DNA Tetrahedra as Functional Nanostructures: From Basic Principles to Applications
Graphical Abstract
Abstract
Self-assembled supramolecular DNA tetrahedra composed of programmed sequence-engineered complementary base-paired strands represent elusive nanostructures having key contributions to the development and diverse applications of DNA nanotechnology. By appropriate engineering of the strands, DNA tetrahedra of tuneable sizes and chemical functionalities were designed. Programmed functionalities for diverse applications were integrated into tetrahedra structures including sequence-specific recognition strands (aptamers), catalytic DNAzymes, nanoparticles, proteins, or fluorophore. The article presents a comprehensive review addressing methods to assemble and characterize the DNA tetrahedra nanostructures, and diverse applications of DNA tetrahedra framework are discussed. Topics being addressed include the application of structurally functionalized DNA tetrahedra nanostructure for the assembly of diverse optical or electrochemical sensing platforms and functionalized intracellular sensing and imaging modules. In addition, the triggered reconfiguration of DNA tetrahedra nanostructures and dynamic networks and circuits emulating biological transformations are introduced. Moreover, the functionalization of DNA tetrahedra frameworks with nanoparticles provides building units for the assembly of optical devices and for the programmed crystallization of nanoparticle superlattices. Finally, diverse applications of DNA tetrahedra in the field of nanomedicine are addressed. These include the DNA tetrahedra-assisted permeation of nanocarriers into cells for imaging, controlled drug release, active chemodynamic/photodynamic treatment of target tissues, and regenerative medicine.
1 Introduction
The information encoded in the base sequence of nucleic acids introduces instructive structural and functional information into the biopolymer.1 Besides the dictated base-paired double helix strands, the control over the stability of the duplexes by number and nature of base pairs,2 the principles of strand displacement processes,3 and the switchable reconfiguration properties of nucleic acids, upon intercommunication between specific sequence strands or in the presence of auxiliary triggers, control the structure and functions of nucleic acid assemblies. These include, for example, the pH-stimulated formation and dissociation of i-motif cytosine-rich strands4 or T−A ⋅ T/C−G−C+ triplex structures,5 the K+ or Sr2+ ions induced assembly of G-quadruplexes and their separation in the presence of crown ethers,6 the co-stabilization of oligonucleotide complexes by metal-ion-bridged mismatched bases, such as C−Ag+−C or T−Hg2+−T units, and their separation by metal-ion uncaging ligands,7 e.g., cysteine, the light-induced stabilization or destabilization of duplex DNA strands by photoisomerizable trans/cis azobenzene intercalator units,8 or the supramolecular assembly of ligand-nucleic acid structures, such as oligo adenine/cyanuric acid assemblies.9 Also, enzymes, such as polymerases,10 nickases,11 ligases12 or endonucleases,13 are used as catalysts to modify and functionalize nucleic acid structures. In addition, the base sequences comprising nucleic acids introduce catalytic and recognition functions into the biopolymer. These include sequence-specific binding of low-molecular-weight substrates or macromolecules by aptamers14 or catalytic functions of DNAzymes15 or nucleoapzymes.16 This rich “tool-box” of functional properties of nucleic acids paved the ground to the development of the topic of DNA nanotechnology that has found diverse applications.17 These include the development of DNA switches18 and machines,19 the assembly of dynamic networks emulating native biological networks,20 and the assembly of logic gates and computing circuits.21 Diverse applications emerged from the reconfiguration, recognition, and catalytic functions of DNA. These included the development of optical22 or electrochemical sensors,23 the engineering of gated nanocarriers for the controlled release of drugs,24 the assembly of soft materials, e.g., DNA-based hydrogels revealing stimuli-responsive stiffness properties,25 shape-memory morphing,26 self-healing27 and tissue engineering,28 controlled drug release,26d, 29 and the design of actuating robotic devices.30
Moreover, the plethora structural and functional properties of oligonucleotides were used to assemble programmable two-dimensional (2D) and three-dimensional (3D) DNA structures, and to synthesize DNA-nanoparticle hybrids for the assembly of nanodevices or hierarchical functional nanostructures. Programmed Y-shaped and Holliday junction DNA modules were used to assemble 2D or 3D complex structures31 and the origami method provided a versatile approach to assemble 2D or 3D DNA frameworks.17d, 32 According to this method, a long DNA scaffold is crosslinked by computer-programmed “staple” crosslinking short nucleic acids to form the desired 2D or 3D shapes.33 By tethering protruding nucleic acid strands to the upper/lower faces of the origami rafts or linking nucleic acid tethers to the edges of the origami frameworks, the functionalization of the origami structures with biomolecules or nanoparticles was realized.34 Diverse applications of the hybrid nanoparticles/origami frameworks were demonstrated, including the design of plasmonic or optical antenna devices,35 waveguides,36 chiroplasmonic systems37 and the assembly of Au nanoparticles crystalline composites.38 Also, the modification of the origami scaffolds with biomolecules established stimuli-responsive drug carriers39 or the operation of switchable biocatalytic cascades.40 Moreover, the sequence-dictated assembly of duplex nucleic acids provides versatile means to construct 3D structures such as cubes,41 capsules,42 or hierarchical self-assembly of DNA tetrahedra, dodecahedron or buckyball assemblies.43 In particular, the self-assembly of DNA tetrahedra structures by means of four complementary DNA strands enabled the modification of the edges or the corners of the DNA tetrahedra and the precise positioning of agents at inner or outer sites of tetrahedra cages, attracted substantial recent research efforts, and diverse nanotechnological applications of DNA tetrahedra were introduced.44 The present review article presents the advances and important applications of the DNA tetrahedra nanostructures in the developing area of DNA nanotechnology. In contrast to several review articles addressing specific research areas implementing DNA tetrahedra as functional constituent,45 the present review article aims to provide a comprehesive essay presenting the diverstity of scientific diciplines where DNA tetraehdra play key roles in their developemnt. Specifically, the scope of different applications of the tetrahedra are introduced and the future perspectives of these nanostructures are addressed. The topics to be addressed are displayed in Scheme 1. These include the assembly principles of the DNA tetrahedra and their properties, the application of the DNA tetrahedra frameworks for sensing and imaging, the reconfiguration of DNA tetrahedra and their integration in dynamic networks, the use of DNA tetrahedra frameworks for the assembly of functional complex structures, and the significance of DNA tetrahedra in the area of nanomedicine.
Functional DNA tetrahedra nanostructures and their applications.
2 Assembly and Characterization of DNA Tetrahedra
The basic DNA tetrahedron nanostructure originally reported by Turberfield46 was spontaneously self-assembled by structurally engineered double helices formed upon annealing four predesigned single-strands (1), (2), (3), (4) exhibiting appropriate base complementarity (Figure 1). Each of the six edges of the tetrahedron consists of a 17-base “edge subsequence” hybridized with its complement. Each strand contains three of these sub-sequences or their complements separated by two-base “hinges” designed to be non-hybridized, and these were introduced into the construct to retain appropriate flexibility to accommodate an angle of 60° between adjacent edges. Figure 1A depicts the DNA tetrahedron TA assembled upon annealing of the strand (1)-(4), panel I. The model of the duplex-assembled tetrahedron is depicted in panel II. By the permutation of the base-order and the complementarity of strands (3) and (4) into (5) and (6), panel I, the self-assembled annealed tetrahedron TB exhibiting enantiomeric relationship to TA is formed. The stepwise assembly of the tetrahedron structure may be followed by electrophoretic separation of the intermediate DNA conjugates (Figure 1B). The structural features of the DNA tetrahedron were initially imaged by atomic force microscopy (AFM)47 (Figure 1C) and later high resolution cryogenic transmission electron microscopy (Cryo-TEM) image and reconstructed structure enabled the detailed determination of the 3D structure of the DNA tetrahedron constructs48 (Figure 1D).
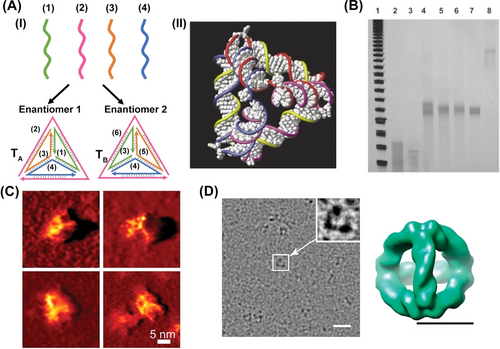
(A) Panel I-Schematic assembly of DNA tetrahedra by four single stranded DNA. Panel II−A space filling view of DNA tetrahedron. (B) Electrophoretic separation of the stepwise assembly of DNA tetrahedron: Lane 2: strands 1+2; Lane 3: strands 3+4; Lane 4: strands 1+2+3; Lane 5: strands 1+2+4; Lane 6: strands 1+3+4; Lane 7: strands 2+3+4; Lane 8: strands 1+2+3+4. (C) AFM image of the purified DNA-tetrahedra structure. (D) An electron cryomicrograph of a DNA-tetrahedron showing 3D density map at 20 Å resolution. Scale bar, 20 nm (left) and 5 nm (right). (A) and (B) Reproduced from Ref. [46] with permission from the Royal Society of Chemistry, copyright (2004). (C) Reproduced from Ref. [47] with permission from the American Association for the Advancement of Science (AAAS), copyright (2005). (D) Reproduced from Ref. [48] with permission from the American Chemical Society, copyright (2009).
The concept to design the tetrahedron structure provided a plethora of approaches to modify tetrahedra structural features and its structural functionalities. The lengths of the duplexes comprising tetrahedral structures control their sizes.49 For example, double-strand consisting of 13, 17, 26, and 37 base-pair revealed tetrahedra sizes corresponding to 4.4 nm, 5.8 nm, 8.8 nm, and 12.6 nm, respectively (Figure 2A). By interaction of the tetrahedra frameworks to auxiliary strands interacting with the edges of the frameworks, the structural distortion of the framework was demonstrated50 (Figure 2B). Treatment of a shrunk tetrahedron framework, (T), that included a “squeezed” edge, controlled by a hairpin structure, with a fuel hairpin that hybridized with the squeezing hairpin element, an extended tetrahedron framework consisting of a toehold-bridged duplex, (T′), was formed. The reverse interaction of extended tetrahedra with the anti-fuel hairpin resulted in the displacement of the toehold-modified duplex edge, leading to the reversed reconfiguration of the squeezed tetrahedral framework. By labeling the edge constituents with the Cy3 and Cy5 fluorophores, the dynamic reversible reconfiguration of the frameworks was followed by the respective Förster resonance energy transfer (FRET) responses of the fluorophores. A similar concept was applied for the dynamic reversible light-induced reconfiguration51 between the extended tetrahedron framework, stabilized by the trans-azobenzene duplex-stabilized edge, and the squeezed tetrahedron framework that displaced the cis-azobenzene-modified strand, upon irradiation of ultraviolent light (UV) (Figure 2C, Panel I). As before, by functionalization of the edge termini with a FAM fluorophore and the Dabcyl quencher, the dynamic reconfiguration of the frameworks were followed by the FAM fluorescence intensities in the two tetrahedra frameworks, Panel II.
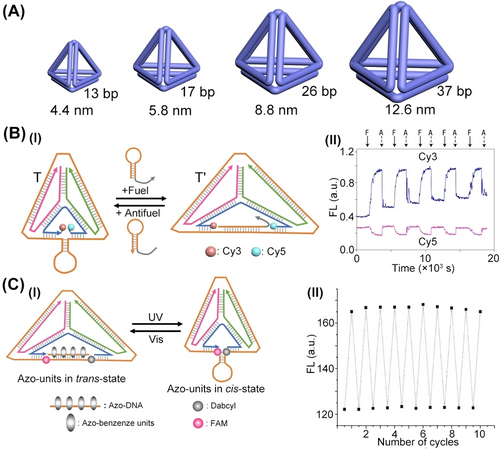
(A) Controlling the sizes of DNA tetrahedra by number of base-pairs comprising tetrahedra edges. (B) Panel I–Dynamic switchable reconfiguration of the DNA tetrahedra frameworks. Panel II−By labelling the edge strand with Cy3/Cy5 fluorophores, the dynamic distortion of tetrahedra was followed by FRET responses of fluorophores. (C) Panel I–Light-stimulated dynamic cyclic reconfiguration of DNA tetrahedron by azobenzene intercalator. Panel II−By labelling edge termini with FAM fluorophore/Dabcyl quencher, the light-stimulated reconfigurations of tetrahedron were followed by fluorescence intensities of FAM. (B) Reproduced from Ref. [50] with permission from Springer Nature, copyright (2008). (C) Reproduced from Ref. [51] with permission from the Royal Society of Chemistry, copyright (2011).
Besides the control over the dimensions and geometrical features of the DNA tetrahedra nanostructures by means of the strands comprising tetrahedra, or by the dictated dynamic perturbation of the edge strands composing the structures, the edges of frameworks provide means to dictate the position of auxiliary loads at defined internal or external positions of the DNA tetrahedra cage.52 This is exemplified in Figure 3A with the assembly of a symmetrical DNA tetrahedron structure composed of four strands S1, S2, S3, and S4 that form the respective duplex edges. The duplex formed between peripheral strand S1 and the strand S4 was engineered, however, to include a systematic broken edge of S4 along the bases T5 to T15 hybridized with the outer strand S1. The protein, cytochrome c, was tethered to each of the ended Ti positions. As the transition of the duplex edge between positions T5 to T15 was designed to yield a double helix revealing a 360° rotation of the cytochrome c load relative to the fixed strand S1, the stepwise transition between the edge position is anticipated to rotate the protein load by 35° around the S1 axis. That is, the transition to step T8 is anticipated to position the protein at the extremely internal cage location whereas the position of cytochrome c at edge position T13 locates the protein load at the extremely external cage position. The dynamic transitions of cytochrome c, in respect to the fixed edge axis of the cage, were followed by the relative migration rates of the protein-functionalized tetrahedra in electrophoresis gel (Figure 3B). While T8-cytochrome c-loaded tetrahedra revealed the fastest migration rate, the T13 cytochrome c-loaded tetrahedra demonstrated the lowest migration rate.
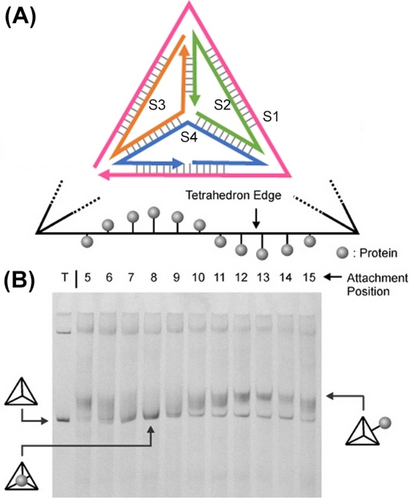
(A) Programmed positioning of a protein (Cytochrome c) to target bases on duplex edges of tetrahedron. (B) Native polyacrylamide gel demonstrating variable mobility of different protein-position modified tetrahedra. Lane T corresponds to naked DNA tetrahedra, inner/outer cytochrome c functionalized tetrahedra are explicitly marked from lane 5 to lane 15. Reproduced from Ref. [52] with permission from the Wiley-VCH, copyright (2006).
A different approach to assemble DNA tetrahedra nanostructures is depicted in Figure 4A with the assembly of a three-point “star” subunit P.43 The assembly of the three-point “star” subunit P comprising of a tripod supramolecular unit that includes toehold tethers at the three-arms leads to the self-assembly of a four-corner tetrahedron composed of four inter-bridged three-arms subunits P. The assembly of tetrahedra was supported by dynamic light scattering (DLS) experiments, demonstrating objects exhibiting a hydrodynamic radius of 10.3±0.6 nm, and AFM images (Figure 4B), and cryo-TEM images that after reconstruction demonstrated the symmetrical four-corner tetrahedral structures (Figure 4C). Moreover, an alternative origami-based approach to construct DNA origami-face tetrahedra cages was reported.53 According to this method, a single origami framework was assembled by appropriate “staple” strands to yield a four-triangle origami scaffold exhibiting edge engineered “hinges” of appropriate complementary for programmed inter-scaffold dictated folding into the 3D caged origami tetrahedral structures (Figure 4D). The formation of the 3D origami-based tetrahedra was supported by DLS and high-resolution TEM images (Figure 4E), revealing tetrahedra cages with edge dimensions of ca. 54 nm.
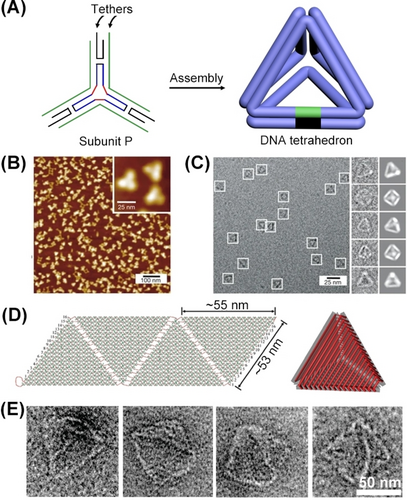
(A) Self-assembly of DNA tetrahedra by three-point “star” subunit. (B) AFM image corresponding to tetrahedra. (C) Cryo-TEM images of DNA tetrahedra particles, the raw enlarged Cryo-TEM images, and reconstructed 3D particle structures. (D) Schematic self-assembly of a 3D origami DNA tetrahedron through the “stapled” folding of four triangular scaffolds. (E) Enlarged TEM images of DNA tetrahedra. (A–C) Reproduced from Ref. [43] with permission from Springer Nature, copyright (2008). (D) and (E) Reproduced from Ref. [53] with permission from the American Chemical Society, copyright (2009).
Beyond the chemical methods to synthesize size-controlled, DNA tetrahedra revealing diverse structural complexities, and the different spectroscopic and microscopic means to characterize the nanostructure, recognizing the programmable features and properties of the tetrahedra nanostructure are important. These are outlined in Figure 5, summarizing the capabilities to modify the tetrahedra corner sites and edge domains towards different functions, and the different physiochemical features of the nanostructure. The present review article implements the DNA tetrahedra features across a broad boundary of scientific disciplines and applications.
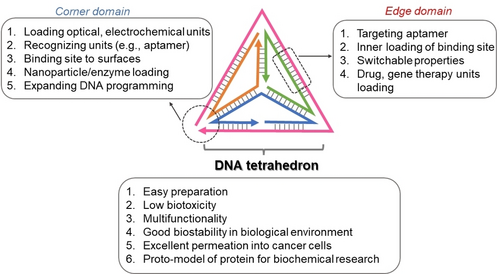
Implementation of structural and physiochemical features of the DNA tetrahedron nanostructure across diverse scientific disciplines.
3 DNA Tetrahedra as Functional Units for Optical Sensing and Imaging
The DNA tetrahedra frameworks provide versatile nanostructures for the integration of sensing recognition elements and optical transducing probes at the edges or corners of the tetrahedron nanostructures. The integration of reconfigurable sensing and transducing elements into the tetrahedra structures provides dynamic means to distort the framework by the sensing events, thereby stimulating modulated optical properties transducing the recognition events. This section introduces the engineering of functional tetrahedron nanostructures enabling optical sensing, multiplexed sensing and amplified sensing.
3.1 Reconfigurable Edge-modified DNA Tetrahedra for Sensing
The functionalization of the edge domains of DNA tetrahedra and their structural distortion upon interactions with auxiliary agents provides versatile means to use the DNA tetrahedra as sensing scaffolds. This is exemplified in Figure 6 with the multiplex analysis of miRNAs, aptamer-ligand complexes, or enzymes by the tetrahedra scaffold.54 Figure 6A depicts a three-hairpins, H1-, H2-, and H3-functionalized analysis of three different miRNAs, e.g., miRNA-21 by hairpin H1, miRNA-221 by H2, and miRNA-155 by H3. The respective edges hybridized with the three hairpins frames were modified at the stem domains of H1 with the FAM fluorophore/BHQ1 quencher, stem domain of H2 with the ROX fluorophore/BHQ2 quencher, and stem domain of H3 with the Cy5 fluorophore/BHQ2 quencher. Under these conditions, the fluorophore units existed in a quenched state. The loop regions of H1, H2, and H3 included the recognition sequence for miRNA-21, miRNA-221 and miRNA-155, respectively. Subjecting the tetrahedra module to any of the miRNAs resulted in the selective opening of the respective hairpin units, and the miRNA concentration guided switching-on of the respective fluorophores, stimulated by the separation of the fluorophore-quencher pair, associated with the respective miRNA-responsive hairpins. Subjecting the tetrahedra sensing module to two different miRNAs or to three miRNAs enabled the multiplexed analysis of the respective miRNAs (Figure 6B). The miRNAs-guided optical sensing was further applied to selectively image miRNAs associated with specific cancer cells (Figure 6C). Subjecting MCF-7 breast cancer cells containing the miRNA-21 and miRNA-155 to the miRNA-responsive tetrahedra switches on the intracellular green fluorescence of FAM and the red fluorescence of Cy5, associated with the concomitant opening of hairpin H1 and H3. In turn, treatment of HepG2 liver cancer cells that include overexpressed miRNA-21 resulted in the selective green fluorescence of FAM, originating from the selective opening of H1. Control experiments applying the tetrahedra sensing module on MCF-10 A epithelial breast cells, lacking any overexpressed miRNA, did not lead to any fluorescent signal, consisted with the caged configuration of the hairpin units in the sensing module.
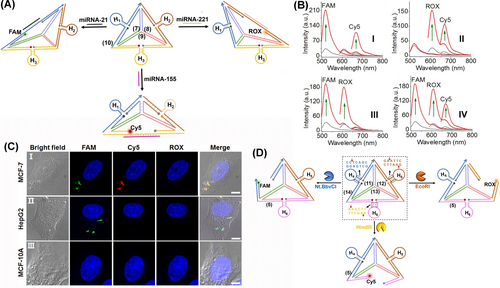
(A) Scheme of DNA tetrahedra for multiplexed optical analysis of miRNAs by functionalizing switchable hairpins. miRNAs-guided opening of the respective hairpins leads to the corresponding fluorescence of respective fluorophores. (B) Multiplexed analysis of: Panel I-miRNA-21 and miRNA-155; Panel II-miRNA-221 and miRNA-155; Panel III-miRNA-21 and miRNA-221; Panel IV-miRNA-21, miRNA-221, and miRNA-155. (C) Intracellular multiplexed analysis of miRNA-21, miRNA-155 in MCF-7 breast cancer cells, miRNA-21 in HepG2 liver cancer cells, and non-malignant MCF-10 A epithelial breast cells by the miRNA-responsive tetrahedra sensing scaffold. Scale bar: 5 μm. (D) DNA tetrahedron modified with three enzyme-responsive hairpins for the multiplexed analysis of EcoRI, Nt.BbvCI, and HindIII nicking enzymes. Reproduced from Ref. [54] with permission from the American Chemical Society, copyright (2020).
In addition, a tetrahedra associated with hairpin on modified at their three edges with pre-engineered hairpins were applied for the optical detection of DNA cleavage enzymes54 (Figure 6D). The tetrahedra edges included the FAM fluorophore/BHQ1 quencher pair, the ROX fluorophore/BHQ2 quencher pair, and the Cy5 fluorophore/BHQ2 quencher pair at the termini edge positions proximate to the hairpin stem domains. The stem regions of H4, H5, and H6 included sequence-specific duplexes that are cleaved by the nicking enzyme Nt.BbvCI, and the endonuclease EcoRI, and HindIII, respectively. Treatment of the reaction module with any of the cleavage enzymes resulted in the selective, concentration-guided cleavage of the respective hairpins, leading to the switched-on fluorescence of FAM, ROX and Cy5, respectively. By subjecting the tetrahedra reaction module to any of the two enzymes or the three enzymes, the multiplexed analysis of the biocatalysts was demonstrated. The concept of hairpin-modified edges of tetrahedra was further realized with the multiplexed analysis of aptamer-ligand complexes.55
The flexibility of designing the DNA tetrahedra structures was further used to develop an ATP sensing module56 (Figure 7A). One of the edges of the tetrahedra was modified with the donor-acceptor pair (rhodamine green and dabcyl) that were spatially extended by the strand L hybridizing with the edge constituents. The strand L included, however, the ATP aptamer recognition sequence. In the presence of ATP, the loop structure of the ATP-aptamer complex forced the donor-acceptor pair into a spatially close configuration that resulted in effective ATP concentration-guided quenching of rhodamine green (Figure 7B).
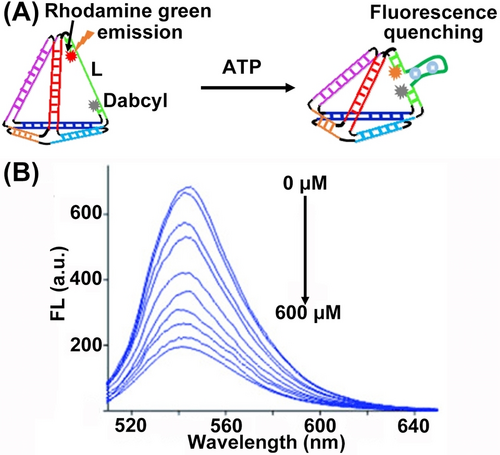
(A) Schematic sensing of ATP by DNA tetrahedron upon ATP-aptamer complex generation. (B) Decaying fluorescence intensities of the DNA tetrahedron under variable concentrations of ATP. Reproduced from Ref. [56] with permission from the Wiley-VCH, copyright (2012).
3.2 Reconfigurable Corner-modified Tetrahedra for Sensing
A versatile approach to apply fluorescent tetrahedra functionalized nanostructures for in vitro sensing and intracellular optical imaging has involved the modification of corners of the tetrahedra with fluorescent labels. According to this approach, tethers associated with the corner fluorophores act as sensing elements that upon the recognition of the target analyte perturb the fluorescent probe associated with the tetrahedra thereby, transducing the sensing events. Alternatively, the edge domains of the tetrahedra were engineered to include the recognition sensing domains. The sensing events perturbed the edge structures, thereby distorting the fluorescent features of the corner fluorophores, thus allowing the optical transduction of the sensing processes.
Figure 8A depicts the multiplexed analysis of three different mRNAs translating c-myc, TK1 and GalNac−T proteins using a tetrahedra module that includes at three corners, tethers consisting of FAM/BHQ1, Cy3/BHQ2, and Cy5/BHQ2 fluorophore/quencher pairs-modified duplexes, where the fluorophore-modified strands are complementary to the C-myc, TK1, and GalNac−T mRNA targets hybridized with quencher-functionalized strand.57 Subjecting the tetrahedra module to any of the respective mRNAs resulted in the concentration-guided removal of the respective fluorescent labels, thus allowing the selective and multiplexed analysis of the respective mRNAs (Figure 8B). This method was then applied to image the C-myc, TK1 and GalNac−T mRNAs overexpressed in MCF-7 breast cancer cells. The same concept was applied to develop a fluorophore/quencher corner-modified miRNA-responsive tetrahedra module for multiplexed sensing and cellular imaging of miRNAs.

(A) Application of corner-modified DNA tetrahedra for multiplexed mRNAs analysis. (B) Panels I and II-Tetrahedron-based optical detection of c-myc mRNA (I–II), TK-1 mRNA (III–IV) and GalNAc−T mRNA (V–VI). (A) and (B) Reproduced from Ref. [57] with permission from the American Chemical Society, copyright (2017).
Tetrahedra modified with different fluorophores were used as mitochondria-targeting probes imaging pH and Ca2+ ion changes in neurons.58 The tetrahedron scaffolds were modified with AF660 fluorescent imaging tracer, fluorescein isothiocyanate (FITC) as responsive fluorophore, fluorescent C-dots (CD) modified with the calcium ligand (CaL) enhancing the fluorescence, and (3-azidopropyl) triphenylphosphonium bromide, TPP, as mitochondria targeting agent (Figure 9A). Under acidic conditions and in the presence of Ca2+-ions the FITC (λem=520 nm) is quenched while the CD fluorescence at 600 nm is enhanced (Figure 9B, Panels I and II). Applying the tetrahedra probe to wild-type neurons allowed the AF660 visualization of TPP targeting mitochondria, and pH and Ca2+-stimulated changes in the mitochondria by the respective probes (Figure 9C).
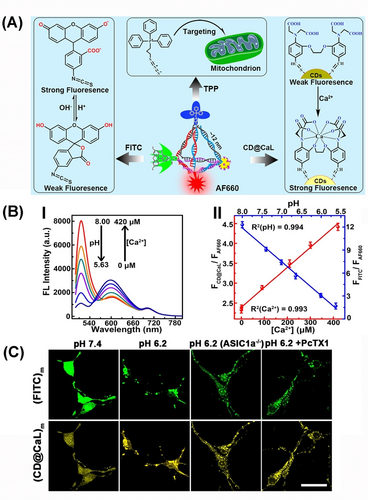
(A) Schematic DNA tetrahedra for intracellular targeting of the mitochondrion and imaging of Ca2+-ions and pH levels. (B) Panel I-Fluorescence changes from DNA tetrahedron under variable levels of Ca2+-ions and pH. Panel II–Calibration curves of fluorescence analysis of Ca2+-ion and pH levels from panel I. (C) Confocal fluorescence microscopy images corresponding to pH changes and Ca2+ changes in mitochondrial containments of wild-type neurons by DNA tetrahedron. Scale bars: 25 μm. Reproduced from Ref. [58] with permission from the American Chemical Society, copyright (2018).
4 DNA Tetrahedra on Surfaces for Electrochemical Sensing Applications
The programmed control over the sizes of the tetrahedra by the base composition (lengths) of tetrahedra constituents, the base sequences comprising the duplex edges of tetrahedra, the chemical functionalities associated with edges or corners of the tetrahedra, and the nature of chemical functionalities integrated into the tetrahedra scaffolds as intercalators or as flexible tethers, provide diverse means to control the functional performance of tetrahedra as sensing scaffold. Particularly, the functionalization of DNA tetrahedra structures with redox labels59 or the control over the interfacial electron transfer properties at DNA tetrahedra-modified electrodes, upon interaction with analyte constituents, provide versatile means to develop DNA-tetrahedra-based electrochemical sensors. In the present section, the structure-function relationships of nucleic acid tetrahedra-modified electrodes will be addressed, and the use of diverse redox-active labels associated with DNA tetrahedra for electrochemical sensing applications are discussed.
4.1 Physical Features of DNA Tetrahedra on Eectrode Surfaces
In general, the functionalization of the corners of tetrahedra scaffold with thiol functionalities provides a versatile means to assemble tetrahedra on metal, e.g., Au surfaces. By the functionalization of the DNA tetrahedra corners with one-, two- or three- thiol functionalities, it was demonstrated that the three thiol-functionalized tetrahedra form a stable dense monolayer on electrode surfaces, allowing the fourth vacant corner to be modified with a free flexible tether for subsequent anchoring of auxiliary agents, such as proteins or nucleic acids.
Understanding the charge transport properties of DNA tetrahedra scaffolds associated with conductive supports is a key feature to develop DNA tetrahedra-based electrochemical sensors.60 This issue was addressed by the integration of Methylene Blue, MB, redox-active intercalator units into duplex sites of DNA tetrahedra scaffolds assembled through three thiol functionalities on an Au surface, where the MB+ units were positioned in spatially close or sterically remote in respect to the electrode surface (Figure 10A, panel I). The voltammetric responses of tetrahedra states were identical (Figure 10A, panel II) implying that the redox-active intercalator units experienced similar charge transport features within the DNA carrier scaffold. The cleavage of one of tetrahedra duplex edges (Figure 10B, panel I), significantly perturbed the voltammetric response of the MB intercalator (Figure 10B, panel II), indicating that an intact duplex configuration of tetrahedra framework is essential to retain the charge transport to the intercalated constituent. The flexible tethering of the redox label to tetrahedra scaffold is, however, significantly affected by the spatial position of the redox label within tetrahedra structure (Figure 10C). The voltammetric responses of ferrocene (a non-intercalating redox label) tethered to DNA tetrahedra at bottom, middle or top positions, panel I, are strongly affected by the distance separating the redox label from the electrode, panel II. The spatially close position redox label revealed a high electronical response, whereas the ferrocene units in a remote position demonstrated inefficient electron transfer communication with the electrode. Furthermore, the sizes of tetrahedra scaffold have an immense effect on the coverage of the electrodes by tetrahedra structures and on the control over the surface properties toward interaction with auxiliary strands, and the subsequent application of tetrahedra-modified surface for sensing.49b As the size of tetrahedra scaffolds increases, the detection limit decreases consistent with the enhanced hybridization efficiencies of the larger tetrahedra scaffolds.
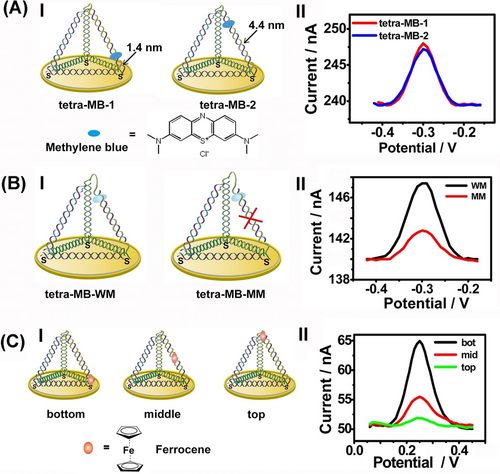
Effects of redox-labels positions in DNA tetrahedra on electrical redox labels: (A) Methylene blue (MB) intercalated in edge duplexes in close or remote positions in respect to electrode sufaces. (B) MB intercalated in duplex-edges reveals superior electron transfer properties as compared to MB intercalated in cleaved duplex edges of tetrahedra. (C) The electron transfer communication efficacies of labeled ferrocene on different positions of tetrahedra. Reproduced from Ref. [60] with permission from the American Chemical Society, copyright (2012).
4.2 Electrochemical Sensing with DNA-tetrahedra Scaffolds
The possibility of modifying the corners of DNA tetrahedra with functional sequence-specific tethers was applied to develop diverse sensing platforms. Figure 11A exemplifies the electrochemical sensing of a target gene T by the DNA tetrahedra scaffold.61 A multi-thiol functionalized DNA tetrahedra structure was assembled on an Au electrode, and the corner of tetrahedra was modified with the tether T′ that was complementary to a part of the target gene, T. In the presence of the target gene T, the hybrid T′/T duplex was formed. The subsequent functionalization of the sensing interface with the biotinylated strand (L) that hybridized with the toehold single-strand of T, followed by the association of the avidin-HRP conjugate to the interface, resulted in the bioelectrocatalytic interface for the voltammetric detection of the gene, T. In the presence of 3, 3′, 5, 5′-Tetramethylbenzidine, TMB, as redox label, the HRP-mediated electrocatalyzed reaction of H2O2 proceeded. The resulting volumetric response related to the concentration of the target gene and enabled the analysis of T the gene with a detection limit corresponding to 1 pM (Figure 11B). Moreover, the DNA-tetrahedra-assisted electrochemical sensing of a target nucleic acid was further developed by demonstrating that integration of the nucleic acid recognition probe in-between the corners of two adjacent tetrahedra frameworks associated with the electrode, provides an efficient means to enhance the sensing efficacy.62 This enhancement was attributed to the stretching of the coiled analyte strand by the recognition probe positioned in between the two tetrahedra.
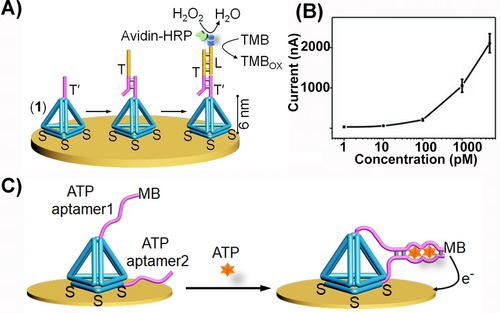
(A) Schematic electrochemical sensing platform for sensing a nucleic acid analyte T by a T′-tethered DNA tetrahedra framework associated with an electrode and using the biotinylated L-strand/avidin-HRP and H2O2/TMB as redox reporter unit. (B) Calibration curve corresponding to the analysis of variable concentrations of T by the sensing platform shown in (A). (C) Schematic configuration of a DNA-tetrahedron framework functionalized with ATP aptamer subunits, where one of the subunits is modified with the MB redox-label. (A) and (B) Reproduced from Ref. [61] with permission from the Wiley-VCH, copyright (2010). (C) Reproduced from Ref. [63] with permission from the Royal Society of Chemistry, copyright (2018).
In addition, the conjugation of nucleic acid tethers to the DNA tetrahedra scaffold was further extended to include aptamer tethers and to develop electrochemical aptasensor devices, e.g., anti-exosome aptamer.63 Alternatively, the structural reconfiguration of redox-labeled aptamer constituents associated with tetrahedra, as a result of formation of the aptamer-ligand complexes on tetrahedra, provided means to develop electrochemical aptasensors. For example, Figure 11C depicts the functionalization of two corners of tetrahedra with subunits of the ATP aptamer, where one of subunits is modified with methylene blue, MB, as redox label.64 In the absence of ATP, the redox-label is spatially apart from the electrode surface, leading to inefficient electron transfer communication with the electrode and low voltammetric response. In the presence of ATP, the resulting reconfiguration of the ATP-aptamer subunits complex led to spatial proximity of the redox label to the electrode and to effective electron transfer communication and high voltammetric response. The ATP guided electrochemical responses of the electrode were controlled by the concentrations of ATP and allowed the analysis of ATP with a detection limit corresponding to 5 nM.
5 Dynamic Reconfiguration of DNA Tetrahedra Structures
The dynamic reconfiguration of DNA nanostructures and the practical applications of these systems attract continuous interest in the area of DNA nanotechnology.19d These included the dynamic operation of supramolecular DNA machines19a, 19c and origami nanostructures,65 and their application as frameworks acting as switchable catalysts,40a chiroplasmonic devices,37b and switchable materials.66 In the present section, dynamic reconfiguration of DNA tetrahedra and the applications of these systems will be addressed.
5.1 Dynamic Reconfiguration of DNA Tetrahedra Structure
The dynamic reversible and switchable oligomerization of DNA tetrahedra structures was accomplished by the triggered formation and dissociation of stimuli responsive bridging units67 (Figure 12). In one system, the pH-stimulated dimerization of DNA tetrahedra structures and the reversible separation of the dimer structures were demonstrated by the application of T−A ⋅ T triplex nucleic acid bridging units (Figure 12A). Two tetrahedra T1-functionzalized with a tether p and T2-modified with a duplex tether q/o yielded the triplex (T−A ⋅ T)-bridged T1−T2 dimer structure at pH=7.0. At pH=10.0, the T−A ⋅ T bridges were dissociated leading to the separated tetrahedra structures. By labeling the tether p associated of T1 with Cy5 and the duplex tether q/o with Cy3, the resulting intramolecular FRET signal in the triplex-stabilized tetrahedra dimer provided a readout signal for the dimerization process (Figure 12B). By the pH-stimulated formation and dissociation of the triplex-bridged T1−T2 dimer, the resulting FRET signal provided a readout signal for the cyclic dimerization/separation of the DNA tetrahedra dimer, inset. Similarly, by tethering to the DNA tetrahedra structures T3 and T4 with G-quadruplex subunits (x and y), the K+-ion-stimulated dimerization of tetrahedra T3−T4 by interlinking the K+-ion-stabilized G-quadruplex was demonstrated (Figure 12C). In the presence of 18-crown-6-ether, the G-quadruplexes were separated to yield the individual tetrahedra. The formation of the K+-ion stabilized G-quadruplex bridging units was followed by the incorporation of hemin into the G-quadruplex bridges yielding the hemin/G-quadruplex DNAzyme bridging units that stimulated the H2O2-catalyzed oxidation of Amplex Red to the fluorescent Resorufin that provided a readout signal for the dimerization of tetrahedra structures (Figure 12D). The cyclic K+-ion induced formation of the T3−T4 dimer, and the reverse crown-ether guided separation of the dimer was then probed by the ON/OFF switching of the DNAzyme-mediated H2O2 oxidation of Amplex Red to Resorufin, inset. The switchable dimerization/separation of tetrahedra T1−T2 and T3−T4 using pH or K+-ion/crown ether triggers was then applied to dictate the selective dimerization of T1−T2 or T3−T4 in the presence of the respective inputs (pH or K+-ions), resulting in the dictated FRET signal or generated by the hemin/G-quadruplex catalyzed oxidation of Amplex Red by H2O2. Alternatively, upon addition of the auxiliary tetrahedra T5 that were modified with the tethers×and duplex q/o, to the mixture of T1 and T4, the oligomerization of tetrahedra into the trimer structure T4−T5−T1 proceeded (Figure 12E). The formation of trimer was confirmed by the parallel transduction of the FRET signal and Resorufin fluorescence output signal and concomitant gel electrophoretic separation experiments. The triggered dynamic oligomerization of DNA tetrahedra structures was further applied to selectively sense miRNAs and to image cancer cells.67
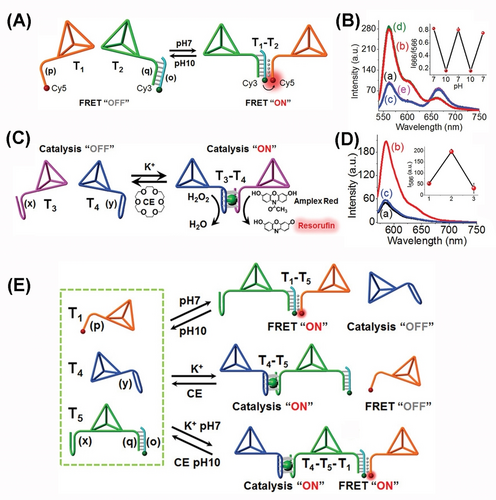
(A) pH-stimulated dimerization and separation of two DNA tetrahedra. (B) Probing the dynamic pH-induced triplex-guided formation/dissociation of the DNA-tetrahedra assembly by FRET fluorescence responses. (C) Switchable dimerization/separation of the DNA tetrahedra by G-quadruplex subunits. (D) The hemin/G-quadruplex catalyzed oxidation of Amplex Red to fluorescent Resorufin providing the readout signal for the switchable formation/dissociation. (E) Optional stimuli-guided oligomerization of DNA tetrahedra nanostructures using a mixture of three DNA tetrahedra units T1, T4, T5, functionalized with respective oligomerization/separation units. Reproduced from Ref. [67] with permission from the Wiley-VCH, copyright (2021).
The dynamic dimerization of DNA tetrahedra structures was further examined in cell-like (protocell) environments by probing biocatalytic functions of the dimer structures and their interactions with biocatalysts (enzymes) in the confined media of the protocells.68 Hydrogel microcapsules loaded with tetrahedra T1 and T2 (functionalized at their corner with G-quadruplex subunits) in the absence, or eventually the presence of an enzyme, e.g., glucose oxidase, were prepared according to Figure 13A. CaCO3 microparticles impregnated with the DNA tetrahedra and enzyme were modified with poly(allylamine hydrochloride), PAH, and subsequently with a promoter nucleic acid strand (15). The particles were subjected to an aqueous solution that included the carboxymethyl cellulose polymer chains P1 and P2 modified with hairpins H1 and H2/(16). The hairpins H1 and H2 were engineered to allow the promoter-induced hybridization chain reaction (HCR) that resulted in the coating of the CaCO3 core particles with a hydrogel film crosslinked by the opened duplexes H1/H2. The subsequent etching of the CaCO3 core with ethylenediaminetetraacetic acid (EDTA) yielded the microcapsule containments loaded with the DNA tetrahedra (and eventually the enzyme). Subjecting the DNA microcapsules loaded with the tetrahedra T1 and T2 to K+-ions and hemin resulted in the dynamic K+-ion-stimulated stabilization of the hemin/G-quadruplex DNAzyme in the protocell assembly that catalyzed the oxidation of Amplex Red to the fluorescent Resorufin (Figure 13B). Subjecting the microcapsules to 18-crown-6-ether, CE, resulted in the separation of the hemin/G-quadruplex-bridged tetrahedra dimer and to the switched-off DNAzyme activity. By the cyclic permeation of K+-ions/CE into the microcapsules, the DNAzyme activity was reversibly switched between “ON” and “OFF” states (Figure 13C). In the presence of co-loaded glucose oxidase, GOx, in the protocell, and treatment of the system with glucose (Figure 13D) the biocatalytic cascade consisting of the aerobic oxidation of glucose to gluconic acid and H2O2, and the subsequent hemin/G-quadruplex-bridged dimer DNAzyme catalyzed oxidation of Amplex-Red to the fluorescent Resorufin proceeded in the protocell. The biocatalytic cascade operating in the protocell was found to be 5-fold enhanced as compared to the biocatalytic cascade operation by an equal content of biocatalysts in a diffusional random configuration (Figure 13E). The enhanced biocatalytic cascade in the confined environment of the protocell was attributed to the effective channeling of the GOx-generated H2O2, formed at high local concentration in the protocell, into the adjacent DNAzyme catalytic units.
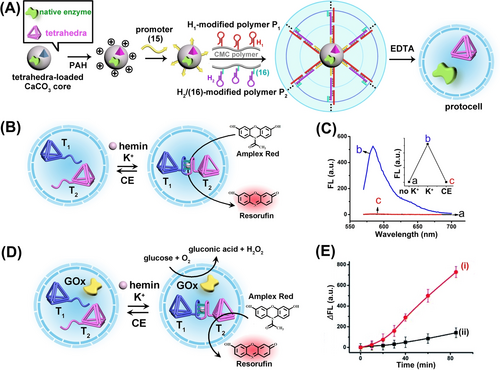
(A) Integration of DNA-tetrahedra (as protein models) and/or native enzymes into carboxymethyl cellulose microcapsules as biocatalysts-loaded protocells. (B) Encapsulation of two DNA tetrahedra T1 and T2 modified each with G-quadruplex subunits as tethers in microcapsule. The switchable K+-ion-stimulated dimerization of tetrahedra constituents indicated by hemin/G-quadruplex catalyzing the oxidation of Amplex Red by H2O2 to form Resorufin. (C) Switchable fluorescence intensities of hemin/G-quadruplex-catalyzed generation of Resorufin in the microcapsules system. (D) Integration of a mixture of DNA-tetrahedra T1, T2 functionalized with G-quadruplex subunits and the enzyme GOx in a microcapsule containment. (E) Time-dependent formation of Resorufin by the intracapsular driven biocatalytic cascade, Curve (i) and by non-confined driven biocatalytic cascade, Curve (ii). Reproduced from Ref. [68] with permission from the Royal Society of Chemistry, copyright (2022).
5.2 DNA Tetrahedra Dynamically Switchable Nucleic Acid Machineries
DNA tetrahedra provide functional frameworks for the dynamic switchable operation of nucleic acid machineries.69 This is exemplified in Figure 14 with the assembly of a DNA tetrahedron that guides the dynamic switchable operation of a transcription machinery. The tetrahedra include a caged inactive partial transcription template composed of the domains G/P domains and modified with the Cy3/Quncher labels, Figure 14A. In the presence of the “key” strand G′/P′/H, the tetrahedron framework is partially unlocked to yield the separated Cy3-labelled separated edge, acting as a promoter-activated transcription template, that in the presence of added T7RNA polymerase/NTPs yields in the presence of 3,5-difluoro-4-hydroxybenzylidene imidazolinone (DFHBI) the fluorescent DFHBI spinach aptamer complex. Subjecting the transcription machinery to the counter-locking strand G/P/H′ displaces the lock strand, resulting in the separation of the transcription template and the reconfiguration of the caged transcription-inactive DNA tetrahedra. The parent DNA tetrahedron and unlocking DNA tetrahedron could be imaged by AFM, Figure 14B, Panels I and II and by the switched fluorescence of the Cy3-labeled uncaged tetrahedra frameworks, Panel III. The “ON”/“OFF” switchable operation of the transcription machinery is displayed in Figure 14C. By mixing two functional DNA tetrahedra engineered to be unlocked selectively by two different “unlocking keys” yielding the DFHBI spinach aptamer- or/and the Malachite Green (MG) aptamer-generation transcription machinery, Figure 14D, the gated switchable operation of the two transcription machineries guided by the two tetrahedra was demonstrated, Figure 14E.
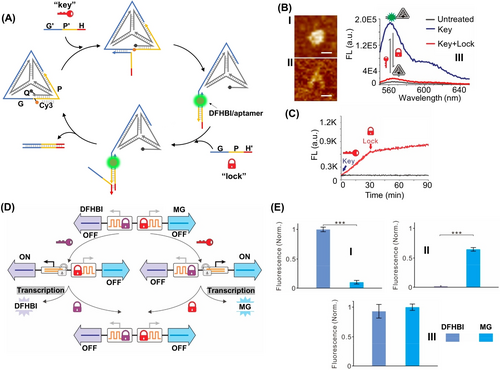
(A) A switchable DNA tetrahedra-stimulated transcription machinery. (B) AFM images corresponding to: Panel I-the caged inactive transcription machinery shown in (A). Panel II-the fueled, uncaged, promoter-functionalized, DNA tetrahedra transcription machinery transcribing the fluorescent DFHBI-aptamer product. Scale bar, 10 nm. Panel III-Switchable fluorescent spectra upon the fuel/anti-fuel uncaging/caging of the fluorophore/quencher modified tetrahedra structures. (C) Time-dependent fluorescence changes upon the switchable uncaging and caging of the DNA tetrahedra transcription machinery transcribing the fluorescent DFHBI/aptamer complex. (D) Schematic application of two different DNA-based tetrahedra machineries guiding the gated transcription of the fluorescent DFHBI-aptamer and/or MG-aptamer complexes, in the presence of specific “unlocking keys” strand inputs. (E) Gated fluorescence outputs upon: Panel I-the input-driven uncaging of the DFHBI transcription machinery. Panel II-the input-driven uncaging of the MG transcription machinery. Panel II-upon the simultaneous operation of DFHBI and MG transcription machineries. Error bars represent standard deviations from at least three tests. *P≤0.05, ***P≤0.001. Reproduced from Ref. [69] with permission from the American Chemical Society, copyright (2020).
5.3 Dynamic Reconfiguration of DNA Tetrahedra Networks
The development of artificial dynamic networks mimicking biological networks attracts recent research interest as a part of the rapidly developing area of System Chemistry.70 Dynamic biological networks reveal characteristic features including adaptive,71 hierarchically adaptive,72 switchable,73 and amplified reactivity,74 signal promotion75 and information transfer capacities.76 A simple dynamic network aiming to emulating some of the features of biological network is depicted in Figure 15. The network includes a mixture of four dynamically equilibrated constituents AA′, BB′, AB′ and BA′ composed of the components A, A′, B, B′. Subjecting the constitutional dynamic network (CDN) X to trigger T1 that stabilizes constituent AA′, provides a promoting signal, guiding the dynamic reconfiguration of CDN X to CDN Y, where constituent AA′ is upregulated due to its stabilization, and on the expense of AB′ and BA′ that are simultaneously downregulated. The resulting separation of AB′ and BA′ yields, however, the concomitant upregulation of BB′, a constituent that does not share components with the stabilized constituent AA′. That is, stabilization of the constituent AA′ results in the adaptive dynamic downregulation of the antagonist constituents AB′ and BA′ and the concomitant upregulation of the agonist constituent BB′. Triggering CDN Y with the counter trigger T1′, that depletes the stabilized trigger T1, reconfigures CDN Y to the parent CDN X. Integrating CDN X to trigger T2 that stabilizes constituent AB′ results in the triggered upregulation of AB′, the adaptive downregulation of the antagonist constituents AA′ and BB′, and the concomitant upregulation of the agonist constituent BA′. The information encoded in the bare sequence of nucleic acid structures provides versatile means to engineer structurally interchangeable equilibrated constituents that include embedded structural switchable motives that are triggered by auxiliary stimuli.18c The structural reconfiguration of the constituents provides, then, versatile means to control the stabilities of constituents, and guide dynamic transitions of DNA networks.
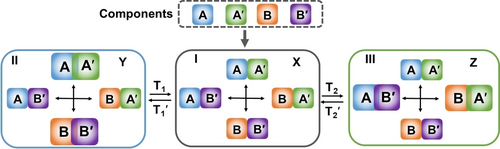
Schematic assembly of a 2×2 constitutional dynamic network (Panel I) and its dynamic reversible reconfiguration into two alternate networks in the presence of T1/T1′ as fuel/anti-fuel triggers, Panel II, or T2/T2′ as fuel/anti-fuel triggers, Panel III.
Indeed, substantial progress in the design and dynamic operation of DNA-based CDNs were reported in recent years.20d Dynamic networks revealing adaptive,77 hierarchically adaptive,78 feedback-driven79 and cascaded and switchable80 reconfigurable properties were demonstrated. Different auxiliary triggers, such as fuel/anti-fuel strands,77 the formation and displacement of triplex structures,81 the K+-ion-induced formation of G-quadruplex and their separation by crown ether77 or the use of photoisomerizable intercalators82 were applied to dynamically reconfigure the CDNs. Different application of dynamically controlled DNA network assemblies were reported, including the operation of switchable CDN-guided biocatalytic cascades83 or the engineering of network guided formation of hydrogels revealing regulated stiffness for controlled drug release.66
The synthetic means to construct DNA tetrahedra of controlled sizes (3–8 nm) close to the dimension of protein scaffolds, and the feasibility to modify tetrahedra with nucleic acid tethers allowing the dynamic switchable dimerization and separation of DNA tetrahedra structures, provides a versatile artificial means to mimic native protein-protein interactions by these nanostructures. Not surprisingly, the DNA tetrahedra frameworks were used as building blocks to construct constitution dynamic networks. The stimuli-triggered reconfiguration of these CDNs provided, then, means to model dynamic control over protein-protein interactions by the DNA tetrahedra nanostructures.
Figure 16A introduces a dynamic network, CDN P, consisting of four equilibrated different-sized tetrahedra dimers AA′, BB′, AB′, and BA′. Each of the dimers is bridged by a different Mg2+-ion-dependent DNAzyme consisting of appropriately engineered subunits.84 The DNAzymes associated with the constituents differ in their substrate binding sites, and the catalytic cleavage rates of the respective fluorophore/quencher-modified substrate (Figure 16A, panel I), provide readout signals quantifying the contents of the equilibrated constituents. That is, by designing appropriate calibration curves relating the rates of the cleavage of the respective substrates to different concentration of the DNAzymes, and monitoring the catalytic rate of the DNAzymes associated with constituents in the CDNs, the contents of the constituents are quantified. Subjecting CDN P to the fuel trigger T1 that hybridizes with AA′ leads to the reconfiguration of CDN P into CDN Q, where AA−T1 is upregulated, AB′ and BA′ are downregulated and concomitantly the agonist tetrahedra-dimer BB′ is upregulated. Treatment of CDN Q with the counter strand T1′ regenerates CDN P. Similarly, subjecting CDN P to the trigger T2 results in the stabilization of BA′−T2, resulting in the dynamic reconfiguration of CDN P into CDN R, where BA′−T2 is upregulated, AA′ and BB′ are downregulated, and BA′ acting as agonist constituent to AB′−T2, is also upregulated. Subjecting CDN R to the counter fuel T2′ regenerates CDN P. The reversible triggered fluorescence changes transduced by the DNAzyme reporter units associated with constituents upon the T1/T1′-triggered dynamic transitions of P→Q→P and the respective fluorescence changes of constituents are summarized in Figure 16B. Similarly, the fluorescence changes of DNAzyme reporter units associated with the constituents upon the dynamic T2/T2′-triggered transitions of P→R→P are presented in Figure 16C. The results demonstrate the dynamic control over the concentrations of the dimer-tetrahedra constituent by means of auxiliary triggers as a model system for guided dynamic control of protein-protein interactions.
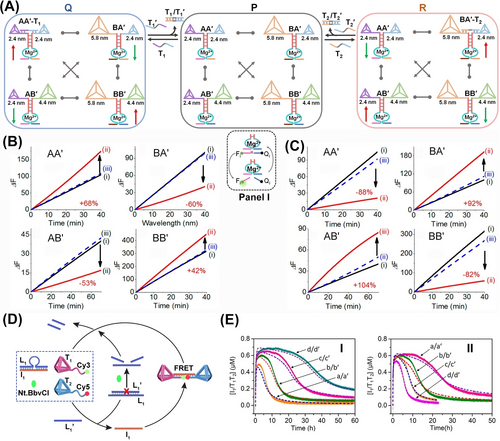
(A) Schematic assembly of a CDN P, consisting of four constituents AA′, BB′, BA′ and AB′ composed of three different sized DNA tetrahedra dimers. The CDN P undergoes dynamic and reversible reconfiguration into CDN Q or R, in the presence of the fuel/anti-fuel triggers T1/T1′ or T2/T2′. Each of the constituents is functionalized with a different Mg2+-ion-dependent DNAzyme catalytic reporter unit used as calculating the concentrations of constituents by cleavage rate, Panel I. Time-dependent DNAzyme reporter units cleavage rates associated with the constituents, upon the cyclic transition between CDN P (i) to CDN Q (ii) and back to CDN P (iii), in the presence of triggers T1/T1′, (B), and upon the cyclic transition between CDN P (i) to CDN R (ii) and back to CDN P (iii), in the presence of triggers T2/T2′, (C). (D) Schematic transient dynamic dimerization and separation of tetrahedra T1 and T2 upon subjecting the reaction module to the fuel strand L1′. (E) Panel I-Transient concentration changes of the T1+T2/I1 in the presence of variable concentrations of the fuel L1′: a/a′-d/d′, 0.2–0.8 μM. Panel II- Transient concentration changes of the T1+T2/I1 in the presence of variable concentrations of the nicking enzyme: a/a′-d/d′, 0.0306–0.0765 μM. (Solid curves, experimental results; dotted curves, computationally-simulated results.) Reproduced from Ref. [84] with permission from the American Chemical Society, copyright (2019). Reproduced from Ref. [90] with permission from the American Chemical Society, copyright (2022).
Moreover, diverse intracellular transformations, such as gene transcription85 and protein translation,86 cell motility,87 and proliferation88 reveal spatial dissipative mechanisms demonstrating transient76c or oscillatory76b processes in clustered networks, leading to temporal structures and catalytic reactions. Substantial recent efforts are directed to develop artificial networks and circuits emulating such natural processes.70a, 70b, 89
The size analogy between DNA tetrahedra and proteins was then employed to develop transient tetrahedra dimerization circuits as models for dynamic, transient, dimerization of proteins in nature90 (Figure 16D). A mute reaction module composed of two tetrahedra T1 and T2, modified with Cy3 and Cy5 fluorophore-functionalized tethers, a duplex L1/I1 and a nicking enzyme (Nt. BbvCI) was employed to mimic dissipative protein dimerization by tetrahedra structures. Subjecting the reaction module to the triggering strand L1′ displaced the duplex L1/I1 yielding the duplex L1/L1′ and the released I1 interlinked tetrahedra T1/T2 into a dimer structure. The duplex L1/L1′ was engineered to be cleaved by the nicking enzyme, leading to the release of L1′ that displaced I1 from tetrahedra dimer, resulting in the separation of dimer and the recovery of inactive reaction module. The transient formation and depletion of tetrahedra structure was followed by the FRET signal changes associated with the Cy3/Cy5 fluorophores linked to the temporal tetrahedra dimer structure (Figure 16E). The transient operation of the circuit was controlled by the concentrations of the activating strand L1′ (Panel I) and the nicking enzyme (Panel II).
6 Loaded DNA-tetrahedra and their Applications
Functionalization of size-tuneable and shape-controlled DNA frameworks with programmed nanoparticle structures37b, 91 or the encapsulation of nanoparticles in DNA caged scaffolds92 finds growing interest as a means to develop functional nanostructures with emergent optical properties,34b, 93 delivery features94 or core-subunits for the built-up of hierarchical structures of enhanced complexities.95 In this section, the use of DNA tetrahedra as scaffold for the assembly of corner-functionalized nanoparticles, caging units of nanoparticles and programmed hierarchical growth of complex structures will be introduced, and the programmed functions of the assemblies will be addressed.
6.1 Nanoparticle-Modified DNA Tetrahedra Structures
Using single-strand functionalized Au NPs (5 nm), the assembly of a DNA-bridged pyramidal tetrahedra structure carrying at each corner the Au NPs was demonstrated.96 By applying DNA conjugated different-sized Au NPs (5, 10, 15, 20 nm) the assembly of asymmetric Au NPs-modified DNA tetrahedra was accomplished. The asymmetric structures were visualized by TEM (Figure 17A and 17B). Although, this structure reveals chiroplasmonic properties, the size homogeneity of the particle limited the intense circular dichroism (CD) response of the structures. Nonetheless, by engineering the length of the DNA edges bridging two-sized AuNPs, a symmetric, non-chiral structure of a pair of 10 nm sized AuNPs and a pair of 20 nm sized AuNPs97 (Figure 17C) structure I, was synthesized. By the appropriate shortening of two mirror-image edges of tetrahedra structures, two asymmetric, chiral mirror-image structures of the four AuNPs were constructed, structures IIa and IIb. As expected, the structure I lacked any circular CD response, yet the asymmetric structures IIa and IIb revealed opposite CD spectra where the CD intensities were controlled by the distances shortening the respective edges, consistent with the distortion degree of the respective tetrahedra (Figure 17C). In a related study, the strand dictated formation of DNA tetrahedra modified at four edges with different types of particles leads to four edges-dictated mirror-images of the asymmetric chiral tetrahedra structures98 (Figure 17D). The chiral structures of the mirror-image NPs-modified tetrahedra was evidenced by the CD bands corresponding to the plasmon absorbance of the AuNPs at λ=530 nm and the AgNPs at λ=410 nm (Figure 17E).
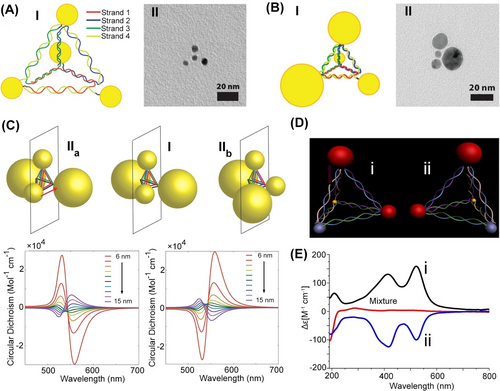
(A) Panel I-Schematic assembly of a symmetric tetrahedron with four 5 nm sized AuNPs. Panel II-TEM image of the symmetric nanostructure. (B) Panel I-Schematic assembly of an asymmetric tetrahedron with four different sized AuNPs (5, 10, 15, 20 nm). Panel II-TEM image of the asymmetric AuNPs-tetrahedron. (C) Mirror images of plasmonic nanostructures IIa and IIb composed of two sets of different sized AuNPs (10 nm and 20 nm) linked to DNA tetrahedra corners consisting of mirror distorted tetrahedra edges, yielding asymmetric mirror-images chiroplasmonic AuNPs structures. The chiroplasmonic structures IIa and IIb reveal opposite circular dichroism bands. (D) Schematic configuration of mirror-image chiroplasmonic nanostructures consisting of different sized and different material nanoparticles (10, 20 nm AuNPs; AgNPs; CdSe/ZnS quantum dot). (E) Opposite circular dichroic spectra of the mirror-image nanostructure shown in (D). (A) and (B) Reproduced from Ref. [99] with permission from the American Chemical Society, copyright (2009). (C) Reproduced from Ref. [102] with permission from the American Chemical Society, copyright (2014). (D) and (E) Reproduced from Ref. [103] with permission from the American Chemical Society, copyright (2012).
6.2 DNA Tetrahedra as Nanocages
The size-programmability of the DNA tetrahedra nanostructure49 and the ability to functionalize intra-edge domains with nucleic acid tether52 provides versatile means to load the tetrahedra with payloads, e.g., nanoparticles99 or enzymes,100 and eventually introduce into the DNA tetrahedra instructive information to guide sequential chemical transformations within tetrahedra frameworks.101
Figure 18A introduces a versatile strategy to encapsulate nanoparticles, e.g., AuNPs into a self-assembled tetrahedra structure.99 A tripod subunit, X, consisting of three arms, where each arm consisting of two duplexes was engineered as functional subunit to assemble the DNA tetrahedra structures. Each of the duplexes associated with the arms was further crosslinked with a nucleic acid strand P that includes domains k and t, and each of duplexes was further functionalized with a tether f. The tether k exhibits self-complementarity features, while the tether k is complementary the nucleic acids k′ coupling the auxiliary AuNPs. The tripod subunits self-assemble into tetrahedra structure, resulting in the intra tetrahedra functionalization of the edges with the tethers k. The permeation of the k′-modified AuNPs through the faces of the nanostructures led to the intra-tetrahedra loading of the structure with AuNPs that hybridized with the tethers k. By applying a counter strand k′′, the AuNPs payload was displaced, resulting in a process that regenerated the vacant tetrahedra structure. The AFM images and cryo-TEM images supported the expected AuNPs-tetrahedron encapsulated structures (Figure 18B, panels I and II). By the further addition of subunits (W) consisting of cross-bridged duplexes extended by tethers g′ and h′ and modified with single strand tethers l, to the tripod subunits (Y) modified with tethers g and h, the inter-bridging of the tripod units into an extended DNA tetrahedra structure was demonstrated102 (Figure 18C). Subjecting the extended DNA tetrahedra structure to AuNPin capped with two functional strands l′ and m′, and then to AuNPout capped with two functional strands m, the gold nanoparticles interacted with the extended tetrahedra assembled into a supramolecular structure consisting of five AuNPs. One AuNPin was positioned in the center of tetrahedra through the hybridization of strand l/l′ and the other four AuNPsout were positioned on the other tethers (m′) of AuNPin through hybridization of the duplexes m/m′. The assembly of a 3D structure of the AuNPs was realized and confirmed by TEM reconstructed cryo-TEM imaging (Figure 18D, panels I and II). Furthermore, the tripod subunits crosslinking concept to assemble DNA tetrahedra cages was further developed to assemble multi-layered tetrahedra nanocages103 (Figure 18E). Towards this goal, three types of tripods X, Y, and Z were designed and two types of extending cross-bridging duplexes W1 and W2 were used for the sequential self-assembly of the multi-layered tetrahedra caged assembly. In the first step, the tripod subunits X were allowed to assemble into the small-sized inner tetrahedra structure. Subjecting the small-sized tetrahedra structure to tripod Y and the extending duplex units W1 resulted in the X-tetrahedra-guided coating of the second layer tetrahedra by the crosslinking of the tripod Y, in the presence of the extending duplexes W1. The medium sized tetrahedra was crosslinked to the inner small tetrahedra by edged strand functionality associated with the two-sized tetrahedra. Similarly, treatment of the two-layered caged tetrahedra structure with the tripod Z and the extending duplex bridging units W2 resulted in the two-layer tetrahedra-guided assembly of the third layer tetrahedra coating where tripod Z were linked by the extender units W2 and concomitantly bridged by duplex formed between tether functionalities associated with the edge of medium sized tetrahedra and the extender units W2. The three-layer caged structure of tetrahedra was confirmed by reconstructed Cryo-TEM images (Figure 18F) and supported by electrophoretic and AFM measurements.
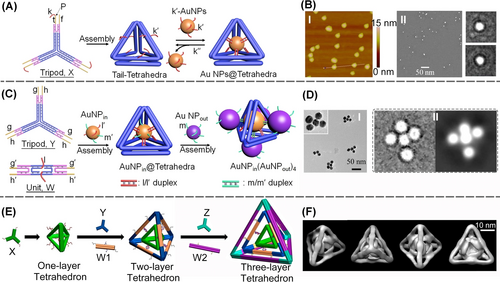
(A) Schemetic assembly of a DNA tetrahedron encapsulating gold nanoparticle. (B) Panel I-AFM image and Panel II–Cryo TEM image of the AuNPs loaded tetrahedra. (C) Schemetic construction of programmed intra-cage deposited AuNPs decorated by four-corner AuNPs aggregates. (D) Panel I-TEM image; Panel II–Cryo TEM image of programmed AuNPs aggregate. (E) Sequential assembly of tetrahedron-in-tetrahedron layer structures. (F) Reconstructed Cryo TEM image of the three-layered DNA tetrahedron structure. (A) and (B) Reproduced from Ref. [99] with permission from the American Chemical Society, copyright (2014). (C) and (D) Reproduced from Ref. [102] with permission from the American Chemical Society, copyright (2015). (E) Reproduced from Ref. [103] with permission from the American Chemical Society, copyright (2015).
DNA tetrahedra were further applied as a structural subunit to self-assemble crystalline Au nanoparticle composites and particularly the challenging diamond superlattice structure104 (Figure 19). Using the M13 phage DNA, as scaffold, and a set of DNA staple strands, a 3D DNA origami bundle-based tetrahedra structure functionalized at its corners with protruding tethers, l, and its edges with protruding tethers, m, was self-assembled (Figure 19A). The reconstructed cryo-TEM image of the assembled tetrahedron subunits, Panel I, reveals an edge length of ca. 36 nm and an internal void volume revealing a cross-section of ca. 9 by 6 nm. Treatment of tetrahedra with nucleic acid m′-functionalized AuNPs resulted in the modification of the internal void containment of tetrahedra with the AuNPs. The cryo-TEM image demonstrated the AuNPs-loaded tetrahedron structures, panel II. Subjecting the unloaded tetrahedra to the l′-tethered AuNPs led to the self-assembly of an ordered face-centered cubic (FCC) crystalline lattice of the aggregated AuNPs. In turn, subjecting the mixture of the AuNPs-loaded tetrahedra to the m′-modified AuNPs resulted in the self-assembly of tetrahedra into a diamond superlattice crystalline composite of the AuNPs (Figure 19A). Tetrahedra-guided assembly of the FCC and diamond crystalline structures was confirmed by small-angle X-ray scattering (SAXS) experiments (Figure 19B). The 2D scattering pattern and the associated structure factor S(q) revealed sharp scattering peaks. The ratio of the positions of the peaks matched …, consistent with an FCC lattice. Similarly, the SAXS peak and associated structure factor S(q) for the diamond superlattice AuNPs aggregate revealed sharp scattering peaks position at ratios corresponding to …, in agreement with the cubic diamond lattice.
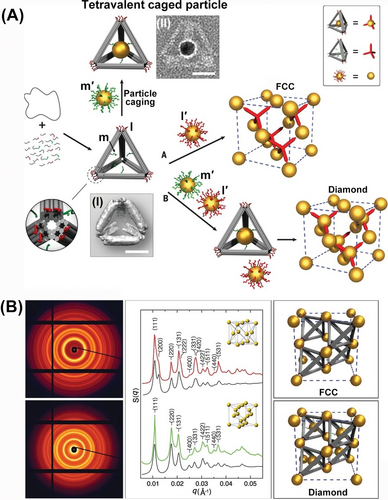
(A) Schematic assembly of FCC and diamond crystalline structure of AuNPs using a 3D DNA origami tetrahedron as scaffold. Scale bars, 20 nm. (B) 2D SAXS patterns of the FCC superlattices of the AuNPs and of the diamond superlattice AuNPs aggregate. Reproduced from Ref. [104] with permission from the American Association for the Advancement of Science (AAAS), copyright (2016).
7 DNA Tetrahedra for Nanomedicine
The functionalization of the DNA tetrahedra corners or edges with nucleic acid tethers provides general means to link recognition aptamer to the DNA tetrahedra for targeting cells or for selective sensing of cellular constituents, e.g., miRNA, while applying the DNA tetrahedra as carriers into the cells. Alternatively, the DNA tetrahedra framework can be used as a platform for the intercalation of drugs or therapeutic agents. These features turn the DNA tetrahedra frameworks as versatile carriers for medical applications. Moreover, the high cell permeation efficacies,105 their biostability,106 the versatile means to modify the tetrahedra corners/edges with fluorophore labels, and the use of the DNA tetrahedra as a confined reaction framework guiding dynamic reconfiguration of therapeutic agents,107 such as siRNA, introduce potential innovative functions for nanomedical applications such as intracellular imaging108 or sense-and-treat gene therapies.107 The present section addresses different uses of the DNA tetrahedra for nanomedicine.
7.1 DNA-tetrahedra as Cell Targeting and Nano-framework Delivery Vehicles
DNA tetrahedra provide an effective 3D structure to interact with cell membrane109 and to assist cell sorting and separation.110 This has been exemplified with the functionalization of the corners of a DNA tetrahedra with one, two or three anti-EpCAM receptor aptamer units105 (Figure 20A). The binding affinity of the different tetrahedra scaffolds to the clustered EpCAM receptor sites associated with circulating tumor cells (CTC) was controlled by the number of aptamer units tethered to tetrahedra backbone (Figure 20B). As the number of aptamer units increased, the synergistic binding of tetrahedra to the clustered receptor was enhanced. The control over the binding affinities of tetrahedra to the receptor sites was, then, applied to select and sort the cancer cells, from a mixture of cells. The different EpCAM aptamer-modified tetrahedra were anchored to magnetic beads and these were used to sort fluorescent (Cy5) stained MCF-7 CTC (Figure 20C). As the number of aptamer units linked to tetrahedra increased the capturing efficiency of the MCF-7 cancer cells was enhanced (Figure 20D). The control over the binding affinities of aptamer-functionalized tetrahedra with cell membranes was further used to separate different cells within a microfluidic device110 (Figure 20E). The glass channels of the device underwent functionalization with two distinct DNA tetrahedra: T1, modified with an aptamer KDED-3a targeting DLD-1 cells, and T2, carrying two aptamers KCHA-10 targeting HCT-116 cells. Despite the similar binding affinities of aptamer KDED-3a and aptamer KCHA-10 towards their respective cells, the microfluidic channel successfully sorted these cell types. Within the channels, tetrahedral scaffolds captured DLD-1 cells labeled with a blue fluorophore and HCT-116 cells labeled with a red fluorophore. The binding affinity of the respective cells to the aptamer-modified tetrahedra was influenced by the number of specific aptamers present. For instance, T1 with one aptamer KDED-3a exhibited weak binding to DLD-1 cells, while T2 with two aptamers KCHA-10 displayed strong binding to HCT-116 cells. Consequently, the separation of the cell mixture was determined by shear forces generated by varying flow rates through the channel. High-flow rates facilitated the release of both DLD-1 and HCT116 cells, releasing a mixture of the two. Conversely, at low flow rates, only weak DLD-1/aptamer complexes were released, with subsequent high flow rates leading to the separation of HCT-116 cells. This mechanism enabled the effective separation of the two cell types, as illustrated in Figure 20F.
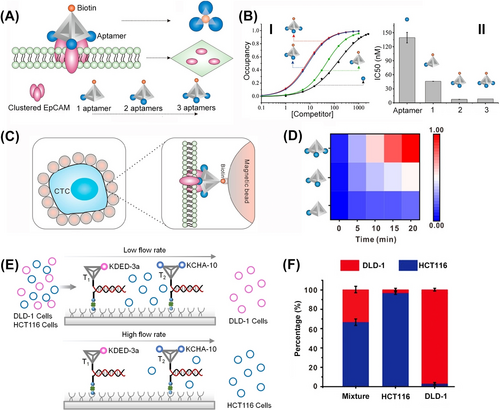
(A) Functionalization of DNA tetrahedra corners with variable numbers of aptamer against the EpCAM receptor associated with cancer cells. (B) Binding affinities of the aptamer-modified tetrahedra to circulating tumor cells (CTC) relating to the number of aptamer units. (C) Schematic separation of EpCAM-functionalized cancer cells from a mixture of cells by the aptamer-modified DNA tetrahedra. (D) Capture efficacy of the EpCAM-containing cancer cells guided by the number of aptamer modified on tetrahedra. (E) Schematic separation of two types of cells (DLD-1; HCT116) by the respective aptamer-modified DNA tetrahedra using microfluidic channels. (F) Separation of the DLD-1 and HCT116 cells by the microfluidic device using controlled binding affinities of the respective DNA-tetrahedra and different flow rates. (A–D) Reproduced from Ref. [105] with permission from the American Chemical Society, copyright (2019). (E) and (F) Reproduced from Ref. [110] with permission from the Wiley-VCH, copyright (2020).
Beyond the adhesion of DNA tetrahedra to cell interfaces by means of aptamer-cell membrane interactions,111 DNA tetrahedra exhibit superior cell permeation properties thus guiding superior permeation of tetrahedra-modified payload-functional carriers.47, 112 Indeed, improved cell permeation of DNA tetrahedra carriers as compared to DNA-duplex functional carriers or Lipofectin transfection means were demonstrated.113 These features were employed to assemble miRNA responsive, doxorubicin (DOX)-loaded N3-UiO-66 DNA-tetrahedra functionalized metal–organic framework nanoparticles, NMOFs114 (Figure 21A). Tetrahedra-modified DOX-loaded NMOFs revealed a ca. four-fold enhanced permeation into MDA-MB-231 cancer cell, as compared to duplex-modified NMOFs, and a ten-fold enhanced permeation as compared to epithelial MCF-10 A breast cells. The enhanced permeation features of the DOX-loaded miRNA-responsive NMOFs were reflected by selective cytotoxicity towards MDA-MB-231 breast cancer cells (miRNA-21 responsive) and HepG2 cancer cells (miRNA-155 responsive). Moreover, Zn(II)-protoporphyrin IX-loaded DNA tetrahedra-functionalized UiO-66 NMOFs, where tetrahedra units are conjugated to the NMOFs by a duplex (17)/(17′) comprising the Vascular endothelial growth factor (VEGF) aptamer in strand (17′), were prepared115 (Figure 21B). In the presence of VEGF, overexpressed in cancer cells, the formation of the VEGF/aptamer complex uncaged the NMOFs, resulting in the release of the Zn(II)-protoporphyrin IX. Its incorporation in the VEGF/aptamer G-quadruplex units resulted in an effective photosensitizer for the light-induced formation of reactive oxygen species (ROS) (Figure 21B). By integrating the Zn(II)-protoporphyrin IX-loaded DNA tetrahedra into MDA-MB-231 cancer cells, effective photodynamic treatment by the ROS agents was demonstrated (Figure 21C). Selective VEGF-triggered photodynamic treatment of the MDA-MB-231 cancer cell, as compared to epithelial MCF-10 A breast cells was realized.
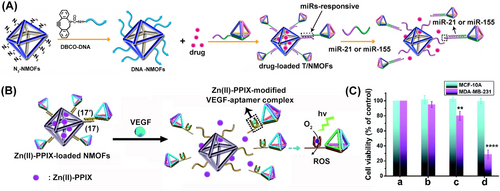
(A) Synthesis of miRNA-responsive, doxorubicin-loaded NMOFs functionalized with DNA tetrahedra for enhanced cell permeation and improved chemotherapeutic treatment of cancer cell. Reproduced from Ref. [113] with permission from the American Chemical Society, copyright (2021). (B) Zn(II)-PPIX-loaded VEGF-responsive DNA tetrahedra gated NMOFs for the photodynamic treatment of cancer cells. (C) Cell viability of MDA-MB-231 breast cancer cells or MCF-10 A epithelial cells upon treatment with: (a) buffer, (b) DNA-tetrahedra-gated Zn(II)-PPIX-loaded NMOFs without irradiation, (c) DNA-duplex-gated Zn(II)-PPIX-loaded NMOFs upon photodynamic irradiation, (d) DNA-tetrahedra-gated NMOFs upon photodynamic treatment. (A) Reproduced from Ref. [114] with permission from the American Chemical Society, copyright (2021). Reproduced from Ref. [115] with permission from the Royal Society of Chemistry, copyright (2021).
The DNA-tetrahedra-stimulated enhanced cell permeation efficacy of drug carrier has been further applied to develop stimuli-responsive lentivirus-loaded DNA-based hydrogel microcapsules for immunogenetic therapy of liver cancer cells116 (Figure 22). DNA-based hydrogel microcapsules loaded with vector-modified lentivirus (green fluorescence protein, GFP or COVID-19 spike protein, SP, vectors) were prepared as outlined in Figure 22A. The microcapsules boundaries were functionalized with the anti-α-fetoprotein aptamer (α-fetoprotein, AFP, is overexpressed biomarker in liver cancer cells), and the DNA tetrahedra, as cell permeating agents. The immunogenetic therapeutic principles of the lentivirus-loaded microcapsules in schematically depicted in Figure 22B. The DNA tetrahedra-facilitated permeation of the microcapsules into liver cancer cells, as compared to normal cells, and the AFP-guided unlocking of the microcapsules in the liver cancer cells resulted in the selective release of the lentivirus in the cancer cells and the superior GFP (or SP) expression in the liver cancer cells. A selective factor of 50,000 for the GFP expression in the cancer cells over healthy liver cells, was observed (Figure 22C). These results originated from the cooperative DNA-tetrahedra-stimulated cancer cell permeation and AFP-induced uncaging of the microcapsules in the liver cancer cells. The effective and selective protein expression in the liver cancer cells was, then, utilized for the efficient immunogenetic therapeutic treatment of liver cancer cells, through the activation of the COVID-19 immune system (Figure 22D). The selectively expressed SP, by the SP-vector-loaded lentivirus permeated into the liver cancer cells, and accumulated in the cancer cell membranes. This led to the activation of the COVID-19 immune system of vaccinated or COVID-19 infected individuals, resulting in the targeted killing of the liver cancer cells. Indeed, the treatment of the liver cancer cells with human Peripheral Blood Mononuclear Cells, PBMCs (White Blood Cells, WBCs) of vaccinated or COVID-19-recovered individuals resulted in ca. 75 %–80 % cancer cell death (within exposing the cells to two doses of the WBCs), whereas no cytotoxic effect on the liver cancer cells was observed upon treatment with WBCs originating from non-vaccinated individuals (Figure 22E).
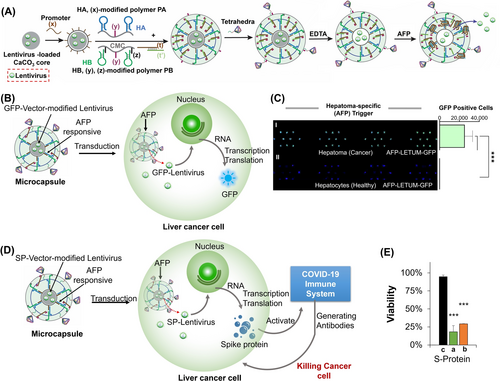
(A) Synthesis of GFP (or SP)-loaded AFP-responsive hydrogel microcapsules functionalized with DNA-tetrahedra. (B) Schematic DNA tetrahedra-guided permeation of the GFP-vector-modified lentivirus-loaded microcapsules into liver cancer cells and the intracellular AFP-triggered unlocking and the intracellular expression of the GFP protein. (C) AFP-triggered release of the GFP-vector-modified lentivirus for selective GFP expression in the liver cancer cells and healthy liver cells. (D) Schematic activation of the COVID-19 immune system by the DNA-tetrahedra-modified SP-vector-lentivirus-loaded microcapsules permeating into the liver cancer cells. (E) Selective killing of liver cancer cells by PMBC cell of: (a) COVID-19 vaccinated individuals. (b) COVID-19 recovered individuals. (c) Non-vaccinated/non-infected COVID-19 individuals. (***p<0.001). Reproduced from Ref. [116] with permission from the Wiley-VCH, copyright (2023).
7.2 DNA-tetrahedra as Therapeutic Carriers
The DNA tetrahedra do not only facilitate the permeation of the nanostructure into cells but protect oligonucleotides against degradation.47, 112 There two features were employed to use DNA tetrahedra as nano-vehicles delivering therapeutic constituents, such as siRNAs into cells (Figure 23A). The edges of DNA tetrahedra were engineered to include duplexes comprising of a target siRNA (e.g., luciferase gene siRNAs) that are further modified with folate ligands targeting KB HeLa cells.117 Treatment of mice bearing xenograft KB tumors with the siRNA-functionalized tetrahedra resulted in over 50 % inhibition of luciferase activity in the tumors as compared to non-treated tumors or tumors treated with the siRNA duplex, in the absence of tetrahedra carrier (Figure 23B) demonstrating the effective delivery and protection of the siRNA by tetrahedra. Similarly, inhibition of the transcription/translation of the mRNAs, e.g., GFP118 or fisZ bacterial gene119 were demonstrated.
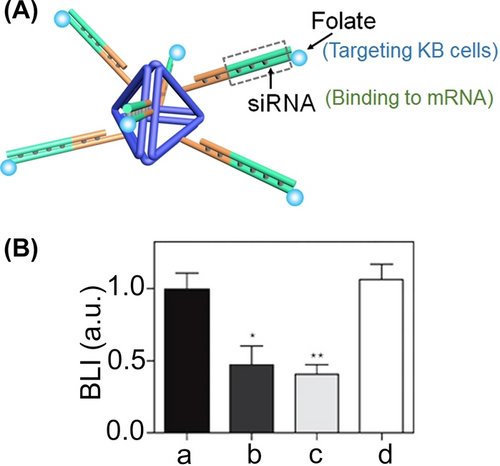
(A) Tetrahedra nanocarrier functionalized with folate-labelled siRNA on its edges for silencing target genes in KB cells. (B) Luciferase silencing in mice bearing KB xenograft tumors treated with: (a) buffer solution, (b) intratumoral tetrahedra modified with folate-conjugated anti-luciferase siRNA, (c) intravenous tetrahedra functionalized with folate-conjugated anti-luciferase siRNA, (d) intravenous folate-conjugated anti-luciferase siRNA (*P<0.138, **P<0.002). (A) and (B) Reproduced from Ref. [117] with permission from Springer Nature, copyright (2023).
The superior cell permeation and stability features of DNA tetrahedra, as compared to other DNA scaffolds introduced useful DNA nanostructures for drug delivery. To reach this goal, the development of means to integrate the drugs as stable non-leaked configurations and the design effective release mechanisms, is essential. In addition, targeting of the drug-loaded tetrahedra to the specific cell environment is important.
One approach to load tetrahedra carrier utilized the double-strand edges of tetrahedra as scaffolds to intercalate the drug.120 This is exemplified in Figure 24A with the assembly of DNA tetrahedra modified at their edges with the nucleic acid tethers that provided anchoring sites for EGFR-receptor nanobodies (fragments of the respective antibody) modified with the tether. The cis-Pt(II)-bisamine/bipyridine anti-cancer drug (56MESS) was intercalated into the duplex edges of tetrahedra duplexes acting as carriers. The supramolecular nanostructures consisting of the nanobody-conjugated, drug-loaded tetrahedra acted as functional targeted carriers that bind to the EGFR receptors associated with A431 skin cancer cells, resulting in tetrahedra facilitated endocytosis. The permeation of the carriers into the lysosome resulted in the enzymatic cleavage of the DNA tetrahedra and the release of the therapeutic drug load. Validation of the efficacy of the drug-loaded nanobody-modified tetrahedra as chemotherapeutic drug carrier toward cytotoxicity of a A431 cancer cell line, as compared to the cytotoxicity of a reference carrier composed of an analog drug-loaded duplex DNA functionalized with the nanobody, was demonstrated (Figure 24B). The cytotoxicity of the drug-loaded tetrahedra was two-fold higher as compared to the reference duplex carrier (90 % cell death as compared to 50 %), due to the enhanced permeation of tetrahedra drug carrier. The enhanced cytotoxicity of the drug-loaded tetrahedra carrier, as compared to the reference duplex-functionalized carrier, was further reflected in in vivo experiments (Figure 24C). In these experiments, mice bearing A431 tumors were subjected to treatment with 56MESS-loaded nanobody-functionalized tetrahedra, and their tumor growth was compared with reference groups. These references included mice treated with unloaded nanobody tetrahedra or 56MESS-loaded nanobody-functionalized duplex carriers. After 20 days, tumors in mice treated with drug-unloaded tetrahedra reached a volume of 1500 mm3, while those treated with drug-loaded duplex carriers reached 1000 mm3. Remarkably, mice treated with 56MESS-loaded nanobody-functionalized tetrahedra exhibited virtually no tumor growth (<20 mm3). This significant inhibition of tumor growth was ascribed to the effective permeation and localized cytotoxicity of the drug-loaded tetrahedra carrier. Related studies121 reported the intercalation of doxorubicin into the duplex domains of DNA tetrahedra carriers, and the use of tetrahedra as a vehicle to enhance the cytotoxic efficacies. Moreover, the DNA tetrahedra scaffold allowed, in addition to the intercalation of the doxorubicin drug, the further modification of tetrahedra corners with the anti-EGFR antibody and the functionalization of one of the corners with a fluorophore (Cy3), as a means to target and visualize the transport and localization of the carrier in cancer cells.
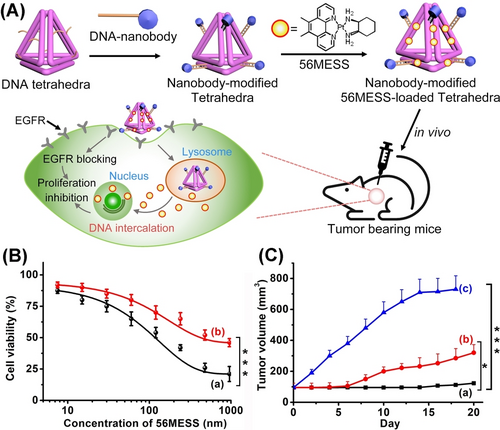
(A) Schematic design of 56MESS intercalated into the duplex domains of DNA tetrahedra and modified with DNA tethers-modified anti-EGFR nanobodies for efficient chemotherapeutic treatment. (B) Cell viability of A431 cancer cells subjected to: (a) tetrahedra carrier. (b) duplex carrier. (C) tumor sizes of A431 tumor bearing mice treated with: (a) tetrahedra carrying 56MESS, (b) duplex carrying 56MESS, (c) 56MESS only. (*P<0.05, ***P<0.001). Reproduced from Ref. [120] with permission from the Wiley-VCH, copyright (2019).
Moreover, an alternative approach has involved the covalent tethering of the anti-cancer drug to the DNA tetrahedra carriers and release of the drug by overexpressed chemical agents present in cancer cells. For example,122 the anti-cancer drug camptothecin was covalently linked to a nucleic acid strand being a part of the DNA tetrahedra carrier using a disulfide bridging bond. The drug-modified DNA tetrahedra was, then, released from the carrier by glutathione (GSH), overexpressed in cancer cells, through cleavage of the S−S bridging bonds. Effective inhibition of tumors growth by the drug-loaded carriers, as compared to treatment with the bare-drug composites, was demonstrated, an effect attributed to the enhanced drug permeation into the cells, assisted by the DNA carrier.
7.3 DNA Tetrahedra for Regenerative Medicine
Recent research activities demonstrated the use of DNA tetrahedra frameworks for regenerative medicine. Particularly, DNA tetrahedra were found to enhance cell proliferation123 and differentiation124 and revealed anti-inflammatory,125 antioxidant126 and immune regulation properties.127 These features of the DNA tetrahedra scaffolds were employed to promote the regeneration of bones128 and blood vessels,129 to induce wound repair130 and tissue regeneration,131 and to affect neurological disorders,132 joint-related inflammatory diseases,133 and immune diseases.127 These broad prospects of DNA tetrahedra nanostructures were addressed in detail in several review articles134 and will be highlighted here with several examples.
Figure 25 introduces the use of the miRNA-2861-loaded DNA tetrahedra as a carrier for controlling bone repair by bone marrow mesenchymal stem cells (BMSCs).128 The DNA tetrahedra carrier were functionalized, at the corners, with the nucleic acid tethers h. The miRNA-2861 that inhibits the expression of the histone deacetylase 5 (HDAC5) protein (that results in the upregulated expression of the runt-related transcription factor 2, Runx2, that ultimately promotes osteogenic differentiation and bone repair) was extended by the tether h′ and was hybridized with tetrahedra framework, yielding a functional carrier for transporting the miRNA into the stem cells (Figure 25A). The mechanism of the bone repair by the BMSCs, interacted with the bone injury, presented schematically in Figure 25B. The cellular RNase H degrades the ribonucleic base domain h′ of the h/h′ resulting in the release of the miRNA-2861 that inhibits the HDAC5 protein, resulting in the promotion of bone injury healing. Figure 25C, panel I, depicts in vitro immunofluorescence images of the HDAC5 protein developed in stem cells treated with the miRNA-2861 functionalized DNA tetrahedra, and respective control systems, and Panel II introduces the statistical analysis of the fluorescence intensities in the respective cell assays. Evidently, the development of the HDAC5 protein is inhibited by the miRNA-functionalized carrier. Similarly, Figure 25D depicts the western blot bands of the HDAC5 proteins formed in the stem cells treated with the miRNA-2861 modified tetrahedra, Panel I, and the quantitative analysis of the bands, Panel II. The results demonstrate significantly inhibited yields of the HDAC5 protein in the cells treated with the miRNA-2861-functionalized carrier, panel II. Figure 25E depicts the effect of the stem cells on the injured bones upon treatment with the miRNA-2861-functionalized tetrahedra frameworks and control systems. After a time-interval of 14 days, the bone-injury treated with BMSCs, interacted with the miRNA-2861 functionalized tetrahedra, is fully healed whereas the control systems did not show a significant bone repair. Furthermore, cells subjected to DNA tetrahedral structure demonstrated enhanced proliferation and migration and retardation of inflammatory processes.135 These features were used to apply DNA tetrahedral framework to promote scarless healing of cutaneous wounds by intervening the AKT-signalling pathway.136 Subjecting keratinocyte (HeCaT cells) or fibroblast (HSF cells) to the DNA tetrahedra frameworks demonstrated enhanced proliferation and migration capacities of the cells (reflected by faster bridging of a bare cell domain, as compared to a control system).
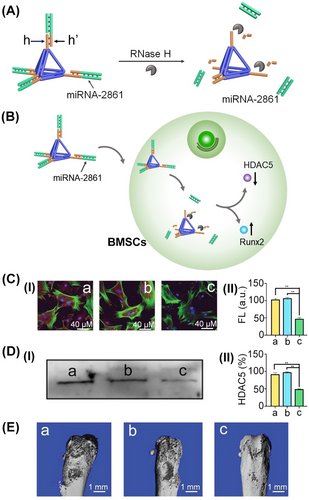
(A) Schematic of a DNA tetrahedra carrier modified at its ends with miRNA-2861 RNA duplexes able to release by RNase H. (B) Schematic inhibition of HDAC5 and the concomitant activation of bone repairing Runx2 in bone marrow mesenchymal stem cells (BMSCs), upon internalization of the miRNA-2861 into stem cells. (C) Immunofluorescence images confirming the inhibition of the HDAC5 upon treatment of the BMSCs with: Panel I-(a) bare DNA tetrahedra. (b) random RNA duplex-modified tetrahedra. (c) miRNA-2861-modified tetrahedra. Panel II–Integrated fluorescence intensities of the images shown in (I). Significance: **p<0.01. (D) Panel I-Western Blot separation of the HDAC5 proteins in the BMSCs treated with: (a) bare tetrahedra. (b) random RNA-functionalized tetrahedra. (c) miRNA-2861-modified tetrahedra. Panel II-Quantitative evaluation of Western Blot stained bands shown in Panel I. Significance: **p<0.01. (E) Effects of BMSCs treated bone injuries subjected to: (a) bare tetrahedra; (b) random RNA-modified tetrahedra; (c) miRNA-2861-functionalized tetrahedra. Reproduced from Ref. [128] with permission from the Wiley-VCH, copyright (2021).
This section has outlined various nanomedical applications of DNA tetrahedra. These applications encompass the utilization of aptamer-modified tetrahedra for targeting and imaging cells, as well as for the controlled separation of cells. Additionally, DNA tetrahedra have been employed to facilitate the permeation of drug-loaded carriers into cells. Specifically, the section introduced the use of DNA tetrahedra to mediate the permeation of drug-loaded nanoparticles that release ROS-generating agents and vector-modified viral agents for the activation of immune-gene therapies targeting cancer cells. Furthermore, it discussed the use of drug-loaded DNA tetrahedra carriers, which are functionalized with specific cell-binding antibodies, for selective chemodynamic and photodynamic treatment. Additionally, the application of DNA tetrahedra carrying bioagents in regenerative medicine presents promising pathways for medical treatment. While the medical functions of DNA tetrahedra as carriers are limited to their small sizes and loading capacities, their major nanomedical advantages are reflected by their integration with carriers exhibiting higher loading capacities, such as porous particles or microcapsules.
8 Controlling Biocatalytic Cascades by DNA Tetrahedra Frameworks
The spatial and topological control of the effectiveness of biocatalytic cascades as a model mimicking effective cellular biocatalytic networks attracts substantial experimental and theoretical interest.137 The operation of biocatalytic cascades in confined environments, such as microdroplets,138 metal–organic framework nanoparticles139 or microcapsules140 were demonstrated. Also, the spatial organization of distance-controlled biocatalytic cascades on supramolecular scaffolds, such as DNA wires,141 2D DNA strips142 or DNA origami frameworks,40b, 143 and the structural positioning of biocatalytic cascades on nanoparticles144 revealed enhanced biocatalytic cascades as compared to the separated biocatalytic constituents. In all these systems, the localized concentration of the products, generated by one catalyst, yielded the substrate that is effectively channeled to the neighbouring biocatalyst, thus providing an effective means for guiding the biocatalytic cascaded transformations. The DNA tetrahedra structures provide a powerful scaffold for controlling biocatalytic cascades. The size-controlled dimensions of tetrahedra, the scaffold-induced ability to precisely position different biocatalyst at tetrahedra corners, and the flexibility to modify the corners of the duplex edges of tetrahedra with chemical functionalities such as −SH or −NH2 or to conjugate to tetrahedra nucleic acid tethers of pre-designed lengths and complementary base compositions provide versatile means to assemble spatially defined biocatalysts-modified DNA tetrahedra systems for controlling biocatalytic cascades.
A related study probed the effect of spatial proximity and structural rigidification of neighboring biocatalysts on the effectiveness of the biocatalytic cascade (Figure 26A).145 The enzymes sarcosine oxidase, SOX, and HRP were randomly assembled on Au surfaces in three different configurations: Configuration I-direct deposition of the enzyme SOX/HRP on the surface, leading to a spatial separation of ca. 50 nm between the biocatalyst; Configuration II-The two enzymes are linked to the Au surface by means of thiolated nucleic acid tether, leading to a spatial separation, exhibiting steric flexibility, of ca. 20 nm and configuration III consisting of random deposition of two DNA tetrahedra nanostructures TM and TL, where tetrahedra TM is substituted at three corners with SH functionalities and the fourth corner functionalized with SOX, and tetrahedra TL is substituted by the three-SH substituents and the fourth corner is modified with HRP, leading to a densely-packed, structurally-rigidified assembly of tetrahedra separated by an inter tetrahedra distance of ca. 10 nm. The enzymatic cascade, (Figure 26B), consisting of the SOX-stimulated demethylation of sarcosine generating H2O2, and the subsequent HRP-catalyzed oxidation TMB by H2O2 to TMBox, was probed electrochemically, and the biocatalytic cascade was controlled by the distance separating the enzymes following the order: configuration III configuration II configuration I.
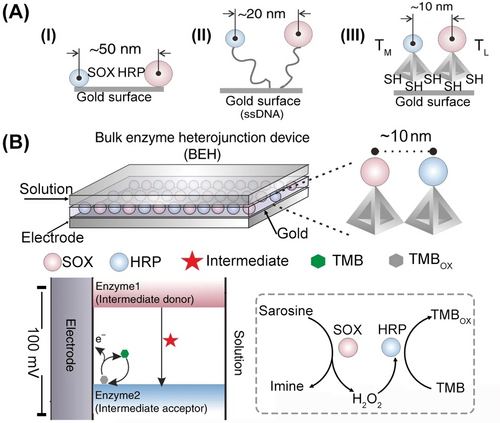
(A) Configuration I-direct deposition of SOX/HRP on the electrode at an average 50 nm separating distance. Configuration II-deposition of thiolated nucleic acid tethered SOX/HRP to the Au-surface with average distance (20 nm). Configuration III-tethering SOX and HRP to thiolated DNA tetrahedra and spatial separation of 10 nm. (B) The bienzyme configurations I, II, and III were allowed to affect the bienzyme cascade where the aerobic SOX-catalyzed oxidation of sarcosine yields H2O2 and subsequently HRP electrocatalyzed the H2O2 oxidation of TMB to TMBOX. The resulting voltammetric response of the different configurations reflects the effect of spatial distance separation on the cascaded bienzyme efficacy. The resulting voltammetric responses followed the order III>II>I. Reproduced from Ref. [145] with permission from Springer Nature, copyright (2020).
9 Conclusions and Outlook
The review article highlighted the plethora of basic scientific concepts introduced by the DNA tetrahedra nanostructures into the field of DNA nanotechnology, and addressed the broad and diverse applications of these frameworks. Three broad topics were addressed. One topic discussed the diverse structural features of DNA tetrahedra including the size tunability of the nanostructures, the availability of geometrically dictated sites including corner, edge, and face positions for integrating spatial functional information into tetrahedra frameworks. These allowed the programmed functionalization of the frameworks with sequence-specific binding strands (aptamers), the modification of the edges with encoded base-sequences exhibiting controlled catalytic properties (DNAzymes) or dictated reactivities toward auxiliary enzymes, such as endonucleases, nickase or ligase. Moreover, the geometrical features of the DNA tetrahedra enabled the programmed and precise positioning of constituents, such as chemical agents or functionalities, e.g., redox labels, thiols, biomaterials (enzymes) or nanoparticles, exhibiting plasmonic or catalytic properties at dictated corner sites, and geometrically-confined inner or outer edge positions. Not surprising, the structural and functional diversity of DNA tetrahedra allowed broad applications, including the development of optical and electrochemical sensors, the use of DNA tetrahedra as scaffolds for programmed catalysis or the application of DNA-tetrahedra as functional bioreactor frameworks, the use of DNA tetrahedra as drug carriers, and the application of DNA tetrahedra frameworks for the self-assembly of crystalline and chiroplasmonic nanoparticle structures. Nevertheless, further applications of the nanostructures may be envisaged. For example, DNA machineries such as polymerase/nickase or the rolling circle amplification find growing interest as biomolecular machineries acting as highly sensitive sensing platforms.146 The integration of these sensing machinery templates as a part of the DNA tetrahedra framework may provide powerful means for point-of-care highly sensitive multiplexed sensing. Alternatively, the DNA tetrahedra might be utilized as vehicle for facilitated permeation of the DNA machineries into cells for multiplexed sensing of genes or imaging of biomarkers. Moreover, DNA tetrahedra provide a promising constituent for future material science applications. Recent research activities applied crossover or Y-shaped DNA subunits147 or oligo-adenine/cyanuric acid supramolecular complexes148 as polydentate constituent to assemble DNA-based hydrogels or DNA-structures, such as fibers. The polydentate configuration of corner-modified DNA tetrahedra may provide a useful building block of hydrogels or material of controllable stiffness properties.
The second topic introduced in the review article addressed the integration of DNA tetrahedra as functional constituents in dynamic networks, e.g., constitutional dynamic networks or transient, dissipative, networks. Specifically, size-similarities of DNA tetrahedra to small proteins, and the recent demonstration of tetrahedra-modified transcription machineries in hydrogel microcapsules140 as protocell models suggest that the integration of dynamic DNA tetrahedra networks into cell-like containments, such as giant fused liposomes149 could provide important steps toward designing synthetic cells.
The third topic discussed in the review article addressed the interactions of DNA tetrahedra with biological environments and specifically their interactions with cells. In fact, the unique structural properties of DNA tetrahedra and their modes of interactions with cells provide the most promising medical and therapeutic applications of these nanostructures. The enhanced cell-permeation features of DNA tetrahedra, and the functionalization of the DNA-tetrahedra with cell-targeting aptamer were broadly used for intracellular sensing, imaging and therapeutic applications. Indeed, DNA tetrahedra acted as effective cell-permeating carriers of drugs, imaging agents or antibodies. The multimode modification of DNA tetrahedra suggests, however, that DNA-tetrahedra structures of enhanced complexities and functionalities could be dynamically generated in cellular environments for exciting future applications. For example, be modification of the corners of DNA tetrahedra with appropriately engineered miRNA (or other biomarker) responsive hairpin structures, the biomarker-triggered intracellular hybridization chain reaction could lead to complex tetrahedra chains, catalytic structures or dynamic DNA networks revealing improved sensing, imaging, and therapeutic applications. Indeed, a recent study150 demonstrated the functionalization of DNA tetrahedra with four nucleic acid hairpin structures that upon permeation into cells led to the miRNA-triggered formation of a constitutional dynamic network for therapeutic applications. Evidently, DNA tetrahedra find pivotal roles in developing DNA nanotechnology across a broad scope of scientific disciplines. The much advancement reporting the use of DNA tetrahedra for imaging biological events at single-cell levels,151 bioanalysis,152 and nanomedicine133, 153 ensure that DNA tetrahedra will provide viable frameworks for exciting innovative applications.
Acknowledgments
We acknowledge funding support from the Israel Science Foundation (project 2049/20).
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Biographical Information
Yu Ouyang received his Ph.D. degree from the Nanjing University of Science and Technology, China, in 2020, working on the assembly of nanomaterials. Since 2020, he holds a postdoctoral position in the laboratory of Prof. Itamar Willner at The Hebrew University of Jerusalem, and his research interests focus on the functionalized nanozymes and dynamic nucleic acid networks and their applications.
Biographical Information
Pu Zhang received her Ph.D. degree from Southwest University, China, working under the supervision of Prof. Ruo Yuan and Prof. Yaqin Chai on DNA nanostructure-based electrochemiluminescent biosensors. From 2018 to 2021, she worked as a postdoctoral fellow in the laboratory of Prof. Itamar Willner at the Hebrew University of Jerusalem, where her research focused on nucleic acid-functionalized nanomaterials and their applications for biosensing, cellular imaging, and cancer therapy. She joined Southwest University in 2021, where she was appointed professor.
Biographical Information
Itamar Willner completed his Ph.D. at The Hebrew University of Jerusalem in 1978. After postdoctoral research at U. C. Berkeley, he joined The Hebrew University of Jerusalem, where he has been Professor since 1986. His research interests include DNA nanotechnology, bioelectronics, nanobiotechnology, artificial photosynthesis, artificial enzymes, dynamic networks, and stimuli-responsive materials. He received the Israel Prize in Chemistry, the Rothschild Prize, the EMET Prize, and the Israel Chemical Society Gold Medal.He is a member of the Israel Academy of Sciences and Humanities, the German Academy of Sciences-Leopoldina, and the Chinese Academy of Sciences.