cytotoxicity in the low micromolar range. Mechanistic studies demonstrated that the compound selectively accumulated in the mitochondria and induced the production of ⋅OH and LPO. Once the antioxidant defenses reliant on GSH and GPX4 are overwhelmed, lipid peroxidation proceeds uncontrollably, ultimately resulting in cell death through ferroptosis. A deeper biological assessment in mouse colon carcinoma multicellular tumor spheroids, that represent a model system for clinical solid tumors, demonstrated a strong therapeutic effect inside the core of the three-dimensional cellular structure.
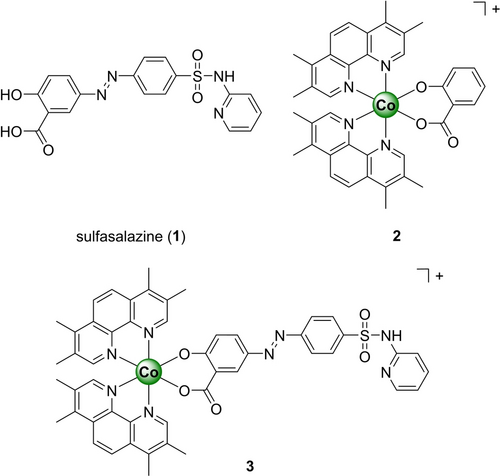
Chemical structures of the compounds investigated in this study. The metal complexes were isolated as hexafluorophosphate salts.
Results and Discussion
Sulfasalazine (1, Figure 1, sold under the brand name Azulfidine) is an anti-inflammatory drug that is clinically applied for the treatment of rheumatoid arthritis, ulcerative colitis, or Crohn's disease.10 Recent studies have reported that the therapeutic effect of commonly applied anticancer drugs such as piperlongumine,11 gemcitabine,12 or cisplatin13 can be enhanced upon combination treatment with sulfasalazine. Mechanistic findings demonstrated that sulfasalazine is able to weaken with the glutathione-dependent antioxidant defense system through the depletion of GSH in the cells, rendering the cancer cells more susceptible to the anticancer treatment.14
Previous studies have demonstrated that the incorporation of an organic therapeutic agent onto a Co(III) complex scaffold can enhance the anticancer effect.15 In particular, Co(III) polypyridine complexes have been previously reported as highly stable and therapeutically active compounds.16 Capitalizing on this, herein, the application of a Co(III) polypyridine sulfasalazine complex 3 as an anticancer agent was proposed and thoroughly investigated.
Co(II) chloride was oxidized with chlorine gas, that was in situ generated by mixing potassium permanganate and concentrated hydrochloric acid, to a Co(III) intermediate. This intermediate was heated at reflux temperatures with two equivalents of the ligand 3,4,7,8-tetramethyl-1,10-phenanthroline to form the [Co(3,4,7,8-tetramethyl-1,10-phenanthroline)2Cl2][Cl] precursor. The precursor was further treated with a mixture of silver(I) triflate and silver(I) oxide and the formed silver chloride, that precipitated from the solution, was removed by filtration. Under basic conditions hydroxybenzoic acid (Scheme S1) or 1 (Scheme S2) were added and the desired metal complexes 2 and 3 were obtained. All compounds were characterized by NMR spectroscopy and electron spray ionization mass spectrometry, and the purity confirmed by high-pressure liquid chromatography (HPLC) and elemental analysis (Figure S2–S5).
The solubility of the metal complexes 2 and 3 was evaluated using dynamic light scattering measurements. Dimethyl sulfoxide stock solutions of the Co(III) complexes were prepared and then diluted in phosphate-buffered saline (PBS) to reach a dimethyl sulfoxide concentration of 0.1 %. The formation of any precipitate or particles was monitored by dynamic light scattering measurements. No particle formation or aggregation was observed, suggesting aqueous solubility under physiological conditions. The stability of compounds under physiological conditions is crucial for a biological application to assure a safe and efficient therapeutic effect as unwanted degradation might lead to low-efficacy and side effects.17 To evaluate the stability of the metal complexes under physiological conditions, the metal complexes were incubated in PBS for 48 h and analyzed by HPLC. No changes in the chromatogram were observed (Figure S6–S7), suggesting stability of these metal complexes under physiological conditions. To investigate the potential ligand release from the metal complex, 3 was incubated with GSH or sodium ascorbate as cellular reducing agents and the stability monitored by HPLC. No significant changes were observed, indicative of the stability under cellular reducing conditions (Figure S8). Overall, the metal complexes were found to be soluble and stable under physiological conditions.
The ability of the components sulfasalazine 1 and 3,4,7,8-tetramethyl-1,10-phenanthroline as well as the Co(III) complexes 2 and 3 to generate ⋅OH radicals, singlet oxygen (1O2) or ⋅O2− was studied by electron spin resonance spectroscopy. The compounds were dissolved in DMEM cell media, that has been supplemented with GSH as a cellular reducing agent, incubated with the ⋅OH and ⋅O2− scavenger 5,5-dimethyl-1-pyrroline N-oxide or the 1O2 scavenger 2,2,6,6-tetramethylpiperidine, and afterwards the electron spin resonance spectrum recorded. While no signals in the spectra for 1, 3,4,7,8-tetramethyl-1,10-phenanthroline, and 2 were monitored, the characteristic signal for ⋅OH radicals upon incubation of 3 was measured (Figure S9–S10). As a complementary technique, the time-dependent production of 1O2 with the specific probe singlet oxygen sensor green, ⋅O2− with the specific probe DHR123, or the ⋅OH with the specific probe 3′-p-(hydroxyphenyl) fluoresceine was studied. While these probes are poorly fluorescent in aqueous solution, the compound are oxidized by the specific type of ROS, forming a highly fluorescent fluoresceine derivative that could be monitored by fluorescence spectroscopy. While no changed for the 1O2 and ⋅O2− specific probes were observed upon incubation with 3, a strong fluorescence signal was observed using the ⋅OH specific probe (Figure S11). Combined these findings suggest that 3 is able to produce ⋅OH species.
The lipophilicity of the Co(III) complexes 2 and 3 was evaluated by measuring their distribution coefficient between the PBS and octanol phase (logP) using the “shake-flask” method. The metal complexes were found in the PBS and the octanol phase and therefore are of combined lipophilic and hydrophilic nature (2: logP=+0.6±0.1, 3: logP=0.4±0.1), which is considered ideal for a drug candidate (Table S1). The cell membrane permeability was simulated using a parallel artificial membrane permeability assay. The metal complexes 2 and 3 were found to exhibit high permeability rates (2: 0.034±0.003 μm/s, 3: 0.047±0.003 μm/s), based on comparisons to well-characterized control compounds with established permeability rates (Table S2). Overall, the metal complexes demonstrated to be partly hydrophilic and partly lipophilic as well as being able to efficiently penetrate an artificial cell membrane.
Based on these promising pharmacological properties, the therapeutic effects of the metal complexes 2 and 3 were studied in comparison to its components (sulfasalazine 1, 3,4,7,8-tetramethyl-1,10-phenanthroline, hydroxybenzoic acid, cobalt(II) chloride) as well as the anticancer drug cisplatin in cancerous mouse colon carcinoma (CT-26), human breast adenocarcinoma (MCF-7), human hepatocellular carcinoma (Hep G2), human leukemia (THP-1), human pancreatic adenocarcinoma (PT-45), and non-cancerous human fibroblast (GM-5657), mouse embryonic fibroblast (MEF), and human embryonic kidney (HEK-293) cells (overview: Table 1, drug response curves: Figure S12–S19). The cells were incubated with the compounds for 4 h, afterwards washed to remove any compound that has not been internalized in this time frame, and the cell viability assessed using the dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). The individual components including the respective ligands and metal precursor 3,4,7,8-tetramethyl-1,10-phenanthroline, hydroxybenzoic acid, and cobalt(II) chloride were found to be non-toxic (IC50>100 μM) in all tested cell lines. The cytotoxicity of 1 demonstrated to be highly dependent on the evaluated cell line. While the compound was found to be non-toxic in Hep G2, PT-45, GM-5657, MEF, and HEK-293 cells (IC50>100 μM), the compound showed a weak therapeutic effect in CT-26 (IC50=71.32±7.46 μM), THP-1 (IC50=41.53±2.40 μM) and MCF-7 (IC50=27.61±1.33 μM) cells. The Co(III) polypyridine complex 2 demonstrated a weak cytotoxicity in all evaluated cell lines (IC50>100 μM). The mixture of 1 and 2, referred to as 1+2, was found to be poorly cytotoxic in the same range as 1 alone. Strikingly, the Co(III) polypyridine sulfasalazine complex 3 was found with a cytotoxic effect in all cancer cell lines in the low micromolar range (CT26: IC50=1.04±0.32 μM, MCF-7: IC50=2.45±0.26 μM, Hep G2: IC50=4.63±0.37 μM, THP-1: IC50=2.33±0.46 μM, PT-45: IC50=1.91±0.13 μM). Interestingly, the compound was found with a higher cytotoxicity towards these cancer cells lines than towards the evaluated non-cancerous cell lines (GM-5657: IC50=12.05±1.37 μM, MEF: IC50=11.16±1.22 μM, HEK-293: IC50=13.69±1.57 μM). Notably, upon prolongation of the incubation time to 48 h, a therapeutic effect of the ligands in the micromolar range and the metal complex in the nanomolar range was observed (Table S3, drug response curves: Figure S20). The cellular uptake of the metal complexes 2 and 3 was determined by incubation for 4 h and analysis of the metal content by inductively coupled plasma optical emission spectroscopy (ICP-OES). The results showed that 3 had a higher cellular uptake than 2 that could partly explain the difference in cytotoxicity (Figure S21).
Compound |
Cancerous Cell Lines |
Non-Cancerous Cell Lines |
||||||
|---|---|---|---|---|---|---|---|---|
CT-26 |
MCF-7 |
Hep G2 |
THP-1 |
PT-45 |
GM-5657 |
MEF |
HEK-293 |
|
cisplatin |
6.04±0.34 |
7.48±0.36 |
9.04±0.45 |
5.86±0.51 |
8.06±1.17 |
8.64±1.03 |
12.17±1.53 |
8.43±0.87 |
phenme [a] |
>100 |
>100 |
>100 |
>100 |
>100 |
>100 |
>100 |
>100 |
sal [b] |
>100 |
>100 |
>100 |
>100 |
>100 |
>100 |
>100 |
>100 |
CoCl2 |
>100 |
>100 |
>100 |
>100 |
>100 |
>100 |
>100 |
>100 |
1 |
71.32±7.46 |
27.61±1.33 |
>100 |
41.53±2.40 |
>100 |
>100 |
>100 |
>100 |
2 |
>100 |
>100 |
>100 |
>100 |
>100 |
>100 |
>100 |
>100 |
1+2 |
64.71±5.30 |
23.29±4.86 |
86.70±8.35 |
36.04±5.27 |
>100 |
>100 |
>100 |
>100 |
3 |
1.04±0.32 |
2.45±0.26 |
4.63±0.37 |
2.33±0.46 |
1.91±0.13 |
12.05±1.37 |
11.16±1.22 |
13.69±1.57 |
- [a] phenme=3,4,7,8-tetramethyl-1,10-phenanthroline. [b] sal=hydroxybenzoic acid.
Based on the high therapeutic effect in CT-26 cells, further studies of 3 were performed in cancerous CT-26 cells. Analogously, the therapeutic effect was concentration-dependent visualized with the cell live/dead calcein AM/propidium iodide stain. While the cell population treated with low concentration of 3 consisted of living cells, the number of dead cells increased upon treatment with higher concentrations of 3 (Figure S22), verifying the therapeutic effect of the metal complex. Combined these results suggest the cytotoxicity of compound 3 in the low micromolar range in comparison to its individual components.
For an insight into the mechanism of action, the subcellular localization of the metal complex was studied upon careful extraction of the major cellular organs (nucleus, lysosomes, mitochondria, and cytoplasm) and determination of the respective metal content by ICP-OES. While only minimal amount of the compound was localizing in the nucleus (4.1±3.4 %) and the lysosomes (10.0±4.2 %), 3 was primarily found in the mitochondria (51.2±7.5 %) (Figure 2). Previous studies have demonstrated that a subcellular localization inside the mitochondria is ideal for anticancer drug development due to its function in energy regulation, metabolism, and induction of programmed cell death pathways.18
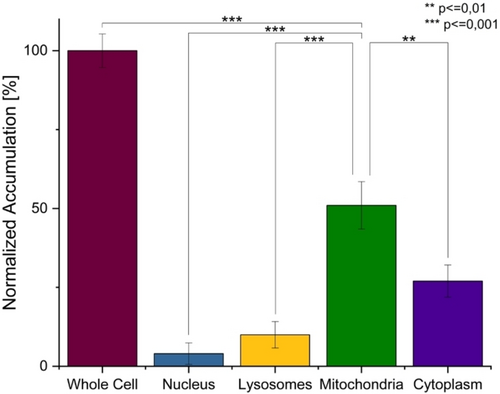
Subcellular accumulation of 3 in CT-26 cells after 4 h of incubation determined by ICP-OES. Statistical analysis was performed by one-way ANOVA with Tukey's multiple comparisons test. The error bars correspond to the standard deviation of the three replicates.
The ability of the metal complexes to generate ROS inside the cancerous cells was assessed by fluorescence microscopy using the ROS specific probe 2′,7′-dichlorodihydrofluorescein diacetate. While this dye is non-emissive in human cells, the fluorophore gets oxidized in the presence of ROS, generating a strongly green-fluorescent molecule. The microscopy images of the cancerous cells that were treated with 1 or 2 did not show any green emission, indicative that these compounds did not generate ROS. Contrary, a strong green fluorescence signal was observed inside the cancerous cells upon treatment with 3, suggestive of its ability to efficiently produce ROS (Figure S23). For a deeper understanding, studies into the identification of the type of ROS, including ⋅OH, 1O2, or ⋅O2−, generated by the metal complex were pursued. The cancerous cells were preincubated with ⋅OH (D-mannitol), 1O2 (sodium azide), or ⋅O2− (4,5-dihydroxy-1,3-benzenedisulfonic acid disodium salt monohydrate) specific scavengers, that supress the ability of the metal complex to generate cytotoxic ROS species, treated with the IC50 value of 3 (1.26 μM) for 4 h, and the cell viability determined using a MTT assay. As no changes in the cell survival upon preincubation with 1O2 and ⋅O2− scavengers were observed, these types of ROS were ruled out. Contrary, the preincubation with ⋅OH scavengers were found to strongly enhance the cell viability (Figure 3A), suggestive of a mechanism of action of 3 involving ⋅OH species. As a complementary technique, the identification of the type of ROS produced by the metal complex was investigated by fluorescence microscopy using the ⋅OH (3′-p-(hydroxyphenyl) fluorescein), 1O2 (singlet oxygen sensor green), or ⋅O2− (dihydroethidium) specific fluorophores. While no emission of the 1O2 and ⋅O2− probe molecules were observed, upon treatment of the cancerous cells with 3 and incubation with the ⋅OH probe strong green emission was observed (Figure 3B). Overall, these results suggest that 3 is able to efficiently produce ⋅OH inside the cancerous cells.
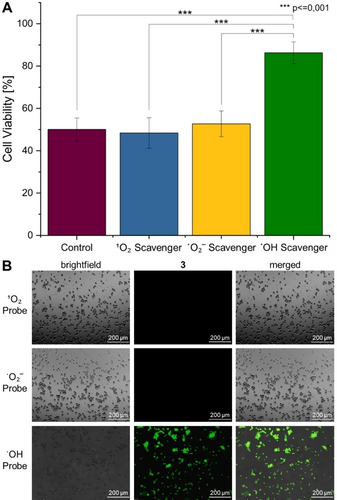
Identification of the type of ROS generated by 3 in CT-26 cells. A) Quantification of the cell viability upon pre-incubation with ⋅OH (50 mM, D-mannitol), 1O2 (5 mM, sodium azide), or ⋅O2− (5 mM, 4,5-dihydroxy-1,3-benzenedisulfonic acid disodium salt monohydrate) specific scavengers, that supress the ability of the metal complex to generate cytotoxic ROS species, treatment with the IC50 value of 3 (1.26 μM), and determination of the cell viability determined using a MTT assay. Statistical analysis was performed by one-way ANOVA with Tukey's multiple comparisons test. The error bars correspond to the standard deviation of the three replicates. B) Fluorescence microscopy images of CT-26 cells upon co-incubation with the IC50 value of 3 (1.26 μM) and the ⋅OH (3′-p-(hydroxyphenyl) fluorescein, λex=460–490 nm, λem=517–527 nm), 1O2 (singlet oxygen sensor green, λex=460–490 nm, λem=517–527 nm), or ⋅O2− (dihydroethidium, λex=545–580 nm, λem=617 nm) specific fluorophores.
The ability to induce oxidative stress inside the mitochondria, as the primary localization of the metal complex, was studied upon monitoring of the mitochondria membrane potential using the specific dye JC-1. While the fluorophore aggregates with a red fluorescence when the cell contains intact mitochondria, the fluorophore forms monomers when the mitochondria membrane potential drops with a green fluorescence. The microscopy images showed that upon treatment of the cancerous cells with 3 only the monomeric species of the dye was observed, suggestive of the loss of the mitochondria membrane potential (Figure S24). The loss of mitochondrial membrane potential could also be verified by morphological examinations using ultrastructural transmission electron microscopy (TEM) analyses. In contrast to the untreated samples (Figure 4A and B, arrows), the ferroptotic cells treated with 3 revealed a dramatic alteration of the mitochondrial ultrastructure, especially in their cristae. The cristae depicted a loosened to complete dissolved cristae arrangement, which is in agreement with the literature (Figure 4C–G, arrows).19 These results indicate that treatment with 3 leads to morphological mitochondrial deformation, resulting in a loss of mitochondrial membrane potential and thus inactive or dysfunctional mitochondria.
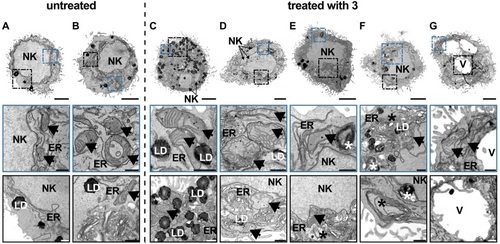
Ultrastructural TEM analysis of CT-26 cells without and after treatment with 3. A–B. Representative TEM images of untreated cells revealed no ultrastructural damage to the cell organelles. C–G. Ultrastructural analysis of CT-26 cells with treatment 3 (n=3) depicted damaged mitochondrial cristae (arrows), enlargement of the cell with no rupture in cell membrane and presumed initial fragmentation of the nucleus (NK). In addition, expanded and increased occurrence of the endoplasmic reticulum (ER) and accumulation of lipid droplets (LD), as well as lysosomes, autophagosomes and multilaminar bodies (asterisks) were visible. Probably advancing ferroptosis also indicated vacuole formation (V). Overview scale bar: 3 μm and detailed images: 0.5 μm.
To further elucidate the mechanism of action of the Co(III) polypyridine sulfasalazine complex, the cell death mechanism triggered by the metal complex was studied. The cancerous cells were preincubated with autophagy (3-methyladenine), apoptosis (Z-VAD-FMK), paraptosis (cycloheximide), necrosis (necrostatin-1), ferroptosis (ferrostatin-1) inhibitors, or an iron chelator (deferoxamine), that supress the ability of the metal complex to induce a specific cell death mechanism, treated with the IC50 value of 3 (1.26 μM) for 4 h, and the cell viability determined using a MTT assay. As the preincubation with autophagy, paraptosis, and necrosis inhibitors did not significantly change the cell survival, these cell death mechanisms were ruled out. In contrast, the preincubation with apoptosis inhibitors caused a slight increase of the cell viability, while with ferroptosis inhibitors and iron chelators a drastic increase was observed (Figure S25). These results suggest that 3 triggers cell death through a multimodal mechanism primarily involving ferroptosis and secondarily apoptosis. For further insight into the apoptosis cell death mechanism its dependency on the caspase 3/7 pathway was studied using a caspase 3/7 glo assay. These caspases are known executers of extrinsic and intrinsic apoptosis pathways.20 The results of the assay showed that the treatment of the cancerous cells with 3 elevated the caspase 3/7 activity (Figure S26).
The ability of the Co(III) polypyridine sulfasalazine complex to trigger cell death by ferroptosis was further investigated upon monitoring of the ferroptosis specific hallmarks. Ferroptosis is particularly characterized by the reaction of ⋅OH species with naturally occurring polyunsaturated fatty acids to generate highly cytotoxic LPO species. The presence of LPO inside of metal complex treated cancer cells was investigated by fluorescence microscopy using the specific probe BODIPY581/591 C11. The microscopy images showed no emission upon treatment with 2 and only slight fluorescence signals in the cells upon treatment with 1. These findings are in agreement with previous studies that have demonstrated that 1 could produce LPOs in certain types of cancer cells.21 Using the same concentration, a much stronger fluorescence signal of the LPO specific probe was observed upon treatment with 3, suggestive of high concentrations of LPO inside the cancer cells (Figure 5).
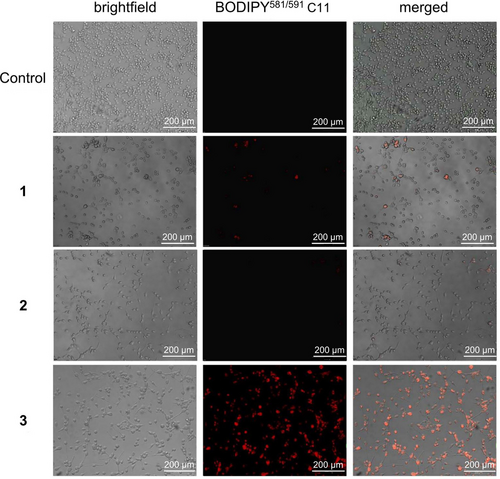
Evaluation of the generation of LPOs by 1–3 in CT-26 cells. Fluorescence microscopy images of CT-26 cells upon co-incubation of 1–3 (1.26 μM) and the LPO specific probe BODIPY581/591 C11 (5 μM, λex=460–490 nm, λem=510 nm).
TEM analyses were then used to examine the effects of the increased LPO concentration morphologically in more detail. These confirmed that the cellular stress caused by the increased LPO concentration resulted in ultrastructural damage to several cell organelles (Figure 4). In addition to the alterations of the mitochondrial cristae (C–G, arrows), other morphological hallmarks of ferroptosis could be observed. TEM analysis revealed an obvious enlargement of the cells with initial deformation of the cell membrane, which, however, was still intact and not ruptured at this point of treatment. Although cellular stress also leads to DNA damage, the nucleus of the ferroptotic cells looked normal and revealed no DNA condensation. Only an early fragmentation of the nucleus (NK) could be suspected. Further hallmarks of ferroptosis were an enlarged and more frequent endoplasmic reticulum (ER) as well as an increased accumulation of lipid droplets (LD), which overall indicates a disturbed homeostasis. As ferroptosis promotes enhanced autophagy, an increased presence of lysosomes, autophagosomes and multilamellar bodies (asterisks) to destroy damaged cell organelles was also observed. It was also found that in a presumably advanced stage of ferroptosis, cellular swelling with accompanying vacuole formation occurs. All these morphological alterations indicate cell death due to ferroptosis and correspond to the findings described in the literature.19
Within cancerous cells naturally occurring LPO are neutralized with GSH as part of the antioxidant defense system, resulting in the decrease of the GSH concentration. Using the GSH specific probe ThiolTrackerTM Violet, the concentration of GSH inside the cell could be estimated by fluorescence microscopy. While the cancer cells treated with 2 revealed a strong violet emission, indicating high concentrations of GSH, the cells treated with 1 or 3 depicted only a very low fluorescence, suggesting a depletion of GSH levels (Figure S27). Complementary, the GSH concentration inside the cancer cells was quantified using a commercial colorimetric assay. In comparison to the control cells, the cancer cells treated with 3 demonstrated a reduction of GSH levels by 67±6 % (Figure S28). The conversion of NADPH to NADP+ was studied by monitoring the NADPH/NADP+ ratio using a commercial glo assay. While the incubation with compounds 1 and 2 did not influence the NADPH/NADP+ ratio, 3 strongly reduced the NADPH/NADP+ ratio (Figure S29). Besides the depletion of GSH during ferroptosis, the expression levels of the enzyme GPX4 is also typically reduced. To investigate whether the intracellular levels of GPX4 are changed during the treatment with the metal complexes, a Western Blot analysis with GAPDH as an internal reference was performed. The Western Blot image showed that treatment with 1 and 2 did not significantly influence the expression levels of GPX4 inside the cancer cells. Contrary, the treatment of the cancer cells with 3 strongly reduced the levels of GPX4 (Figure S30). Overall, these findings suggest that the generated ⋅OH species, that are generated upon treatment with 3, are able to produce LPOs that overwhelm the antioxidation system of the cancer cells through depletion of GSH and GPX4 levels. Based on these findings, as the biological mechanism of action the reduction of the Co(III) complex to a stable Co(II) intermediate with GSH is proposed. Using Fenton-like chemistry, this intermediate could produce ⋅OH which promote the generation of LPO and oxidative stress inside the cancer cells. As cellular antioxidants GSH and GPX4 are able to transform these highly reactive species into harmless alcohols, triggering the depletion of GSH, NADPH, and the reduction of the expression of GPX4 (Figure S31). Combined these effects results in the efficient induction of cell death by ferroptosis.
Based on the promising biological effects in two-dimensional monolayer cancer cells, the therapeutic properties were further studied in CT-26 multicellular tumor spheroids. Multicellular tumor spheroids (MCTS) are a widely used model to mimic the pathological conditions of solid tumors.22 To evaluate the therapeutic efficiency of 3, a commercial CellTiter-Glo 3D Cell Viability kit, which determines the intracellular ATP concentration in living cells, was used. The Co(III) polypyridine sulfasalazine complex 3 was found with a cytotoxic effect in the low micromolar range (IC50=6.34±1.87 μM) against CT-26 multicellular tumor spheroids with an average diameter of approximately 250 μm. For a deeper understanding of the long-term therapeutic effects, CT-26 multicellular tumor spheroids were treated with 1–3 or cisplatin (6.34 μM) and the tumor growth of the spheroid daily monitored over a period of nine days (Figure S32). The treatment with 1 or 2 showed an exponential tumor growth similar as the control multicellular tumor spheroids that were treated with PBS. The treatment with cisplatin demonstrated a slightly reduced tumor growth. Contrary, the treatment with 3 had a strong tumor growth inhibition effect (Figure 6A), indicative of the superior therapeutic efficiency of 3 in comparison to cisplatin. For an insight into the treatment of larger tumors, the therapeutic effects of the compounds were further studied in CT-26 multicellular tumor spheroids with an average diameter of approximately 500 μm. Previous studies have demonstrated that larger multicellular tumor spheroids are significant more challenging to be eradicated as smaller multicellular tumor spheroids due to proliferation gradients and hypoxic regions in the tumor center.23 While the treatment with 1–2 or cisplatin did not show a statistical significant therapeutic effect (the treatment with cisplatin was within the standard deviation of the experiment), the treatment with 3 demonstrated a strong tumor growth inhibition effect (Figure 6B). Combined these findings indicate the ability of 3 to therapeutically intervene in small as well as big multicellular tumor spheroids. For a mechanistic understanding of the observed tumor growth inhibition effect of the metal complex, the multicellular tumor spheroids with an average diameter of approximately 500 μm were treated with 3 (1.26 μM), stained with fluorophores specific for cell death and in particular ferroptosis hallmarks, and then the biological effects visualized by fluorescence microscopy. The cell live/dead calcein AM/propidium iodide stain demonstrated that the treatment with 3 resulted in a mixture of living and dead cells within the tumor spheroid. Remarkably, dead cells were also observed in the center of the spheroid, that is considered challenging to be eradicated, suggesting good penetration of the Co(III)-sulfasalazine complex (Figure 6C). The ability of the metal complex to generate ROS in the tumor spheroids was evaluated by fluorescence microscopy using the ROS specific probe 2′,7′-dichlorodihydrofluorescein diacetate. The microscopy images of the spheroids treated with 3 showed the characteristic green emission, indicative of the efficient ROS generation of 3 (Figure 6D). The ability of the metal complex to trigger lipid peroxidation in the tumor spheroids was evaluated by fluorescence microscopy using the specific probe BODIPY581/591 C11. The microscopy images showed a strong fluorescence signal upon treatment with 3, indicative of high LPO levels in the entire tumor spheroid (Figure 6E). The GSH depletion was evaluated by fluorescence microscopy using the specific probe ThiolTrackerTM Violet. The microscopy images did not show the distinct violet emission, that is characteristic for the presence of GSH, in the core of the tumor spheroid after treatment with 3 (Figure 6F), suggesting the GSH depletion. Combined these findings suggest the ability of 3 to efficiently induce cell death of the tumor spheroid upon induction of ferroptosis.
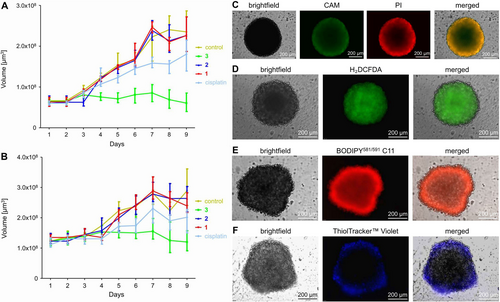
Evaluation of the biological effects on CT-26 multicellular tumor spheroids. A) Tumor growth inhibition curves of CT-26 multicellular tumor spheroids with an average diameter of 250 μm upon treatment with 1–3 or cisplatin (IC50=6.34 μM) and daily monitoring of the tumor growth. B) Tumor growth inhibition curves of CT-26 multicellular tumor spheroids with an average diameter of 500 μm upon treatment with 1–3 or cisplatin (IC50=6.34 μM) and daily monitoring of the tumor growth. The error bars correspond to the standard deviation of the three replicates. C) Fluorescence microscopy images upon co-incubation of 3 with cell live stain calcein (CAM, λex=460–490 nm, λem=515 nm) and cell death stain propidium iodide (PI, λex=545–580 nm, λem=617 nm). D) Fluorescence microscopy images upon co-incubation of 3 with the ROS specific probe 2′,7′-dichlorodihydrofluorescein diacetate (λex=460–490 nm, λem=527 nm). E) Fluorescence microscopy images upon co-incubation of 3 with the LPO specific probe BODIPY581/591 C11 (λex=460–490 nm, λem=510 nm). F) Fluorescence microscopy images upon co-incubation of 3 with the GSH specific probe ThiolTracker™ Violet (λex=330–385 nm, λem=526 nm). MCTS with an average diameter of approximately 500 μm were treated with 3 (1.26 μM).
Conclusion
This study reports on the chemical synthesis and biological evaluation of a Co(III) polypyridine sulfasalazine complex for anticancer therapy. The compound demonstrated to be highly stable under physiological conditions. The metal complex was found to be partly hydrophilic and partly lipophilic, allowing for a high water solubility under biological conditions as well as high cellular penetration. While the individual components such as the respective ligands, metal salts as well as metal precursors were found to be non-toxic in a variety of cancer cells, the lead compound showed a cytotoxic effect in the low micromolar range. Insights into the biological mechanism of action revealed that the metal complex preferably accumulated in the mitochondria of the cancerous cells, causing the generation of cytotoxic reactive oxygen species. Further analysis revealed that the compound selectively produced hydroxy radicals that ultimately cause the formation of lipid peroxides. Once the antioxidant defenses reliant on glutathione and the glutathione peroxidase 4 enzyme are overwhelmed, lipid peroxidation proceeds uncontrollably, ultimately resulting in cell death through ferroptosis. Based on these promising biological effects, the therapeutic properties were further evaluated in small and large multicellular tumor spheroids as a tumor tissue culture model for clinical solid tumors. The metal complex revealed a cytotoxic effect resulting in tumor growth inhibition over several days. We are confident that the development of ferroptosis inducing metal complexes will open new avenues for the treatment of cancer. The ability to therapeutically intervene in multimodal mechanism of action involving high cytotoxic hydroxyl radicals could present a promising alternative for the treatment of drug resistant or hypoxic tumors.
Acknowledgments
J.K. gratefully acknowledges the financial support provided by the Liebig fellowship from the Chemical Industry Fund of the German Chemical Industry Association, the Life Sciences Bridge Award from the Aventis Foundation, and the Paul Ehrlich & Ludwig Darmstaedter Early Career Award 2024—a prize awarded by the Paul Ehrlich Foundation, Germany. Open Access funding enabled and organized by Projekt DEAL.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.