Anilines Formation via Molybdenum-Catalyzed Intermolecular Reaction of Ynones with Allylic Amines
Yi-Zhe Yu
State Key Laboratory of Physical Chemistry of Solid Surfaces, Key Laboratory of Chemical Biology of Fujian Province, and College of Chemistry and Chemical Engineering, Xiamen University, 361005 Xiamen, P. R. China
Search for more papers by this authorHong-Yi Su
State Key Laboratory of Physical Chemistry of Solid Surfaces, Key Laboratory of Chemical Biology of Fujian Province, and College of Chemistry and Chemical Engineering, Xiamen University, 361005 Xiamen, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Chun-Xiang Zhuo
State Key Laboratory of Physical Chemistry of Solid Surfaces, Key Laboratory of Chemical Biology of Fujian Province, and College of Chemistry and Chemical Engineering, Xiamen University, 361005 Xiamen, P. R. China
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 200032 Shanghai, P. R. China
Search for more papers by this authorYi-Zhe Yu
State Key Laboratory of Physical Chemistry of Solid Surfaces, Key Laboratory of Chemical Biology of Fujian Province, and College of Chemistry and Chemical Engineering, Xiamen University, 361005 Xiamen, P. R. China
Search for more papers by this authorHong-Yi Su
State Key Laboratory of Physical Chemistry of Solid Surfaces, Key Laboratory of Chemical Biology of Fujian Province, and College of Chemistry and Chemical Engineering, Xiamen University, 361005 Xiamen, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Chun-Xiang Zhuo
State Key Laboratory of Physical Chemistry of Solid Surfaces, Key Laboratory of Chemical Biology of Fujian Province, and College of Chemistry and Chemical Engineering, Xiamen University, 361005 Xiamen, P. R. China
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 200032 Shanghai, P. R. China
Search for more papers by this authorGraphical Abstract
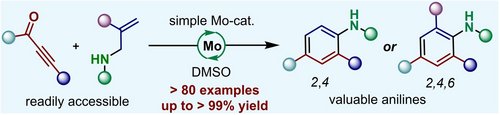
An unprecedented and efficient approach for the synthesis of multi-substituted anilines was reported. With a simple Mo-catalyst, a wide range of 2,4-di and 2,4,6-trisubstituted anilines, some of which were otherwise challenging to access, were efficiently prepared in generally good yields from readily accessible ynones and allylic amines. This process was robust, modular and ready for the syntheses of various valuable multi-substituted anilines.
Abstract
The multi-substituted anilines are widely found in organic synthesis, medicinal chemistry and material science. The quest for robust and efficient methods to construct a diverse array of these compounds using readily accessible starting materials under simple reaction conditions is of utmost importance. Here, we report an unprecedented and efficient approach for the synthesis of 2,4-di and 2,4,6-trisubstituted anilines. With a simple molybdenum(VI) catalyst, a wide range of 2,4-di and 2,4,6-trisubstituted anilines were efficiently prepared in generally good to excellent yields from readily accessible ynones and allylic amines. The synthetic potential of this methodology was further underscored by its applications in several synthetic transformations, gram-scale reactions, and derivatization of bioactive molecules. Preliminary mechanistic studies suggested that this aniline formation might involve a cascade of aza-Michael addition, [1,6]-proton shift, cyclization, dehydration, 6π-electrocyclization, and aromatization. This novel strategy provided a robust, simple, and modular approach for the syntheses of various valuable di- or trisubstituted anilines, some of which were otherwise challenging to access.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202412299-sup-0001-misc_information.pdf16.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. Ruiz-Castillo, S. L. Buchwald, Chem. Rev. 2016, 116, 12564–12649;
- 1bJ. F. Hartwig, K. H. Shaughnessy, S. Shekhar, R. A. Green, Organic Reactions; Wiley, 2020, Vol. 100, S. E. Denmark, Ed. chap. 14, pp 853–958.
- 2
- 2aF. Ullmann, Ber. Dtsch. Chem. Ges. 1903, 36, 2382–2384;
- 2bD. Ma, Y. Zhang, J. Yao, S. Wu, F. Tao, J. Am. Chem. Soc. 1998, 120, 12459–12467;
- 2cE. K. Creutz, J. G. Lotito, C. Fu, J. C. Peters, Science 2012, 338, 647–651.
- 3For selected reviews:
- 3aD. Ma, Q. Cai, Acc. Chem. Res. 2008, 41, 1450–1460;
- 3bF. Monnier, M. Taillefer, Angew. Chem. Int. Ed. 2009, 48, 6954–6971; Angew. Chem. 2009, 121, 7088–7105;
- 3cC. Sambiagio, S. P. Marsden, M. A. Blacker, P. C. McGowan, Chem. Soc. Rev. 2014, 43, 3525–3550;
- 3dS. Bhunia, G. G. Pawar, S. V. Kumar, Y. Jiang, D. Ma, Angew. Chem. Int. Ed. 2017, 56, 16136–16179; Angew. Chem. 2017, 129, 16352–16369;
- 3eQ. Cai, W. Zhou, Chin. J. Chem. 2020, 38, 879–893.
- 4
- 4aF. Paul, J. Patt, J. F. Hartwig, J. Am. Chem. Soc. 1994, 116, 5969–5970;
- 4bA. S. Guram, S. L. Buchwald, J. Am. Chem. Soc. 1994, 116, 7901–7902.
- 5For selected reviews:
- 5aJ. P. Wolfe, S. Wagaw, J.-F. Marcoux, S. L. Buchwald, Acc. Chem. Res. 1998, 31, 805–818;
- 5bJ. F. Hartwig, Acc. Chem. Res. 1998, 31, 852–860;
- 5cR. Dorel, C. P. Grugel, A. M. Haydl, Angew. Chem. Int. Ed. 2019, 58, 17118–17129; Angew. Chem. 2019, 131, 17276–17287.
- 6
- 6aD. M. T. Chan, Tetrahedron Lett. 1996, 37, 9013–9016;
- 6bP. Y. S. Lam, S. Deudon, K. M. Averill, R. Li, M. Y. He, P. DeShong, C. G. Clark, J. Am. Chem. Soc. 2000, 122, 7600–7601;
- 6cFor a recent review: M. J. West, J. W. B. Fyfe, J. C. Vantourout, A. J. B. Watson, Chem. Rev. 2019, 119, 12491–12523.
- 7
- 7aA. Nishizawa, T. Takahira, K. Yasui, H. Fujimoto, T. Iwai, M. Sawamura, N. Chatani, M. Tobisu, J. Am. Chem. Soc. 2019, 141, 7261–7265;
- 7bJ. Liu, C. Zhang, Z. Zhang, X. Wen, X. Dou, J. Wei, X. Qiu, S. Song, N. Jiao, Science 2020, 367, 281–285;
- 7cP. Onnuch, K. Ramagonolla, R. Y. Liu, Science 2024, 383, 1019–1024.
- 8
- 8aFor a recent review: Y. Park, Y. Kim, S. Chang, Chem. Rev. 2017, 117, 9247–9301;
- 8bN. A. Romero, K. A. Margrey, N. E. Tay, D. A. Nicewicz, Science 2015, 349, 1326–1330;
- 8cA. Ruffoni, F. Juliá, T. D. Svejstrup, A. J. McMillan, J. J. Douglas, D. Leonori, Nat. Chem. 2019, 11, 426–433;
- 8dL. Zhang, L. Liardet, J. Luo, D. Ren, M. Grätzel, X. Hu, Nat. Catal. 2019, 2, 366–373;
- 8eR. R. Anugu, S. Munnuri, J. R. Falck, J. Am. Chem. Soc. 2020, 142, 5266–5271;
- 8fZ.-W. Hou, H. Yan, J. Song, H.-C. Xu, Green Chem. 2023, 25, 7959–7962;
- 8gQ.-C. Gan, J. Qiao, C. Zhou, R.-N. Ci, J.-D. Guo, B. Chen, C.-H. Tung, L.-Z. Wu, Angew. Chem. Int. Ed. 2023, 62, e202218391;
- 8hQ. Lv, Z. Hu, Y. Zhang, Z. Zhang, H. Lei, J. Am. Chem. Soc. 2024, 146, 1735–1741.
- 9
- 9aG. A. Olah, V. P. Reddy, G. K. S. Prakash, Friedel–Crafts reactions. In Kirk-Othmer encyclopedia of chemical technology; Wiley: New York, 2000, pp 159–199;
- 9bI. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy, J. F. Hartwig, Chem. Rev. 2010, 110, 890–931.
- 10
- 10aW. P. Hong, A. V. Iosub, S. S. Stahl, J. Am. Chem. Soc. 2013, 135, 13664–13667;
- 10bU. S. Dighe, F. Julia, A. Luridiana, J. J. Douglas, D. Leonori, Nature 2020, 584, 75–81;
- 10cY. Shi, C. Yi, X. Huang, Y. Tang, J. Jiao, Y. Li, J. Org. Chem. 2023, 88, 9554–9564;
- 10dH. Wu, L. Zhao, W. Wang, Y. Yu, G. Wu, Org. Chem. Front. 2023, 10, 5484–5489;
- 10eJ. Hu, R. Ma, J. Hu, X. Liu, X. Liu, H. He, H. Yi, A. Lei, Green Chem. 2024, 26, 4684–4690;
- 10fB.-Y. Zhao, Q. Jia, Y.-Q. Wang, Nat. Commun. 2024, 15, 2415–2422;
- 10gY.-F. Ren, X.-Y. Chen, H.-W. Du, W. Shu, Chem Catalysis 2024, 4, 100873.
- 11
- 11aD. B. Ramachary, K. Ramakumar, V. V. Narayana, J. Org. Chem. 2007, 72, 1458–1463;
- 11bS. Kiren, A. Padwa, J. Org. Chem. 2009, 74, 7781–7789;
- 11cL. Li, M.-N. Zhao, Z.-H. Ren, J.-L. Li, Z.-H. Guan, Org. Lett. 2012, 14, 3506–3509;
- 11dS. Yaragorla, R. Dada, ACS Omega 2017, 2, 4859–4869;
- 11eY. Xia, H. Huang, F. Zhang, G.-J. Deng, Org. Lett. 2019, 21, 7489–7492;
- 11fP. G. Dalai, N. Panda, Adv. Synth. Catal. 2022, 364, 3736–3742;
- 11gS. A. Makarov, N. A. Bakiev, D. A. Eshemeteva, Org. Chem. Front. 2023, 10, 2760–2765;
- 11hW. Cai, Y. Huang, Angew. Chem. Int. Ed. 2023, 62, e202310133;
- 11iJ. Wei, T. Pham, E. I. Attah, M. Liu, T. Yaroshuk, H. Chen, L. Wojtas, X. Shi, Angew. Chem. Int. Ed. 2024, e202407360.
- 12
- 12aL.-Y. Cao, J.-N. Luo, J.-S. Yao, D.-K. Wang, Y.-Q. Dong, C. Zheng, C.-X. Zhuo, Angew. Chem. Int. Ed. 2021, 60, 15254–15259;
- 12bY.-Q. Dong, K. Wang, C.-X. Zhuo, ACS Catal. 2022, 12, 11428–11435;
- 12cY.-Q. Dong, X.-N. Shi, L.-Y. Cao, J. Bai, C.-X Zhuo, Org. Chem. Front. 2023, 10, 3544–3552;
- 12dY.-Z. Yu, J. Bai, J.-M. Peng, J.-S. Yao, C.-X. Zhuo, J. Am. Chem. Soc. 2023, 145, 8781–8787;
- 12eL.-Y. Cao, J.-L. Wang, K. Wang, J.-B. Wu, D.-K. Wang, J.-M. Peng, J. Bai, C.-X. Zhuo, J. Am. Chem. Soc. 2023, 145, 2765–2772;
- 12fJ.-L. Wang, G.-Y. Wu, J.-N. Luo, J.-L. Liu, C.-X. Zhuo, J. Am. Chem. Soc. 2024, 146, 5605–5613.
- 13
- 13aS. Asako, S. Ishihara, K. Hirata, K. Takai, J. Am. Chem. Soc. 2019, 141, 9832–9836;
- 13bS. Asako, T. Kobayashi, S. Ishihara, K. Takai, Asian J. Org. Chem. 2021, 10, 753–756;
- 13cS. Banerjee, T. Kobayashi, K. Takai, S. Asako, L. Ilies, Org. Lett. 2022, 24, 7242–7246.
- 14K. A. Hall, J. M. Mayer, J. Am. Chem. Soc. 1992, 114, 10402–10411.
- 15For a review on the use of dioxomolybdenum(VI) complexes in organic synthesis, see: R. Hernández-Ruiz, R. Sanz, Synthesis 2018, 50, 4019–4036.
- 16Selected recent examples:
- 16aS. Suárez-Pantiga, R. Hernández-Ruiz, C. Virumbrales, M. R. Pedrosa, R. Sanz, Angew. Chem. Int. Ed. 2019, 58, 2129–2133; Angew. Chem. 2019, 131, 2151–2155;
- 16bB. F. S. Nunes, M. C. Oliveira, A. C. Fernandes, Green Chem. 2020, 22, 2419–2425;
- 16cJ. Li, M. Lutz, R. J. M. Klein Gebbink, ChemCatChem 2020, 12, 6356–6365;
- 16dP. Zhou, Y. Li, T. Xu, Org. Lett. 2022, 24, 4218–4223.
- 17B. Trost, Science 1991, 254, 1471–1477.
- 18R. D. Taylor, M. MacCoss, A. D. Lawson, J. Med. Chem. 2014, 57, 5845–5859.
- 19For details, see the Supporting Information.
- 20
- 20aL. A. Paquette, D. E. Kuhla, J. H. Barrett, J. Org. Chem. 1969, 34, 2879–2884;
- 20bH. Jansen, J. C. Slootweg, K. Lammertsma, Beilstein J. Org. Chem. 2011, 7, 1713–1721;
- 20cN. Mandal, A. Das, C. Hajra, A. Datta, Chem. Sci. 2022, 13, 704–712;
- 20dG. Li, M. N. Lavagnino, S. Z. Ali, S. Hu, A. T. Radosevich, J. Am. Chem. Soc. 2023, 145, 41–46.
- 21L. A. Paquette, Angew. Chem. Int. Ed. 1971, 10, 11–20; Angew. Chem. 1971, 83, 11–20.
- 22
- 22aV. E. Vogel, H. Altenbach, J. Drossard, H. Schmickler, H. Stegelmeier, Angew. Chem. Int. Ed. Engl. 1980, 19, 1016–1018; Angew. Chem. 1980, 92, 1053–1054;
- 22bK. Satake, R. Okuda, M. Hashimoto, Y. Fujiwara, H. Oka-moto, M. Kimura, S. Morosawa, J. Chem. Soc.-Perkin Trans. 1994, 1, 1753–1757.
- 23J. A. Jordan-Hore, C. C. Johansson, E. M. Beck, M. J. Gaunt, J. Am. Chem. Soc. 2008, 130, 16184–16186.
- 24Z. Liang, L. Ju, Y. Xie, L. Huang, Y. Zhang, Chem. Eur. J. 2012, 18, 15816–15821.
- 25M. Bu, G. Lu, C. Cai, Org. Chem. Front. 2016, 3, 630–634.
- 26Z. Zuo, J. Liu, J. Nan, L. Fan, W. Sun, Y. Wang, X. Luan, Angew. Chem. Int. Ed. 2015, 54, 15385–15389; Angew. Chem. 2015, 127, 15605–15609.
- 27Z. Chen, Q. Yan, Z. Liu, Y. Xu, Y. Zhang, Angew. Chem. Int. Ed. 2013, 52, 13324–13328; Angew. Chem. 2013, 125, 13566–13570.
- 28K. Beydoun, H. Doucet, Eur. J. Org. Chem. 2012, 34, 6745–6751.
- 29Z. Zhang, J.-T. Deng, J.-Y. Feng, J.-Y. Liang, X.-T. Xu, J.-B. Peng, J. Org. Chem. 2023, 88, 12054–12063.
- 30R. D. Viirre, G. Evindar, R. A. Batey, J. Org. Chem. 2008, 73, 3452–3459.
- 31Y. Yang, L. Mao, W. Guan, X. Wei, Y. Shao, Z. Luo, X. Lin, L. Li, J. Sci. Food Agric. 2021, 101, 2210–2217.
- 32J. Liu, R. Ma, F. Bi, F. Zhang, C. Hu, H. Venter, S. J. Semple, S. Ma, Bioorg. Med. Chem. Lett. 2018, 28, 1825–1831.
- 33D. Liang, Z. Hu, J. Peng, J. Huang, Q. Zhu, Chem. Commun. 2013, 49, 173–175.
- 34L. J. Scott, Drugs 2019, 79, 1905–1909.