Experimental Observation of a Terminal Borylene-Dinitrogen Adduct via Cleavage of a 1,2,3,4,5-Diboratriazoline
Dr. Arumugam Jayaraman
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Department of Chemistry and Biochemistry, University of Nevada Las Vegas, 89154 Las Vegas, United States
These authors contributed equally.
Search for more papers by this authorDr. Benedikt Ritschel
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
These authors contributed equally.
Search for more papers by this authorDr. Merle Arrowsmith
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorChristian Markl
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorMalte Jürgensen
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorAnel Halkić
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorYannick Konrad
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorDr. Andreas Stoy
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorDr. Krzysztof Radacki
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Holger Braunschweig
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorDr. Arumugam Jayaraman
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Department of Chemistry and Biochemistry, University of Nevada Las Vegas, 89154 Las Vegas, United States
These authors contributed equally.
Search for more papers by this authorDr. Benedikt Ritschel
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
These authors contributed equally.
Search for more papers by this authorDr. Merle Arrowsmith
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorChristian Markl
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorMalte Jürgensen
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorAnel Halkić
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorYannick Konrad
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorDr. Andreas Stoy
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorDr. Krzysztof Radacki
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Holger Braunschweig
Institute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Institute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
Search for more papers by this authorGraphical Abstract
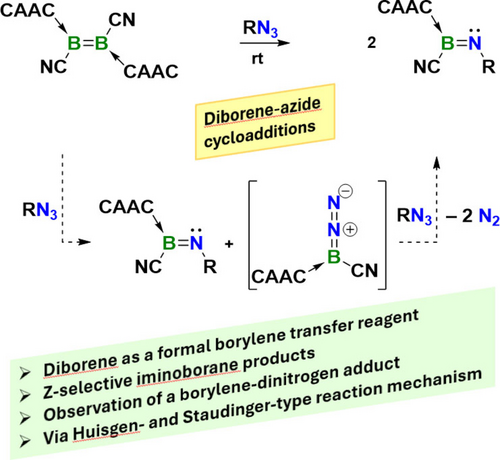
The reaction of a doubly cyclic alkyl(amino)carbene (CAAC)-stabilized dicyanodiborene with organic azides yields stable CAAC-iminoborane adducts. Experimental and computational data confirm the involvement of a relatively long-lived terminal borylene-dinitrogen adduct (i.e. diazoborane), [(CAAC)B(CN)(η1-N2)] as a key intermediate in the reaction.
Abstract
While azides do not react with simple alkenes except under harsh conditions, a diboron alkene analogue, the doubly cyclic alkyl(amino)carbene (CAAC)-stabilized dicyanodiborene 1, reacts spontaneously with organic azides (7–10 equiv.) at room temperature to yield two equivalents of stable CAAC-imino(cyano)boranes (2-R). NMR-spectroscopic monitoring of the reaction mixtures shows the initial formation of a 1 : 1 mixture of 2-R and a relatively long-lived intermediate (Int), which in the presence of excess azide is converted into a second equivalent of 2-R. In the absence of excess azide, however, Int decomposes to 3, the product of an intramolecular C−H activation by a putative dicoordinate borylene intermediate “(CAAC)B(CN)”. Mechanistic insights from trapping experiments, NMR-spectroscopic and high-resolution mass spectrometry data, as well as DFT computations reveal that Int is the terminal borylene end-on-dinitrogen adduct [(CAAC)B(CN)(η1-N2)]. The formation of the iminoboranes 2-R from diborene 1 and RN3 proceeds via an azide-diborene Huisgen-type [3+2] cycloaddition reaction, followed by a retro-[3+2] cycloaddition, yielding 2-R and [(CAAC)B(CN)(η1-N2)]. The latter then undergoes either N2 extrusion and intramolecular C−H activation to generate 3, or a Staudinger-type reaction with a second equivalent of azide to generate a second equivalent of the iminoborane 2-R.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202412307-sup-0001-Combined_cif_files.cif12.4 MB | Supporting Information |
| anie202412307-sup-0001-misc_information.pdf4.3 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aF. Zhang, P. Maksyutenko, R. I. Kaiser, A. M. Mebel, A. Gregǔsová, S. A. Perera, R. J. Bartlett, J. Phys. Chem. A 2010, 114, 12148–12154;
- 1bO. Mó, M. Yáñez, A. Martín Pendás, J. E. Del Bene, I. Alkortad, J. Elguero, Phys. Chem. Chem. Phys. 2007, 9, 3970–3977;
- 1cE. R. Lory, R. F. Porter, J. Am. Chem. Soc. 1973, 95, 1766–1770;
- 1dC. N. Baird, R. K. Datta, Inorg. Chem. 1972, 11, 17–19.
- 2Selected reviews:
- 2aP. Paetzold, Adv. Inorg. Chem. 1987, 31, 123–170;
- 2bH. Nöth, Angew. Chem. Int. Ed. Engl. 1988, 27, 1603–1623;
- 2cY. Fan, J. Cui, L. Kong, Eur. J. Org. Chem. 2022, e202201086.
- 3
- 3aB. Borthakur, H. Braunschweig, A. Deißenberger, T. Dellermann, R. D. Dewhurst, I. Krummenacher, M. Nutz, A. K. Phukan, M. Schäfer, Angew. Chem. Int. Ed. 2017, 56, 7975–7979;
- 3bB. Kröckert, K.-H. van Bonn, P. Paetzold, Z. Anorg. Allg. Chem. 2005, 631, 866–868;
- 3cC. Klöfkorn, M. Schmidt, T. Spaniol, T. Wagner, O. Costisor, P. Paetzold, Chem. Ber. 1995, 128, 1037–1043;
- 3dP. Paetzold, D. Hahnfeld, U. Englert, Chem. Ber. 1992, 125, 1079–1081;
- 3eP. Paetzold, D. Hahnfeld, U. Englert, W. Wojnowski, B. Dreczewski, Z. Pawelec, L. Walz, Chem. Ber. 1992, 125, 1073–1078.
- 4
- 4aL. Winner, W. C. Ewing, K. Geetharani, T. Dellermann, B. Jouppi, T. Kupfer, M. Schäfer, H. Braunschweig, Angew. Chem. Int. Ed. 2018, 57, 12275–12279;
- 4bB. Böck, U. Braun, T. Habereder, P. Mayer, H. Nöth, Z. Naturforsch. 2004, 59b, 681–684;
10.1515/znb-2004-0608 Google Scholar
- 4cE. v. Steuber, G. Elter, M. Noltemeyer, H.-G. Schmidt, A. Meller, Organometallics 2000, 19, 5083–5091;
- 4dE. Bulak, P. Paetzold, Z. Anorg. Allg. Chem. 2000, 626, 1277–1278;
10.1002/(SICI)1521-3749(200006)626:6<1277::AID-ZAAC1277>3.0.CO;2-1 CAS Web of Science® Google Scholar
- 4eJ. Kiesgen, J. Münster, P. Paetzold, Chem. Ber. 1993, 126, 1559–1563.
- 5
- 5aM. Schäfer, N. A. Beattie, K. Geetharani, J. Schäfer, W. C. Ewing, M. Krahfuß, C. Hörl, R. D. Dewhurst, S. A. Macgregor, C. Lambert, H. Braunschweig, J. Am. Chem. Soc. 2016, 138, 8212–8220;
- 5bM. Schäfer, J. Schäfer, R. D. Dewhurst, W. C. Ewing, M. Krahfuß, M. W. Kuntze-Fechner, M. Wehner, C. Lambert, H. Braunschweig, Chem. Eur. J. 2016, 22, 8603–8609;
- 5cH. Braunschweig, K. Geetharani, J. O. C. Jiménez-Halla, M. Schäfer, Angew. Chem. Int. Ed. 2014, 53, 3500–3504.
- 6
- 6aT. Thiess, G. Bélanger-Chabot, F. Fantuzzi, M. Michel, M. Ernst, B. Engels, H. Braunschweig, Angew. Chem. Int. Ed. 2020, 59, 15480–15486;
- 6bP. Paetzold, Phosphorus, Sulfur and Silicon 1994, 93–94, 39–50;
- 6cP. Paetzold, J. Kiesgen, K. Krahé, H.-U. Meier, R. Boese, Z. Naturforsch. 1991, 46b, 853–860.
10.1515/znb-1991-0703 Google Scholar
- 7
- 7aA. K. Swarnakar, C. Hering-Junghans, K. Nagata, M. J. Ferguson, R. McDonald, N. Tokitoh, E. Rivard, Angew. Chem. Int. Ed. 2015, 54, 10666–10669;
- 7bB. L. Frenette, A. A. Omaña, M. J. Ferguson, Y. Zhou, E. Rivard, Chem. Commun. 2021, 57, 10895–10898;
- 7cA. A. Omaña, R. Watt, Y. Zhou, M. J. Ferguson, E. Rivard, Inorg. Chem. 2022, 61, 16430–16440.
- 8
- 8aH. Braunschweig, W. C. Ewing, K. Geetharani, M. Schäfer, Angew. Chem. Int. Ed. 2015, 54, 1662–1665;
- 8bH. Braunschweig, R. D. Dewhurst, W. E. Ewing, M. Schäfer, Chem. Eur. J. 2017, 23, 5953–5956.
- 9L. Winner, A. Hermann, G. Bélanger-Chabot, O. F. González-Belman, J. O. C. Jiménez-Halla, H. Kelch, H. Braunschweig, Chem. Commun. 2018, 54, 8210–8213.
- 10
- 10aF. Dahcheh, D. Martin, D. W. Stephan, G. Bertrand, Angew. Chem. Int. Ed. 2014, 53, 13159–13163;
- 10bF. Dahcheh, D. W. Stephan, G. Bertrand, Chem. Eur. J. 2015, 21, 199–204;
- 10cX. Yue, L. Wu, H. Wang, Chem. Asian J. 2023, 18, e202300411.
- 11
- 11aP. Cui, R. Guo, L. Kong, C. Cui, Inorg. Chem. 2020, 59, 5261–5265;
- 11bR. Guo, X. Zhang, T. Li, Q. Li, D. A. Ruiz, L. L. Liu, C. H. Tung, L. Kong, Chem. Sci. 2022, 13, 2303–2309.
- 12L. Xie, J. Zhang, H. Hu, C. Cui, Organometallics 2013, 32, 6875–6878.
- 13Winner, G. Bélanger-Chabot, M. A. Celik, M. Schäfer, H. Braunschweig, Chem. Commun. 2018, 54, 9349–9351.
- 14D. Raiser, H. Schubert, H. F. Bettinger, L. Wesemann, Chem. Eur. J. 2021, 27, 1981–1983.
- 15L. Zhu, R. Kinjo, Angew. Chem. Int. Ed. 2022, 61, e202207631.
- 16M. Arrowsmith, H. Braunschweig, T. E. Stennett, Angew. Chem. Int. Ed. 2017, 56, 96–115.
- 17J. Böhnke, H. Braunschweig, T. Dellermann, W. C. Ewing, T. Kramer, I. Krummenacher, A. Vargas, Angew. Chem. Int. Ed. 2015, 54, 4469–4473.
- 18M. Arrowsmith, D. Auerhammer, R. Bertermann, H. Braunschweig, G. Bringmann, M. A. Celik, R. D. Dewhurst, M. Finze, M. Grüne, M. Hailmann, T. Hertle, I. Krummenacher, Angew. Chem. Int. Ed. 2016, 55, 14464–14468.
- 19D. Auerhammer, M. Arrowsmith, J. Böhnke, H. Braunschweig, T. Kupfer, Chem. Sci. 2018, 9, 2252–2260.
- 20C. Zhang, C. C. Cummins, R. J. Gilliard Jr., Science 2024, 385, 327–331.
- 21
- 21aP. Bhattacharya, Z. M. Heiden, G. M. Chambers, S. I. Johnson, R. M. Bullock, M. T. Mock, Angew. Chem. Int. Ed. 2019, 58, 11618–11624;
- 21bJ. O. Friedrich, R. E. Wasylishen, J. Chem. Phys. 1985, 83, 3707–3708.
- 22
- 22aH. Braunschweig, I. Krummenacher, M.-A. Légaré, A. Matler, K. Radacki, Q. Ye, J. Am. Chem. Soc. 2017, 139, 1802–1805;
- 22bM.-A. Légaré, G. Bélanger-Chabot, R. D. Dewhurst, E. Welz, I. Krummenacher, B. Engels, H. Braunschweig, Science 2018, 359, 896–900;
- 22cM.-A. Légaré, M. Rang, G. Bélanger-Chabot, J. I. Schweizer, I. Krummenacher, R. Bertermann, M. Arrowsmith, M. C. Holthausen, H. Braunschweig, Science 2019, 363, 1329–1332.
- 23S. Berski, Z. Latajka, A. J. Gordon, New J. Chem. 2011, 35, 89–96.
- 24A. Gärtner, L. Meier, M. Arrowsmith, M. Dietz, I. Krummenacher, R. Bertermann, F. Fantuzzi, H. Braunschweig, J. Am. Chem. Soc. 2022, 144, 21363–21370.
- 25D. Prieschl, M. Arrowsmith, M. Dietz, A. Rempel, M. Müller, H. Braunschweig, Chem. Commun. 2020, 56, 5681–5684.
- 26M. Yamamoto, W. C. Chan, Z. Lin, M. Yamashita, Chem. Eur. J. 2023, 29, e202302027.
- 27R. Witte, S. Kar, K. Radacki, M. Härterich, M. Rang, M. Michel, C. Mihm, C. Czernetzki, T. Brückner, E. Beck, S. Lutz, R. D. Dewhurst, H. Braunschweig, Chem. Commun. 2024, DOI: 10.1039/d4cc02923b.
- 28
- 28aP. Scheiner, Tetrahedron 1967, 24, 349–356;
- 28bP. K. Kadaba, S. B. Edelstein, J. Org. Chem. 1990, 55, 5891–5894.
- 29Selected examples:
- 29aM. E. Munk, Y. K. Kim, J. Am. Chem. Soc. 1964, 86, 2213–2217;
- 29bR. Huisgen, L. Möbius, G. Szeimies, Chem. Ber. 1965, 98, 1153–1158;
- 29cP. K. Kabada, J. Org. Chem. 1992, 57, 3075–3078;
- 29dT. Slagbrand, A. Volkov, P. Trillo, F. Tinnis, H. Adolfsson, ACS Catal. 2017, 7, 1771–1775.
- 30Mass-spectrometric (LIFDI-MS) analysis of the initial reaction mixture of 1 with 2 equiv. of PhN3 in toluene showed no mass peak corresponding to the diboratriazoline intermediate A-Ph.
- 31Selected examples:
- 31aR. Huisgen, G. Szeimies, L. Möbius, Chem. Ber. 1966, 99, 475–490;
- 31bW. H. Pearson, S. C. Bergmeier, S. Degan, K.-C. Lin, Y.-F. Poon, J. M. Scheryantz, J. P. Williams, J. Org. Chem. 1990, 55, 5719–5738;
- 31cD. R. Roque, J. L. Neill, J. W. Antoon, E. P. Stevens, Synthesis 2005, 2497–2502;
- 31dB. Wei-Qiang, S. Chiba, Org. Lett. 2009, 11, 729–732;
- 31eS. Xie, S. A. Lopez, O. Ramström, M. Yan, K. N. Houk, J. Am. Chem. Soc. 2015, 137, 2958–2966.
- 32LIFDI-MS analysis of the initial reaction mixtures of 1 with 2 equiv. of PhN3 and MesN3 did not show any mass peak for Int as the N2 ligand likely dissociates too readily under the LIFDI-MS conditions.
- 33It is noteworthy that the dicoordinate borylene D has been postulated as an intermediate in the twofold reduction of [(CAAC)BBr2(CN)] to the self-stabilizing cyanoborylene tetramer [(CAAC)B(CN)]4.[18,34] The latter, however, was never observed in any of the reactions of 1 with azides, suggesting that the formation of [(CAAC)B(CN)]4 proceeds via a different mechanism, which does not involve D. One possibility is that the reduction of [(CAAC)BBr2(CN)] generates the bromo(cyano)boryl radical [(CAAC)BBr(CN)]⋅, which oligomerizes by intramolecular Br-CN ligand exchange (via ionic diradical intermediates such as [{(CAAC)BBr(CN)}⋅→{B(CN)(CAAC)}⋅]Br), before further reduction to the borylene tetramer [(CAAC)B(CN)]4. Attempts to generate the diazoborane Int by reduction of [(CAAC)BBr2(CN)] in dilute solutions under pressurized N2 atmosphere and at low temperatures invariably yielded [(CAAC)B(CN)]4, without any trace of Int, thus further supporting the hypothesis that D is not an intermediate in the reduction of [(CAAC)BBr2(CN)] to [(CAAC)B(CN)]4.
- 34F. Fantuzzi, Y. Jiao, R. D. Dewhurst, F. Weinhold, H. Braunschweig, B. Engels, Chem. Sci. 2022, 13, 5118–5129.
- 35C. Tang, Q. Liang, A. R. Jupp, T. C. Johnstone, R. C. Neu, D. Song, S. Grimme, D. W. Stephan, Angew. Chem. Int. Ed. 2017, 56, 16588–16592.
- 36J. M. Hopkins, M. Bowdridge, K. N. Robertson, T. S. Cameron, H. A. Jenkins, J. A. C. Clyburne, J. Org. Chem. 2001, 66, 5173–5716.
- 37A. Gärtner, U. S. Karaca, M. Rang, M. Heinz, P. D. Engel, I. Krummenacher, M. Arrowsmith, A. Hermann, A. Matler, A. Rempel, R. Witte, H. Braunschweig, M. C. Holthausen, M.-A. Légaré, J. Am. Chem. Soc. 2023, 145, 8231–8241.
- 38Deposition numbers 2253319 (for 2-Ph), 2253318 (for 3), 2326268 (for 2-PhF5), 2253314 (for 2-PhCN), 2253315 (for 2-PhNMe2), 2253313 (for 2-TolBr2), 2253317 (for 2-Cy) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 39
- 39aS. W. Kwok, J. R. Fotsing, R. J. Fraser, V. O. Rodionov, V. V. Fokin, Org. Lett. 2010, 12, 4217–4219;
- 39bL.-M. Jin, H. Lu, Y. Cui, C. L. Lizardi, T. N. Arzua, L. Wojtas, X. Cui, X. P. Zhang, Chem. Sci. 2014, 5, 2422–2427;
- 39cS. Xie, Y. Zhang, O. Ramström, M. Yan, Chem. Sci. 2016, 7, 713–718.
- 40N. Kuhn, T. Kratz, Synthesis 1993, 561–562.
- 41G. Sheldrick, Acta Crystallogr. 2015, A71, 3–8.
- 42G. Sheldrick, Acta Crystallogr. 2008, A64, 112–122.
- 43M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, N. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr. J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, D. J. Fox, Gaussian 16, Revision E.01; Gaussian, Inc. Wallingford CT, 2016.
- 44
- 44aA. D. Becke, Phys. Rev. A 1988, 38, 3098–3100;
- 44bA. D. Becke, J. Chem. Phys. 1993, 98, 5648–5652;
- 44cC. Lee, W. Yang, R. G. Parr, Phys. Rev. B 1988, 37, 785–789.
- 45
- 45aS. Grimme, J. Antony, S. Ehrlich, H. J. Krieg, Chem. Phys. 2010, 132, 154104;
- 45bS. Grimme, S. Ehrlich, L. Goerigk, J. Comput. Chem. 2011, 32, 1456–1465.
- 46F. Weigend, R. Ahlrichs, Phys. Chem. Chem. Phys. 2005, 7, 3297–3305.
- 47J. Baker, J. Comput. Chem. 1986, 7, 385–395.
- 48
- 48aA. L. L. East, G. M. Berner, A. D. Morcom, L. Mihichuk, J. Chem. Theory Comput. 2008, 4, 1274–1282;
- 48bA. Jayaraman, A. L. L. East, J. Org. Chem. 2012, 77, 351–356;
- 48cY. P. Budiman, A. Jayaraman, A. Friedrich, F. Kerner, U. Radius, T. B. Marder, J. Am. Chem. Soc. 2020, 142, 6036–6050;
- 48dT. E. Stennett, A. Jayaraman, T. Brückner, L. Schneider, H. Braunschweig, Chem. Sci. 2020, 11, 1335–1341.
- 49J. D. Chai, M. Head-Gordon, Phys. Chem. Chem. Phys. 2008, 10, 6615–6620.
- 50A. V. Marenich, C. J. Cramer, D. G. Truhlar, J. Phys. Chem. B 2009, 113, 6378–6396.
- 51
- 51aR. Ditchfield, Mol. Phys. 1974, 27, 789–807;
- 51bK. Wolinski, J. F. Hinton, P. Pulay, J. Am. Chem. Soc. 1990, 112, 8251–8260;
- 51cJ. R. Cheeseman, G. W. Trucks, T. A. Keith, M. J. Frisch, J. Chem. Phys. 1996, 104, 5497–5509.